Abstract
Background:
The aqueous extract of Cucurbita ficifolia (C. ficifolia) fruit has demonstrated hypoglycemic effect, which may be attributed to some components in the extract. However, the major secondary metabolites in this fruit have not yet been identified and little is known about its extra-pancreatic action, in particular, on liver carbohydrate metabolism. Therefore, in addition to the isolation and structural elucidation of the principal components in the aqueous extract of C. ficifolia, the aim of this study was to determine whether or not the hypoglycemic effect of the aqueous extract of Cucurbita ficifolia (C. ficifolia) fruit is due to accumulation of liver glycogen in diabetic mice.
Materials and Methods:
The aqueous extract from fruit of C. ficifolia was fractionated and its main secondary metabolites were purified and chemically characterized (NMR and GC-MS). Alloxan-induced diabetic mice received daily by gavage the aqueous extract (30 days). The liver glycogen content was quantified by spectroscopic method and by PAS stain; ALT and AST by spectrometric method; glycogen synthase, glycogen phosphorylase and GLUT2 by Western blot; the mRNA expression of GLUT2 and glucagon-receptor by RT-PCR; while serum insulin was quantified by ELISA method. A liver histological analysis was also performed by H&E stain.
Results:
Chemical fingerprint showed five majoritarian compounds in the aqueous extract of C. ficifolia: p-coumaric acid, p-hydroxybenzoic acid, salicin, stigmast-7,2,2-dien-3-ol and stigmast-7-en-3-ol. The histological analysis showed accumulation of liver glycogen. Also, increased glycogen synthase and decreased glycogen phosphorylase were observed. Interestingly, the histological architecture evidenced a liver-protective effect due the extract.
Conclusion:
Five compounds were identified in C. ficifolia aqueous extract. The hypoglycemic effect of this extract may be partially explained by liver glycogen accumulation. The bioactive compound responsible for the hypoglycemic effect of this extract will be elucidated in subsequent studies.
Keywords: Cucurbita ficifolia, Cucurbitaceae, liver glycogen, hypoglycemic plants, p-coumaric acid, salicin, p-hydroxybenzoic acid
Introduction
Cucurbita ficifolia Bouche (Cucurbitaceae), popularly known as “chilacayote” in Mexico, is a plant whose edible fruits are empirically used as anti-diabetic remedy (Alarcon et al., 2002). C. ficifolia has demonstrated hypoglycemic effects in several diabetes animal models, and in type-2 diabetic patients (Acosta et al., 2001; Alarcon et al., 2002; Xia and Wang, 2006a). This hypoglycemic effect of C. ficifolia has been associated with promoting insulin secretion and rise in mRNA expression of insulin and Kir6.2 in RINm5F cells (Miranda et al., 2013). Despite evidence supporting the hypoglycemic effect of C. ficifolia at pancreatic level, little is known about its extra-pancreatic actions, particularly on the carbohydrate metabolism modulated by the liver. In this sense, Xia and Wang (2006b) suggested that the hypoglycemic effect of C. ficifolia might implicate changes in hepatic glycogen. However, this has not yet been confirmed through histological and biochemical studies that include the quantification of glycogen and evaluation of the enzymes involved in the liver glycogen synthesis and its degradation.
In relation to the phytochemical studies of C. ficifolia fruit, Xia and Wang (2006b) proposed to D-chiro-inositol (DCI) as one of the principal components and as one of the probable responsible of its hypoglycemic effect. In addition, nicotinamide, polyphenols, flavonoids and cucurbitacin triterpenes have been identified in C. ficifolia aqueous extract, which also might participate in this hypoglycemic effect; in fact, a hypoglycemic effect of similar intensity has been observed in a free DCI aqueous extract (Banderas et al., 2012; Fortis et al., 2013; Roman et al., 2012). Therefore, apart of DCI, other compounds should be participating in the hypoglycemic effect of C. ficifolia. As in different phytochemical studies with the aqueous extract of C. ficifolia have been observed differences in its composition, it is necessary to perform a chemical analysis to determine the majoritarian compounds in this extract through of its chemical fingerprint. Therefore, in addition to the isolation and structural elucidation of the main components in this extract, the aim of this study was to establish whether the hypoglycemic effect of C. ficifolia involves the accumulation of liver glycogen in diabetic mice through the analysis of the key enzymes involved in the synthesis and degradation of liver glycogen and examination of liver histological architecture.
Materials and Methods
Authentication of plant
Fresh mature fruits of C. ficifolia Bouche (Cucurbitaceae) were collected, taking into account the international and Mexican rules concerning the biodiversity rights, in May 2012, at San Bartolo el Chico, Acolman, Estado de Mexico, Mexico. Experts at Mexican Institute of Social Security carried out the botanical identification (IMSSM-Herbarium, Voucher Specimen Num. 11119).
Extract preparation
The aqueous extract was obtained in accordance with the method reported by Fortis et al., 2013. The dried ripe fruit (100 g) was macerated with water (1:l) for 72 h; the aqueous phase was recovered every 24 h. The supernatant was filtered and freeze-dried (yield 5%).
Structural characterization of the main components in C. ficifolia
The chromatographic fingerprint of the active extract was taken by HPLC, in a Waters liquid chromatography system equipped with a separation module system (Waters, Massachusetts, USA) and a photodiode array detector (Waters, Massachusetts, USA), using the Empower Pro software. Separation was carried out using a Prevail C-18 column (Grace, 150 x 4.6 mm; 5 μm; Columbia, Illinois, USA). The mobile phase consisted of a mixture of trifluoroacetic acid solution (0.5 %, solvent A) and acetonitrile (solvent B) in several ratios. The detection wavelength was scanned at 190-600 nm. As a concentration of 3 mg/ml of the aqueous extract gave a low fingerprint 100 mg of dry extract was suspended in 5 ml methanol (20 mg/ml) to increase the concentration of secondary metabolites. Commercial standards (Sigma Aldrich, Missouri, USA) of p-coumaric acid, gallic acid, catechin, nicotinamide and rutin were compared with the aqueous extract under these chromatographic conditions. Quantification of p-coumaric acid was achieved using several doses of a commercial standard following the same described HPLC method.
