Abstract
Background:
Pestiviruses in general, and Bovine Viral Diarrhea (BVD) in particular, present several potential targets for directed antiviral therapy.
Material and Methods:
The antiviral effect of Cynanchum paniculatum (Bge.) Kitag (Dog strangling vine: DS) extract on the bovine viral diarrhea (BVD) virus was tested. First, a cytotoxicity test in MDBK (Madin-Darby bovine kidney) cells was done with all organic extract concentrations.
Results:
The cytotoxic concentration CC50 for the ethyl acetate (EA) extracts was 18.2 ug/ml. In the tissue culture, infectious dose (TCID50) assay, the BVD virus decreased when treated with 18.2 ug/ml of the ethyl acetate extracts.
Conclusion:
Ethyl acetate extracts and fractions of the DS extract could be used as a potential antiviral for BVD.
Keywords: Antiviral, Bovine viral diarrhoea(BVD) virus, Cynanchum paniculatum (Bge.) Kitag. Ethly acetate extract
Introduction
Bovine viral diarrhea (BVD) virus is an economically-important cattle disease. Cattle persistently infected with BVDV (BVDV-PI) are the primary reservoir for BVDV infection. BVD viruses are classified into two genetic species (BVDV- 1 and -2) that are further divided into numerous subgroups, particularly for BVDV-PI (Bachofen et al., 2010). BVDV is a pathogen that affects multiple organ systems in many animal species. Infection, disease, or both have been described in cattle, sheep, goats, pigs, bison, alpacas, llamas, and white-tailed deer, among others (Walz et al., 2010). The BVD virus is an enveloped positive-strand RNA virus that has generic status in the family Flaviviridae (Collett et al., 1988). It can lead to a variety of clinical outcomes that range from subclinical infections to more severe presentations including abortion, infertility and the fatal mucosal disease (Baker, 1995).
DS is a perennial herb. Its roots are tenuous and dense, with a peculiar scent. Its stem is tiny, erect, unbranched, hairless or slightly haired. Its leaves are sessile and opposite; the blades are lanceolate to linear, 4-13 cm long, 3-15 mm wide, attenuate apically, tapered at the base, hairless on both sides or sparsely soft-haired on the upper side, somewhat rolled backward and ciliolate at the margin, dark green on the upper side and light green underneath and the main veins being raised. The thyrse occurs from the leaf axil near the top of the stem and comprises 10-odd flowers; the calyx is 5-partite with the lobes ovally lanceolate; the corolla is yellowish green and 5-partite, its lobes are broad-ovate, spread or rolled outward; each flower possesses 5 kidney-shaped yellow succulent paracorolla segments (which are united with the stamens at the base), 5 stamens whose filaments are fused into a tube and topped by 2-celled anthers (with each cell having 1 pollen mass, which is drooping and whose stalk is short and stretched), and 1 pistil with a superior ovary (composed of 2 separate carpels) and 2 styles topped by pentagonal stigmas which are slightly protruding at the top. The follicles are horn-shaped, solitary, light brown, with many seeds. The seeds are ovoid, flattened and dark brown, each exhibiting a cluster of minute long white hairs on the top (WANG Jian-sheng, 2009).
DS an important Chinese traditional drug plant, is widely used in traditional prescriptions. Its root and rhizome have been used for rheumatoid arthritis, a sedative, traumatic injuries, urticaria and eczema (ChemicalIndustryPress, 2005). Recently, it was found that the extract of DS could inhibit the growth of human cancer cells (Lee et al., 2003). The present study was done to develop a new natural product reagent that has antiviral effects against BVD; thus, we assayed fractions from DS extracts.
Material and Methods
Plant Material
Cynanchum paniculatum (Bunge) Kitag roots were collected from the Jaechun Yakcho market, Chungchungbuk-do, South Korea, in september 2015, and were identified by Department of Plant Resources, Kongju National University. Voucher specimen (number 15SE2422) has been deposited at the Department of Plant Resources in Kongju National University.
Solvent Fractionation
Cynanchum paniculatum (Bunge) Kitag root was dried and ground into a powder. The air-dried plant of Agrimonia pilosa Ledeb. (525 g) was fractioned with methanol (1.5 L χ 3) at room temperature. The plant fractions were filtered through filter paper (Whatman, 47 mm, USA) and then evaporated to dryness using a rotary vacuum evaporator at 45 °C in a water bath. The methanol fraction of the plant was partitioned with organic solvents of different polarities to yield n-hexane (Hex), chloroform (CHCl3), ethyl acetate (EtOAc), n-butanol (BuOH) and distilled water (H2O) fractions. The concentrate was recovered with a small volume of solvent, and the container was kept open at room temperature until all the residual solvent had evaporated. The dried crude fractions were dissolved in dimethyl sulfoxide (DMSO, Biosesang, Korea, >99%) at a final concentration of 20 mg/ml. The samples were stored at 4 °C.