For DCI detection, it was used the previously HPLC method described by Banderas et al. (2012). The limit of detection (LoD= 50 μg/ml) and limit of quantification (LoQ=137 μg/ml) were determined (signal-noise ratio) and under these analytical parameters, the DCI compound was not detected in this C. ficifolia extract. All NMR spectra were recorded on an Agilent DD2 600 spectrometer (Agilent Technologies, California, USA), at 600 MHz for H NMR, 1H-1H COSY, HSQC and HMBC, and at 150 MHz for 13C NMR in CDCl3.
Spectrometric analysis was carried out on a gas chromatography-mass spectrometry (GC-MS) (Agilent Technology 6890, California, USA) equipped with a quadrupole mass detector in electron impact mode at 70 eV. Volatile compounds were separated on an HP 5MS capillary column and compared with their mass spectra of the National Institute of Standards and Technology (NIST) 1.7 Library.
Isolation and identification of major compounds from C. ficifolia
A portion of the aqueous extract (70 g) was fractionated by silica gel 60 in an open chromatographic column (100 g, 70-230 mesh, Merck Millipore, Massachusetts, USA). The mobile phase consisted of a mixture of n-hexane: isopropanol, resulting in two fractions: CfC1F1 (1.8 g) and CfC1F2 (1.7 g). The fraction CfC1F1 (1.8 g) was loaded onto open column chromatography with silica gel 60 (15 g, 70-230 mesh, Merck Millipore, Massachusetts, USA), using a gradient system with n-hexane: ethyl acetate-methanol mixture, resulting in 12 fractions. The spectrometric analysis of CfC2F5 showed the presence of stigmast-7,22-dien-3-ol and stigmast-7-en-3-ol, respectively (Table 1). The chemical structure of these phytosterols was confirmed by 1H and 13C NMR (Jahan et al., 1995; Seo et al., 1987).
Table 1.
13C NMR spectral data (ppm) of stigmast-7,22-dien-3-ol and stigmast-7-en-ol in fraction CfC2F5 and previously reported data by Jahan et al., 1995., and Seo et al., 1987.
| Carbon number | Stigmast-7,22-dien-3-ol | Stigmast-7-en-ol | ||
|---|---|---|---|---|
| CfC2F5 fraction | Jahan et al., 1995 | CfC2F5 Fraction | Seo et al.,1987 | |
| 1 | 37.91 | 37.0 | 37.11 | 37.16 |
| 2 | 31.41 | 27.6 | 31.85 | 31.47 |
| 3 | 71.06 | 74.0 | 71.06 | 71.05 |
| 4 | 37.91 | 33.9 | 37.91 | 37.97 |
| 5 | 40.24 | 40.4 | 40.24 | 40.27 |
| 6 | 29.68 | 29.4 | 29.68 | 29.67 |
| 7 | 117.43 | 117.2 | 117.43 | 117.43 |
| 8 | 139.45 | 139.6 | 139.45 | 139.59 |
| 9 | 49.43 | 49.0 | 49.43 | 49.47 |
| 10 | 34.2 | 34.4 | 34.2 | 34.21 |
| 11 | 21.53 | --- | 21.53 | 21.57 |
| 12 | 39.54 | 39.6 | 39.45 | 39.58 |
| 13 | 43.37 | 41.3 | 43.26 | 43.38 |
| 14 | 55.02 | 55.6 | 51.23 | 55.05 |
| 15 | 23.05 | 23.1 | 22.95 | 22.99 |
| 16 | 28.49 | 28.4 | 27.95 | 27.97 |
| 17 | 55.88 | 55.8 | 56.07 | 56.08 |
| 18 | 12.23 | 12.0 | 11.82 | 11.85 |
| 19 | 13.02 | 12.5 | 13.02 | 13.04 |
| 20 | 40.8 | 40.2 | 36.57 | 36.73 |
| 21 | 21.07 | 21.0 | 18.97 | 18.98 |
| 22 | 138.14 | 138.0 | 33.87 | 33.88 |
| 23 | 129.42 | 129.6 | 26.18 | 26.52 |
| 24 | 51.23 | 51.3 | 45.82 | 46.07 |
| 25 | 31.85 | 31.8 | 29.14 | 28.97 |
| 26 | 19.02 | 19.2 | 18.97 | 18.98 |
| 27 | 21.53 | --- | 19.81 | 19.61 |
| 28 | 25.38 | 25.5 | 23.05 | 23.01 |
| 29 | 12.03 | 12.6 | 12.23 | 12.33 |
The CfC1F2 fraction (1.7 g) was again fractionated on a RP-18 silica gel column (10 g, 230-400 mesh, POLYGOPREP C18, Macherey-Nagel, Pennsylvania, USA), eluted with a gradient system of water: acetonitrile to give 49 fractions (CfC3F1-CfC3F49). Since the fractions CfC3F9-CfC3F12 (58.9 mg) contained the major secondary metabolites of the active extract, this mixture was subjected to a new chromatographic process on a reverse phase column (6 g of silica gel 60-50 POLYGOPREP, Macherey-Nagel, Pennsylvania, USA) by means of a gradient system of water: acetonitrile mixture, which yielded fractions CfC4F1-CfC4F24. Analysis by 1H and 13C NMR of the fraction CfC4F7 (10.2 mg) showed the presence of salicin (Table 2) in the active extract (Kim et al., 2015). Finally, the CfC4F14- CfC4F18 fractions were purified to obtain CfC5F9 by the same reverse phase chromatographic process, whose 1H and 13C NMR analysis resulted in p-hydroxybenzoic acid (Table 3). The CfC5F20 fraction showed a retention time and a UV spectrum similar to p-coumaric acid.
Table 2.