Cell Culture
Madin-Darby bovine kidney (MDBK) cells were purchased from the American Type Culture Collection (ATCC). MDBK cells were grown in a 75T-Flask using Minimum Essential Medium (MEMA, Thermo, USA) supplemented with 5% (v/v) fetal bovine serum (FBS, GIBCO/BRL, USA) and 1% (v/v) Anti-anti (Antibiotic-Anti mycotic, GIBCO/ USA).
Virus Culture
The Bovine Viral Diarrhea (BVD) virus was purchased from the American Type Culture Collection (ATCC). The BVD virus was propagated using the MDBK cells; the cytopathic effect (CPE) was observed. Samples were subject to repeated freezing and thawing twice and then centrifuged for 5 min at 2000 rpm. We removed the aqueous layer and put the materials into cryogenic vials; these were stored at -70 °C until use. A titer of the virus infection was performed using the Spearman-Karrer method; the 50% tissue culture infective dose (TCID50) was calculated as 1.5× 106/ml.
MTT Assay (MDBK cell)
Cell viability was measured using the Microculture tetrazolium (MTT) assay method. MDBK cells were maintained upon reaching 90% confluence; the cells were trypsinized, and 2x104 cells/ml were seeded in 96 well-plates and incubated at 37 °C in a humidified 5% CO2 atmosphere. After 24 hr, the cells were washed with warmed phosphate buffered saline (PBS) twice before the culture medium was replaced. For the fraction cytotoxicity assay, 20 mg/ml Cynanchum paniculatum(Bunge) Kitag. fraction was diluted in 0.7% DMSO. The fraction samples were diluted with cell growth medium at 10 mg/ml, 5 mg/ml, 2.5 mg/ml, 1.2 mg/ml, 0.6 mg/ml, and 0.3 mg/ml, and 100 ul was added to each well. The plate was returned to the incubator (37 °C and 5% CO2) for 24 hr. After incubation, the cytopathic effect (CPE) was observed, and 50 ul of MTT (3-(4, 5-Dimethylthiazol-2-yl)-2) solution were added to each well and reacted for 4 hr in the incubator (37 °C, 5% CO2). After the reaction, we removed the MTT solution and added dimethyl sulfoxide (DMSO) at a volume of 100 ul to each well. The absorbance at 570 nm was measured using the Microplate Spectrophotometer (Biotech, Eon, USA). The fraction sample of the solvents for the negative control and the cell proliferation effect from the difference in the absorbance were compared. CPE was observed, and the maximum non-cytotoxic concentration (MNCC) of the fraction was determined.
Antiviral activity Test
The tissue culture infectious dose assay (TCID50) assay was done. The antiviral activity of the Cynanchum paniculatum(Bunge) Kitag. fractions was also assayed in 96-well plates. The initial dilution of the fractions was performed in 0.7% DMSO, and the MNCC of the fractions in the MDBK cells was used. The virus was serially diluted in MEMA containing 1% (v/v) Anti-anti, and the virus titer was determined as 104 TCID50/ml. BVD virus growth medium (50 ul) was placed in each well, and 50 ul doses of each fraction for the serial dilutions corresponding to the MNCC were added. We added the BVD virus suspension (104 TCID50/ml) at a volume of 50 ul to each well and then incubated these samples at 37 °C and 5% CO2 allowing them to adsorb for 90 min. We added the MDBK cells (2.5× 103 cells/well) at a volume of 100 ul to each well and incubated the samples at 37 °C in a humidified 5% CO2 atmosphere; viral plaques were counted after 72 hr. The BVD virus growth medium containing the MDBK cells was used as a positive control. The BVD virus growth medium containing the MDBK cells and the virus was used as a negative control.
Statistical Analysis
All experiments were repeated 3 times, and all data were expressed as the mean ± standard error (SE). A P value of < 0.05 was considered to be statistically significant.
Results
Plant Extracts
Cynanchum paniculatum(Bunge) Kitag Root (1kg) was partitioned with different polaritic organic solvents (n-hexane, CHCl3, EtOAc, n-BUOH and water fractions). The mass of each fraction was 352.1 g for the MeOH fraction, 32.3 g for the n-Hex fraction, 25.2 g for the CHCl3 fraction, 41.4 g for the EtOAc fraction, 10.6 g for the n-Buthyl alcohol fraction, and 91.1 g for the D.W. fraction (Table 1).
Table 1.