1H NMR spectral data (ppm) of salicin in the CfC4F7 fraction of C. ficifolia and reported data by Kim et al., 2015.
| 1H RMN | Salicin | |
|---|---|---|
| CfC4F7 fraction | Kim et al., 2015 | |
| 1 | --- | --- |
| 2 | --- | --- |
| 3 | 7.41 (brd, 6.94) | 7.32 (dd, 7.7, 1.4) |
| 4 | 7.28 (brd, 6.94) | 7.01 (td, 7.7, 0.7) |
| 5 | 7.04 (d 8.4) | 7.19 (td, 7.7, 1.4) |
| 6 | 7.36 (d, 8.4) | 7.12 (brd, 7.7) |
| 7a | 4.66 (d,11.56) | 4.78 (d, 12.6) |
| 7b | 4.93 (d, 11.56) | 4.57 (d, 12.6) |
| 1 | 4.35 (d, 7.7) | 4.83 (d, 7.7) |
| 2´ | 3.34 (dd, 7.71, 8.4) | 3.51 (dd, 8.4, 7.7) |
| 3´ | 3.35 (t, 8.4) | 3.46 (t, 8.4) |
| 4´ | 3.31 (overlap) | 3.39 (overlap) |
| 5´ | 3.25 (m) | 3.65 (ddd, 9.8, 6.3, 2.1) |
| 6a | 3.69 (dd, 5.4, 11.56) | 4.49 (dd, 11.9, 2.1) |
| 6’b | 3.89 (dd 2.31, 11.5) | 4.32 (dd, 11.9, 6.3) |
Table 3.
13C and 1H NMR spectral data (ppm) of the p-hydroxybenzoic acid in CfC5F9 fraction and reported data by Pianaro et al., 2007
| Carbon number | p-hydroxybenzoic acid | |||
|---|---|---|---|---|
| CfC5F9 fraction | Pianaro et al., 2007. | |||
| 13C | 1H | 13C | 1H | |
| 1 | 170.18 | --- | 170.07 | --- |
| 2 | 122.83 | --- | 122.73 | --- |
| 3 | 132.97 | 7.87 | 132.99 | 7.86 |
| 4 | 116.00 | 6.82 | 116.03 | 6.80 |
| 5 | 163.27 | --- | 163.36 | --- |
| 6 | 116.00 | 6.83 | 116.03 | 6.80 |
| 7 | 132.97 | 7.88 | 132.73 | 7.86 |
Laboratory animals
Male CD-1 strain mice (Harlan, Indiana, USA), weighing 30-35 g, and 6-8 weeks of age were obtained of the laboratory animal center at Autonomous Metropolitan University campus Iztapalapa. The mice were housed in a 12-h light/dark cycle; basic diet and drinking water were given ad libitum. The handling of laboratory animals was performed in agreement with the statutes of the ICCUA (Institutional Committee for the Care and Use of the Animals, session 8.10, revised in 2010) and the Official Mexican Rule (NOM-062-Z00-1999, revised in 2001).
Acute hypoglycemic effect of C. ficifolia in normal and alloxan-induced diabetic mice
The hypoglycemic effect of C. ficifolia extract was evaluated in normal mice and alloxan-induced diabetic mice (two single intravenous doses of 70 mg/kg of alloxan dissolved in ISS). After one week of alloxan administration, normal mice in fasting (3 groups of 5 animals each) and alloxan-induced diabetic mice with free access to food (3 groups of 5 animals each) were subjected to the following treatments: The Groups-1 received 4 ml/kg of isotonic saline solution (Normal control and Diabetic control); in agreement with previous reports in which an dose of 200 mg/kg was used to induce hypoglycemic effect in experimental animals (Banderas et al., 2012; Fortis et al., 2013), the Groups-2 received a dose of C. ficifolia extract of 200 mg/kg, equivalent to 11.6 μg/kg of p-coumaric acid (Normal C. ficifolia and Diabetic C. ficifolia); while Groups-3 received 5 mg/kg of glibenclamide (Normal glibenclamide and Diabetic glibenclamide), a sulphonylurea agent with hypoglycemic action that acts at pancreatic level as insulin secretagogue (Krentz and Bailey, 2005; Inzucchi et al., 2012). The administration of the treatments was by intra-peritoneal route; before the administration of the treatments, the blood glucose levels were determined at start, and after at different time intervals (120, 240 and 360 min).
Sub-chronic hypoglycemic effect of C. ficifolia in alloxan-induced diabetes mice
Four groups of mice were used: Group 1 consisted of normal mice (4 ml/kg of ISS); while Groups 2, 3 and 4 were alloxan-induced diabetic mice. After one week of the administration of alloxan, Group-1 received ISS (4 ml/kg/day, Normal control); Group- 2 also received ISS (4 ml/kg/day, Diabetic control); Group-3 received the extract of C. ficifolia (200 mg/kg/day, equivalent to 11.6 μg/kg/day of p-coumaric acid); and the Group-4 received 50 mg/kg/day of metformin, a biguanide whose anti-hyperglycemic effect, mainly extra-pancreatic, has been in part attributed to inhibition of the liver glycogenolysis because reduces hepatic glucose production (Carroll et al., 2002; Krentz and Bailey, 2005;). All treatments were given by gavage for 30 days.
Measurements of parameters
Biochemical parameters were quantified from the blood samples obtained from the retro-orbital venous sinus in anesthetized mice. Accu-Check Sensor Comfort (Roche Diag., Indiana, USA) was used to measure glycemia, and both ALT and AST were determined using a Reflotron Plus system (Roche Diag., Indiana, USA). Insulin was quantified in serum samples using enzyme-linked immune-absorbent assay kits for mouse insulin (Lynco Research, Missouri, USA). The glycogen was extracted from liver by digestion with 30% KOH followed by precipitation with ethanol 95%. The absorbance spectrum of a glycogen standard (Sigma Aldrich, Missouri, USA) was recorded at 620 nm. The samples were diluted 1:50 in distilled water. The results reflect the absorbance obtained and are expressed as mg of glycogen per g of dry liver (Passonneau and Lauderdale, 1974).
Histology
Liver tissue was fixed and dehydrated. After its inclusion with Paraplast (Oxford Labware, Missouri, USA), the tissue was cut transversally and stained with hematoxilyn-eosin (H&E) (Presnell and Schreibman, 1997) and Periodic Acid Schiff (PAS) (Prophet et al., 1992). Slices were analyzed using a light microscope Axioskope 2 (Carl Zeiss, New York, USA). Quantification of the positive area of PAS-stained was performed using the Axiovison Program 4.8 (Carl Zeiss, New York, USA) by analyzing 5 samples of each treatment at 400x magnification using a range of Red 58-196, Green 0-90, and Blue 46-203.
RNA extraction and real-time RT-PCR analysis
RNA was isolated from liver tissue by using RNeasy Tissue Mini RNA extraction kit (Qiagen Sciences, Maryland, USA) according to the manufacturer’s protocols. Two micrograms of total RNA were reverse transcribed using the ImProm II reverse transcription system (Promega, Wisconsin, USA). The reaction (20 μl) was incubated in a thermocycler (Applied Biosystems Gene Amp PCR System 2700HT; Thermo Fisher Scientific, Massachusetts, USA).
Then, cDNA was amplified with SYBR Green master mix (Roche Diag., Indiana, USA) containing 0.5 mM of custom primers for the glucagon receptor (Gcgr) (F5’-CCAGAGTCTGCTGATCTGCG-3’, R5’-GCCACCTCTTTGCTCTGCTC-3 ’), GLUT-2 (Slc2a2) (F5’- ATGCCAGTACTGCCGTTTTC-3 ’, R5’-GGCCTTGACCTTGTTCATGT-3’) or a housekeeping gene 36B4 (F5’-CCGCAGGGGCAGCAGTGGT-3’, R5’-AAGCGCGTCCTGGCATTGTCT-3 ’) as an internal control, Fast Star Enzyme, PCR buffer and 3.5 mM MgCl2 in a final volume of 10 μl. The reactions were measured in the Rotor-Gene real-time (Corbett Life Science, Concorde, NSW, Australia). PCR was conducted using the protocol reported by Almanza et al. (2010). The abundances of Gcgr mRNA were normalized by the abundance of mRNA encoding 36B4. The ACt values were calculated in every sample for each gene of interest as follow: Ctinterest - Ctreference with 36B4 as the reference gene (mRNA of reference remained stable throughout the experiments). Relative changes in the expression level of one specific gene (AACt) were calculated as ACtsample minus ACtreference and then presented as 2-AACt (Almanza et al., 2010).
Western blotting
Total protein was isolated from liver tissue using a protein extraction reagent (Thermo Fisher Scientific, Massachusetts, USA), containing 1% halt protease inhibitor mixture (Thermo Fisher Scientific, Massachusetts, USA) and 100 mM sodium orthovanadate. Two hundred milligrams of total protein were separated on NuPAGEnovex 4-20 % SDS gels (Invitrogen, California, USA) transferred to PVDF membranes (Invitrogen, California, USA) and probed with glycogen synthase 2-antibody (G-8), glycogen phosphorylase antibody (PYGB/L/M Antibody N-20) (Santa Cruz Biotechnology, Texas, USA) and GLUT-2 (Abcam, Massachusetts, USA) according to manufacturers’ instructions. Immune-reactive bands were identified with ECL-Plus Western blotting detection reagent (GE Healthcare, Illinois, USA), using anti-mouse and anti-goat horseradish peroxidase-conjugated secondary antibody. Equal loading was verified by probing the membrane with anti-actin (Clavijo et al., 2014).
Statistical analysis
Data are expressed as mean±S.E.M. and were processed using NCSS 2007 software (Kaysville, UT, USA). Differences between groups were analyzed by two-way ANOVA and post hoc Tukey-Kramer test (p<0.05).
Results
Phytochemical analysis of C. ficifolia
A comparison of the fingerprints of aqueous extract versus methanol extract confirmed the necessity of enriching the secondary metabolites by methanol precipitation. Of all the principles analyzed, only the presence of p-coumaric acid was confirmed; therefore, the other compounds were excluded as marker compounds for standardization of the extract. The CfC2F5 fraction from column 2 was analyzed by GC-MS, and two major compounds at 35.4 min and 36.6 min were observed, which corresponded to stigmast-7,22-dien-3-ol and stigmast-7-en-3-ol, respectively. Spectroscopic data of these phytosterols are described in Table 1. The CfC5F7 fraction contained a chemical compound (R.T.= 8.54 min) with identical 1H and 13C NMR data (Table 2) to salicin (Kim et al., 2015).
The CfC5F9 fraction corresponded to one of the major peaks in the chromatogram of C. ficifolia (R.T.= 10.0 min). The structure of this compound was identified by 2D NMR experiments (1H-1H COSY, 13C-HSQC and HMBC, Table 3) as p-hydroxybenzoic acid. The CfC5F20 fraction had the same retention time (15.9 min) and ultraviolet spectra (Xmax = 310 nm) as coumaric acid. The amount of p-coumaric acid in 1 g of extract was quantified using a previously described standard curve, which indicated 58 μg of p-coumaric acid/g of extract (11.6 μg in 200 mg of C. ficifolia extract).
Acute and sub-chronic hypoglycemic effect of C. ficifolia in normal and alloxan-induced diabetic mice
In the acute study, the basal glycemia was similar among groups (Figure 1A). When C. ficifolia aqueous extract was administered, there were significant reductions in glycemia (p<0.05) at 240 and 360 min in normal mice. Glibenclamide produced a significant reduction in glycemia at 120, 240 and 360 min. In diabetic mice, there were significant reductions in glycemia (p<0.05) at 360 min with both C. ficifolia and glibenclamide (Figure 1B). In the sub-chronic test, the glycemic values were significantly higher in diabetic mice (Alloxan ISS) than in Normal control mice. C. ficifolia decreased glucose levels by 25% with respect to the diabetic control (p<0.05) (Figure 1C). In contrast, metformin provoked a non-significant decrease in glucose levels. Insulin levels were not modified with any of the treatments (data not shown).
Figure 1.