Cynanchum paniculatum(Bunge) Kitag extract mass for each solvent
| Solvent | Mass of Extracts (g) |
|---|---|
| Methyl alcohol (ME) | 352.1 |
| n-Hexane (HE) | 32.3 |
| Chloroform (CH) | 25.2 |
| Ethyl acetate (EA) | 41.4 |
| n-Buthyl alcohol (BU) | 10.6 |
| H2O (WT) | 91.1 |
The MTT assay for Cytotoxicity Effects
The potential cytotoxicity of all fractions of Cynanchum paniculatum(Bunge) Kitag in MDBK cells was evaluated by MTT assays. MDBK cells were grown (3.5 × 103) in 96-well plates for 48 hr. The medium was then replaced with medium containing serially diluted solvents of the total extract of Cynanchum paniculatum(Bunge) Kitag. The cells were further incubated for 72 hr. Next, the culture medium was removed, and added media to each well and then incubated at 37°C for 4 hr. After the incubation period, the cytopathic effect (CPE) was observed, and the plate was examined under a binocular microscope. The dead cells in each well were counted. The monolayers of MDBK cells incubated only with growth medium were used as a control. The DMSO control was non-cytotoxic in the MDBK cells. The results show that treatment with the Ethyl acetate extracts (EA) reduced the viability of the MDBK cells and the CC50 of the EA in MDBK cells was 18.2 μg/ml (Figure 1).
Figure 1.
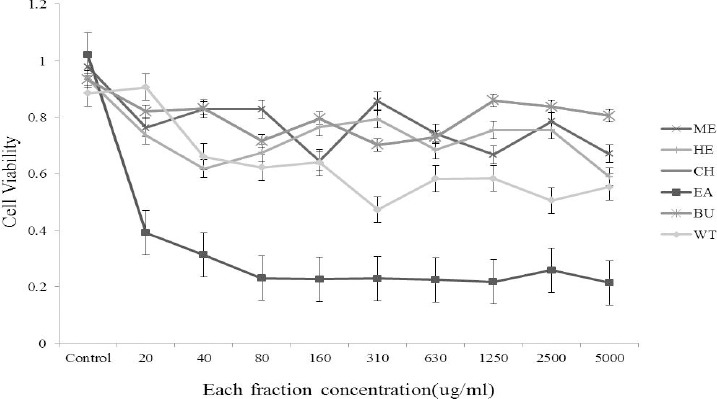
The cytotoxic effect of each fraction in MDBK cells. The cells were treated with various concentrations of each fraction, and the cell viability was determined by MTT assays.
The antiviral activity of the EA fractions was evaluated. Microscopic examination showed that MDBK cells infected with the BVD virus exhibited cytopathic effects including rounding, detachment and death of the cells. Treating the MDBK cells with 18.2 μg/ml EA significantly reduced the cytopathic effect produced by the virus (Figure 2).
Figure 2.
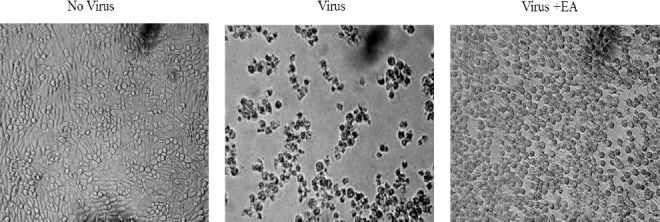
Microscopic examination of the MDCK cells infected with BVD virus
The Antiviral Activity
The antiviral activity of all Cynanchum paniculatum (Bunge) Kitag extracts against the BVD virus is presented in Table 2. The EA MNTD is 18.2 ug/ml. In particular, the extract at low concentrations had a strong antiviral effect against the BVD virus because the BVD virus is a RNA virus. Thus, the Cynanchum paniculatum (Bunge) Kitag extract has an antiviral effect against an RNA virus.
Table 2.
Antivirus activity of the sample against the BVD virus
| Sample | Concentration (ug/ml) | **MNTD (ug/ml) | ||||
|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 80 | 160 | ||
| EA | - | ++ | ++ | ++ | ++ | 18.2 |
| *control (P.C) | - | |||||
| †control (N.C) | - | |||||
MNTD: Maximum nontoxic dose;
P.C: Positive control(Amantadine);
N.C: Negative control(DMSO) ++: Strong toxicity; +: Toxicity; -: Non-toxicity.
Discussion
Bovine viral diarrhea virus (BVDV) is a major economic pathogen in cattle populations throughout the world, with an incidence of infection that is often in excess of 70% (Houe, 1999). Additionally, this virus is responsible for considerable animal suffering and economic damage to farmers (Duffell et al., 1986). The BVD virus is a member of the Pestivirus genus in the virus family Flaviviridae which is the same as other pestiviruses including classical swine fever virus and sheep disease virus. BVDV has a single-stranded, positive-sense RNA genome of approximately 12.5 kilobases. BVDV is most commonly associated with cattle populations, but infection of a variety of domesticated and exotic ungulates has also been reported (L0ken, 1995).