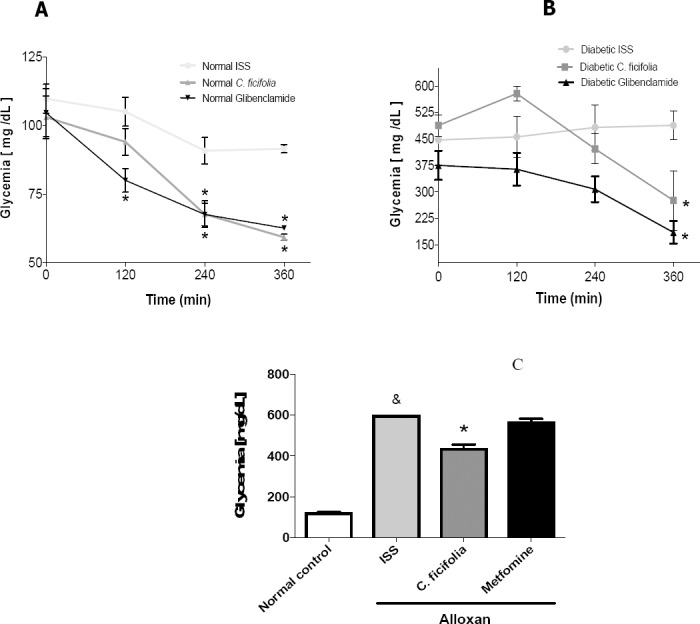
Acute hypoglycemic effect of C. ficifolia extract (200 mg/kg equivalent to 11.6 μg of p-coumaric acid/kg) and glibenclamide (5 mg/kg) in: A) Normo-glycemic mice, *significant difference against normal control (Normal ISS); B) Alloxan-induced diabetic mice, *significant difference against diabetic control (Diabetic ISS). C) Sub-chronic hypoglycemic effect by administration during 30 days of C. ficifolia-extract (200 mg/kg/day, equivalent to 11.6 μg/kg/day of p-coumaric acid) or metformin (50 mg/kg/day) in alloxan-induced diabetic mice, *significant difference against Normal control, *significant difference against diabetic control (Alloxan ISS). Mean±S.E.M. n=5 (p<0.05).
Effect of C. ficifolia on liver glycogen storage
The PAS stain was used to determine the liver glycogen content, which is shown in pink (Figure 2A-D); the distribution and accumulation of carbohydrates, and primarily glycogen, is evident. In the C. ficifolia group, the glycogen accumulation was increased and heterogeneously distributed throughout the liver parenchyma (Figure 2C). Metformin showed a similar result (Figure 2D). Diabetic control showed less glycogen distributed through the parenchyma (Figure 2B). C. ficifolia quantitatively increased glycogen liver content by 195% (p<0.05) with respect to diabetic control (Alloxan ISS) (Figure 2E). Metformin augmented the glycogen liver content by 158% against diabetic control (p<0.05) (Figure 2E). The PAS-staining showed an increase in area dye in the C. ficifolia group compared with the diabetic control (p<0.05) (Figure 2F).
Figure 2.
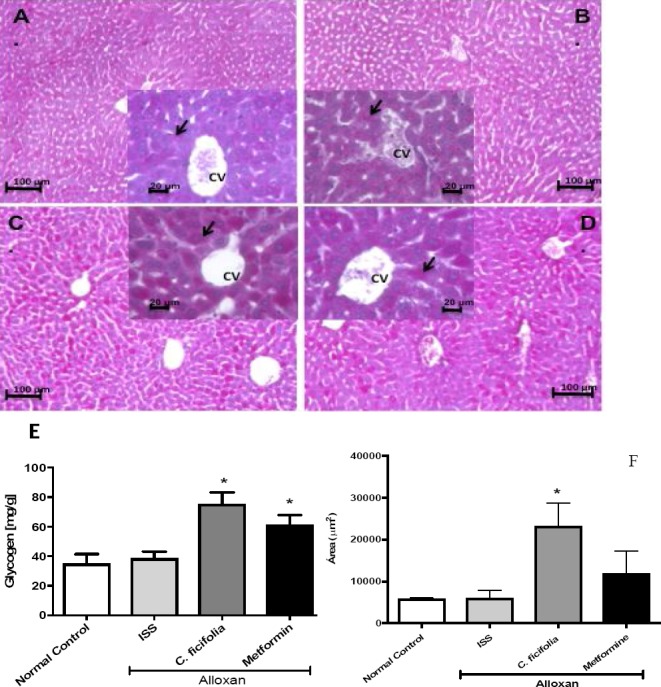
Photomicrographs with PAS staining in pink of transversal liver sections by administration during 30 days of C. ficifolia-extract (200 mg/kg/day, equivalent to 11.6 μg/kg/day of p-coumaric acid) or metformin (50 mg/kg/day) in alloxan-induced diabetic mice. A) Normal control. B) Diabetic control (Alloxan ISS). C) Alloxan treated with C. ficifolia. D) Alloxan treated with metformin. C. ficifolia extract increased the accumulation of liver glycogen. Hepatocyte cytoplasm with dense glycogen deposits (arrow) around the central vein (CV) can be observed. These accumulations are seen as pink PAS-positive areas throughout the section (original magnification 100x and box 400x). E) Quantitative analysis of glycogen content in diabetic mice liver tissue treated for 30 days with C. ficifolia extract. F) Quantitative analysis of positive PAS stained area. Mean±S.E.M. (n=5). *Significant difference against diabetic control (Alloxan ISS) (p<0.05).
Influence of C. ficifolia in glycogen synthase and glycogen phosphorylase of liver
C. ficifolia provoked a significant (p<0.05) increase in glycogen synthase enzyme levels against diabetic control (Figure 3A and 3B). Metformin showed a non-significant increase. C. ficifolia and metformin reduced glycogen phosphorylase (PYGB) compared with the control alloxan group (Figure 3A and 3C). C. ficifolia and metformin increased in a non-significant manner GLUT-2 (Figure 3A and 3D).
Figure 3.
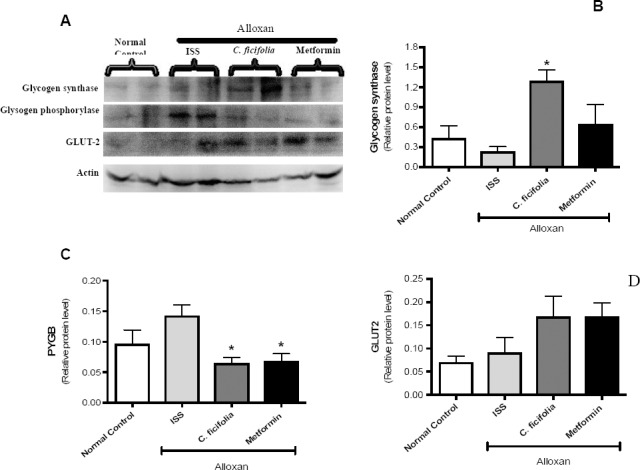
Effect of C. ficifolia extract (200 mg/kg/day, equivalent to 11.6 μg/kg/day of p-coumaric acid) or metformin (50 mg/kg/day) on key enzymes in the synthesis and degradation of glycogen and on GLUT2 in sub-chronic study. Liver tissue lysates were obtained and processed by western blotting. A) Representative image of an immunoblot. B) Relative level by densitometry of glycogen synthase-2. C) Relative level by densitometry of glycogen phosphorylase (PYGB). D) Relative level by densitometry of GLUT2. Densitometry analysis of protein content was made in relation to actin, used as a loading control. Values are expressed as Mean±S.E.M. (n=5). *Significant difference against diabetic control (alloxan ISS). p<0.05.
Effect of C. ficifolia extract on mRNA expression of GLUT-2 and glucagon
The extract of C. ficifolia did not change the expression levels of GLUT-2 nor of glucagon receptor after 30 days of treatment in diabetic control (Figure 4A and 4B). Metformin improved the expression levels of GLUT-2 and glucagon receptor.
Figure 4.
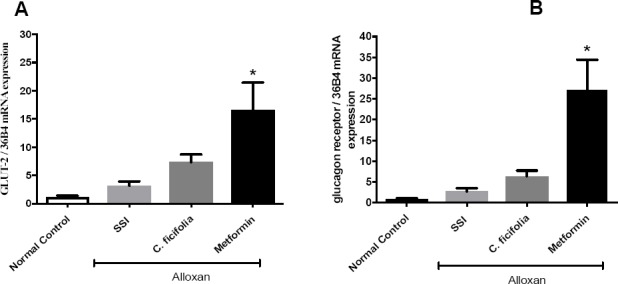
Effect of C. ficifolia extract (200 mg/kg/day, equivalent to 11.6 μg/kg/day of p-coumaric acid) or metformin (50 mg/kg/day) on mRNA expression of: A) GLUT-2; B) glucagon receptor, in liver tissues of normal control and alloxan-induced diabetic mice. Mean±S.E.M. (n=5). *Significant difference against diabetic control (Alloxan ISS). p<0.05.
Effect of C. ficifolia on liver functionality and histology
The ALT and AST levels were observed significantly increased (p<0.05) in diabetic control compared with normal control mice (Figure 5A and 5B). C. ficifolia and metformin caused a significant decrease in the ALT and AST levels (p<0.05). The photomicrographs of liver are shown in Figures 5C-F. The nuclei and nucleoli of hepatocytes were visualized eu-chromatics. The liver lobules (box), the liver central vein, the hepatic cords and some Kupffer cells located in the sinusoidal space, as well as some endothelial cells lining the vein centercan be seen in Figure 5C. After 30 days of treatment, C. ficifolia did not show changes in liver histology (Figure 5E), did not show changes in liver histology, preserving the overall structure of the hepatic lobe. Diabetic control showed disarrayed lobes, cellular damage and ballooning degeneration, which was confirmed by the presence of typical vesiculation, hydropic degeneration, karyorrhexis, karyolysis and altered cytoplasmic regions, as well as increased cytoplasmic acidophilia, vascular congestion, a state of hyperemia, and hydropic degeneration (Figure 5D). Metformin provoked leukocyte infiltration mainly by neutrophils, which is characteristic of an inflammatory condition (Figure 5F).
Figure 5.
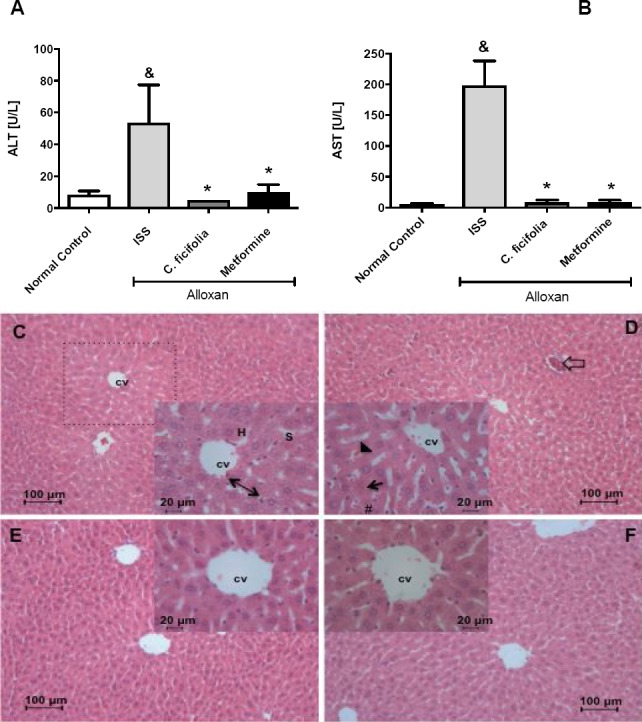
Effect of C. ficifolia extract (200 mg/kg/day, equivalent to 11.6 μg/kg/day of p-coumaric acid) or metformin (50 mg/kg/day) on liver enzymes content in sub-chronic study: A) ALT; B) AST. Mean±S.E.M. (n=5). *Significant difference against Normal control; *Significant difference against diabetic control (Alloxan ISS). p<0.05. C-F) Photomicrographs of liver transversal section: C) Control mice (ISS); D) Alloxan-diabetic mice treated with ISS; E) Alloxan-diabetic mice treated with C. ficifolia. F) Alloxan-diabetic mice treated with metformin. Hepatocyte (H), central vein (CV), hepatic cord (reversible arrow), sinusoid (S), hepatic lobule (dashed line), hyperemia (arrow head), vascular congestion (unfiled arrow), karyorrhexis (arrow) and hydropic degeneration (#). (H&E, original magnification main 100x and box 400x).