Cynanchum paniculatum (Bunge) Kitag is a vivacious plant distributed across all of Korea and also cultivated in China. The root of Cynanchum paniculatum (Bunge) Kitag is used as a Chinese traditional medicine. Cynanchum paniculatum(Bunge) Kitag produces compounds with anti-bacterial and selective anti-fungal properties, and inhibit the growth of many pathogens. The roots contain hemolytic glycosides, which are toxic to mammals, including livestock. Cynanchum paniculatum (Bunge) Kitag is also distasteful to many insect larvae and toxic to some, including monarchs (Choi et al., 2006).
In this study, we first measured the Cynanchum paniculatum (Bunge) Kitag extract mass. The mass of each fraction was 352.1 g for the MeOH fraction high, 32.3 g for the n-Hex fraction, 25.2 g for the CHCl3 fraction, 41.4 g for the EtOAc fraction, 10.6 g for the n-Buthyl alcohol fraction, and 91.1 g for the Water fraction. Then, we tested the cytotoxicity with the MTT assay in MDBK cells using the Cynanchum paniculatum (Bunge) Kitag fraction. The EA fraction cytotoxic concentration CC50 level is 18.2ug/ml which is the same level as the effective level for the antiviral effect. One limitation of the present study is that data on the composition of each organic solvent extract were not collected. Despite this limitation, this research data suggest that the functional fraction (EtOAC) has one single compound responsible for the antiviral effect and no cytotoxicity. In conclusion, it appears that the antivirus activity of the Cynanchum paniculatum (Bunge) Kitag extract is from secondary metabolites. This study supports the possibility that the Cynanchum paniculatum (Bunge) Kitag could be an alternative antiviral drug for BVDV.
References
- 1.Bachofen C, U Braun, M Hilbe, F Ehrensperger, H Stalder, E Peterhans. Clinical appearance and pathology of cattle persistently infected with bovine viral diarrhoea virus of different genetic subgroups. Veterinary microbiology. 2010;141:258–267. doi: 10.1016/j.vetmic.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker J. C. The clinical manifestations of bovine viral diarrhea infection. The Veterinary clinics of North America. Food animal practice. 1995;11:425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- 3.ChemicalIndustryPress B. The State Pharmacopoeia Commission of the People’s Republic of Chin. 2005 [Google Scholar]
- 4.Choi J. H, Jung B. H, Kang O. H, Choi H. J, Park P. S, Cho S. H, Kim Y. C, Sohn D. H, Park H, Lee J. H, Kwon D. Y. The anti-inflammatory and anti-nociceptive effects of ethyl acetate fraction of cynanchi paniculati radix. Biological & pharmaceutical bulletin. 2006;29:971–975. doi: 10.1248/bpb.29.971. [DOI] [PubMed] [Google Scholar]
- 5.Collett M. S, R Larson, S. K Belzer, E Retzel. Proteins encoded by bovine viral diarrhea virus: the genomic organization of a pestivirus. Virology. 1988;165:200–208. doi: 10.1016/0042-6822(88)90673-3. [DOI] [PubMed] [Google Scholar]
- 6.Duffell S. J, M. W Sharp, D Bates. Financial loss resulting from BVD-MD virus infection in a dairy herd. The Veterinary record. 1986;118:38–39. doi: 10.1136/vr.118.2.38. [DOI] [PubMed] [Google Scholar]
- 7.Houe H. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Veterinary microbiology. 1999;64:89–107. doi: 10.1016/s0378-1135(98)00262-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee S. K, K. A Nam, Y. H Heo. Cytotoxic activity and G2/M cell cycle arrest mediated by antofine, a phenanthroindolizidine alkaloid isolated from Cynanchum paniculatum. Planta medica. 2003;69:21–25. doi: 10.1055/s-2003-37021. [DOI] [PubMed] [Google Scholar]
- 9.Walz P. H, D. L Grooms, T Passler, J. F Ridpath, R Tremblay, D. L Step, R. J Callan, M. D Givens, M American, College of Veterinary Internal Control of bovine viral diarrhea virus in ruminants. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. 2010;24:476–486. doi: 10.1111/j.1939-1676.2010.0502.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Jian-sheng C. Z.-r, LI Ji-xiang. Identification between Paniculate Swallowwort and a false one. Tianjin Journal of Traditional Chinese Medicine. 2009;03:208. [Google Scholar]