Discussion
The fruit of C. ficifolia has been reported to possess hypoglycemic activity in different experimental models, including rats, mice and rabbits, with moderate and severe experimental diabetes, and in type-2 diabetic patients (Alarcon et al., 2002; Acosta et al., 2001). These earlier studies have established that an oral dose of 200 mg/kg of C. ficifolia is enough to produce hypoglycemic effect. Therefore, this same dose was used in the present study. Results showed a hypoglycemic effect in acute and sub-chronic test by the aqueous extract of C. ficifolia. In addition, the chemical study of this extract allowed the identification of five majoritarian compounds: two phytosterols, i.e., stigmast-7,22-dien-3-ol and stigmast-7-en-3-ol, as well as p-coumaric acid, p-hydroxybenzoic acid and salicin. However, DCI was not detected in this active extract. As the p-hydroxybenzoic acid resulted be one of the main components in the extract and due to that it has been reported with hypoglycemic effect in streptozotocin-induced diabetic rats (Manuja et al., 2013), this compound might be the responsible of the hypoglycemic effect of C. ficifolia. However, this should be confirmed in further studies and the other components in the extract cannot be yet discarded.
The results of the present study indicate that the C. ficifolia hypoglycemic effect might be due, at least in part, to an increased storage of glycogen in the liver, which was confirmed by PAS staining, like a consequence of increased glycogen synthase. This finding is similar to those of other Cucurbitaceae species with similar effects, such as Cucurbita moschata, which also increased muscle glycogen levels and improved the physical performance in mice (Wang et al., 2012). The response of a normal liver to a glucose load involves the sequential inactivation of glycogen phosphorylase and the activation of glycogen synthase, which is the rate-limiting enzyme in glycogen synthesis. In contrast, in alloxan-induced diabetes model, the liver response to glucose becomes deficient a few days after the administration of alloxan, with the impaired activation of glycogen synthase (Ou et al., 2012).
Interestingly, in this study the administration of C. ficifolia extract increased the glycogen synthase levels and, consequently, the liver glycogen of diabetic mice. High insulin levels promote the storage of glucose as glycogen in the liver through the dephosphorylation and activation of glycogen synthase. In fact, insulin exerts a rapid effect, causing increased glycogen synthase and consequently glycogen accumulation (Wilcox, 2005). In this study, we observed low levels of glucagon receptor in mice treated with C. ficifolia, decreased levels of glycogen phosphorylase and, in consequence, inhibition of glycogenolysis that resulted in decreased plasma glucose. Although these results do not permit directly determinate activation of glycogen synthesis or inhibition of glycogenolysis, the two changes result in increased liver glycogen, which clearly was evident with the PAS stain. On the other hand, GLUT2 expression did not change by the C. ficifolia extract, probably due to increased hepatic glycogen storage by the extract, which makes unnecessary the incorporation of liver glucose (Im et al., 2005).
Alloxan-induced diabetes may produce hepatotoxicity and necrosis (Sawants et al., 2006). In light of this, the hepatic functionality of diabetic mice treated with C. ficifolia by monitoring the levels of ALT and AST in serum was determined. Elevated levels of ALT are related to liver damage and disordered hepatocytes in type 2 diabetes patients, whereas the AST levels are maintained mainly by hepatic sinusoidal cells in the development of fibrosis, which leads to relative increases in serum AST (Judi et al., 2010). In the present study, alloxan-induced diabetic mice showed elevated levels of both enzymes. Histological analysis also showed disordered hepatocytes, loss of radial disposition of the hepatic lobules, increased sinusoidal space and extensive degeneration, as well as necrosis markers such as karyolysis and karyorrhexis, which are characteristics of an inflammatory condition. The opposite was observed in the group treated with C. ficifolia extract, which showed a radial arrangement of the hepatic lobules, no disordered hepatocytes, and no necrosis. The overall hepatic structure remained unaltered, showing a protective effect from the extract.
This effect could be because the C. ficifolia extract contains phytochemical compounds that offer protection against liver toxicity, or it could be due antioxidant and anti-inflammatory effects previously reported for C. ficifolia, that improves the redox state and modulates the GSH/GSSG ratio in liver, pancreas and kidney of diabetic mice and 3T3-L1 adipocytes (Fortis et al., 2013; Roman et al., 2012; Diaz et al., 2012). There are many antioxidant compounds reported in Cucurbitaceae, as vitamins, carotenoids and flavonoids, which function like scavengers or inductors to eliminate reactive oxygen substances, which may be useful in the treatment of various diseases (Diaz et al., 2012; Hancock et al., 2008; Azevedo and Rodriguez 2007; Pereira et al., 2009), cucurbitanes and cucurbitane-type glycosides, which are now recognized for their anti-cancer activities. They also have a range of pharmacological effects both in vitro and in vivo, including anti-inflammatory, hepatoprotective, antimicrobial and anti-hyperglycemic effects (Chang et al., 2008; Wang et al., 2008).
Phenolic components such as p-hydroxybenzoic acid have been identified as antioxidants, which have a peroxyl radical-scavenging ability that counteracts the damaging effects of reactive oxygen species and because of their anti-inflammatory effects and antimicrobial activity against a number of microorganisms (Manuja et al., 2013). The antioxidant activity reported for p-coumaric acid could help reduce oxidative stress and, consequently, the liver cellular damage (Pereira et al., 2009). In addition, it is likely that p-coumaric acid in the extract inhibits gluconeogenesis in the liver by inhibiting pyruvate transport into the mitochondria, as reported Lima et al. (2006).
Other of the major compounds of C. ficifolia extract, salicin, also could contribute to the liver-protective effect due its reported neuroprotective and anti-inflammatory activity. In fact, its hydroxyl groups can interact with ciclooxygenase-2, causing the inhibition of this enzyme and subsequently deregulating inflammation (Passonneau and Lauderdale, 1974; Mahdi, 2014). In addition, two phytosterols, i.e., stigmast-7,22-dien-3-ol and stigmast-7-en-3-ol, were found in the aqueous C. ficifolia extract. These compounds have been also reported in pumpkin seeds from Cucurbita pepo L., which is often eaten as a snack and may prevent chronic diseases; it has also been reported to alleviate the signs of benign prostatic hyperplasia (Kim et al., 2012; Abdel 2006). It is likely that these compounds act either, alone or synergistically, to reduce blood glucose levels, which can reverse or reduce the damage caused by DM. C. ficifolia represents an alternative therapy for treatment and control of DM, with a hypoglycemic effect and liver-protective properties and with antioxidant and anti-inflammatory properties that could potentially help improve the control of DM and prevent the development of vascular complications while improving the quality of life for patients.
Conclusions
The hypoglycemic effect of a chemically characterized extract of C. ficifolia, caused hepatic glycogen storage without changes in liver function or normal liver architecture, showing a liver-protective effect in alloxan-induced diabetic mice. This extract represents an alternative therapy for DM patients with liver complications because it exhibits hypoglycemic and liver-protective effects, while also possessing antioxidant and anti-inflammatory properties. Phytochemical study permitted the identification of five majoritarian compounds: two phytosterols (stigmast-7,22-dien-3-ol and stigmast-7-en-3-ol), p-coumaric acid, p-hydroxybenzoic acid and salicin, which have reports of pharmacological activity. In further studies, should be determined if these compounds are the active principles.
Acknowledgements
The present study was supported by a grant from CONACyT to Jessica Garcia Gonzalez (with fellowship number 234251) as part of her PhD degree in Experimental Biology and PRODEP-SEP. The authors appreciate to the Laboratory Divisional of Molecular Biology of the DCBS at Autonomous Metropolitan University, by the given technical support.
References
- 1.Abdel R. Effect of pumpkin seed (Cucurbita pepo L.) diets on benign prostatic hyperplasia (BPH): chemical and morphometric evaluation in rats. W. J. Chem. 2006;1:33–40. [Google Scholar]
- 2.Acosta J.L, Jimenez B.E, Juarez M.A, Diaz J.C. Hypoglycemic action of Cucurbita ficifolia on type 2 diabetic patients with moderately high blood glucose levels. J. Ethnopharmacol. 2001;77:99–101. doi: 10.1016/s0378-8741(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon F.J, Hernandez E, Campos A.E, Xolalpa S, Rivas J.F, Vazquez L.I, Roman R. Evaluation of the hypoglycemic effect of Cucurbita ficifolia Bouché(Cucurbitaceae) in different experimental models. J. Ethnopharmacol. 2002;82:185–189. doi: 10.1016/s0378-8741(02)00176-9. [DOI] [PubMed] [Google Scholar]
- 4.Almanza J.C, Alarcon F.J, Blancas G, Campos A.E, Roman R, Garcia R, Cruz M. Glycine regulates inflammatory markers modifying the energetic balance through PPAR and UCP-2. Biomed. Pharmacother. 2010;64:534–540. doi: 10.1016/j.biopha.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Azevedo C.H, Rodriguez D.B. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima and Cucurbita pepo. J. Agr. Food Chem. 2007;55:4027–4033. doi: 10.1021/jf063413d. [DOI] [PubMed] [Google Scholar]
- 6.Banderas T.R, Roman R, Zamilpa A, Garcia R, Diaz M, Campos G, Tortoriello J, Alarcon F.J. Influence of two hypoglycemic Cucurbitaceae (Cucurbita ficifolia Bouchéand Ibervillea sonorae Greene) on ATP-sensitive potassium channels in rat aortic rings. Bol. Latinoam. Car. Plant Med. Arom. 2012;11:510–519. [Google Scholar]
- 7.Carroll M.F, Izard A, Riboni K, Burge M.R, Schade D.S. Control of postprandial hyperglycemia. Diabetes Care. 2002;25:2147–2152. doi: 10.2337/diacare.25.12.2147. [DOI] [PubMed] [Google Scholar]
- 8.Chang C.I, Chen C.R, Liao Y.W, Cheng H.L, Chen Y.C, Chou C.H. Cucurbitane-type triterpenoids from the stems of Momordica charantia. J. Nat. Prod. 2008;71:1327–1330. doi: 10.1021/np070532u. [DOI] [PubMed] [Google Scholar]
- 9.Clavijo D, Gutiérrez M, Palestino M, Domínguez M, Nuño N, Souza V, Miranda U, Kershenobich D, Gutiérrez M.C, Bucio L, Gomez L.E. Acetaldehyde targets superoxide dismutase 2 in liver cancer cells inducing transient enzyme impairment and a rapid transcriptional recovery. Food Chem. Toxicol. 2014;69:102–108. doi: 10.1016/j.fct.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Diaz M, Angeles S, Baiza L.A, Medina R, Hernandez D, Ortega C, Roman R, Cruz M, Alarcon F.J. Effect of an aqueous extract of Cucurbita ficifolia Bouchéon the glutathione redox cycle in mice with STZ-induced diabetes. J. Ethnopharmacol. 2012;144:101–108. doi: 10.1016/j.jep.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Fortis A, Alarcon F.J, Banderas T, Diaz M, Roman R, Cruz M, Garcia R. Cucurbita ficifolia Bouche (Cucurbitaceae) and D-chiro-inositol modulate the redox state and inflammation in 3T3-L1 adipocytes. J. Pharm. Pharmacol. 2013;65:1563–1576. doi: 10.1111/jphp.12119. [DOI] [PubMed] [Google Scholar]
- 12.Hancock D, Chudek A, Walker G, Pont D, Viola R. Ascorbic acid conjugates isolated from the phloem of Cucurbitaceae. Phytochemistry. 2008;69:1850–1858. doi: 10.1016/j.phytochem.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Im S.S, Kang S.S, Kim S.Y, Kim H.I, Kim J.W, Kim K.S, Ahn Y.H. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005;54:1684–1691. doi: 10.2337/diabetes.54.6.1684. [DOI] [PubMed] [Google Scholar]
- 14.Inzucchi S.E, Bergenstal R.M, Buse J.B, Diamant M, Ferrannini E, Nauck M, Peters A.L, Tsapas A, Wender R, Matthews D.R. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahan N, Ahmed W, Malik A. New steroidal glycosides from Mimusops elengi. J. Nat. Prod. 1995;58:1244–1247. [Google Scholar]
- 16.Judi L, Toukan A, Khander Y, Ajlouni K, Khatib M.A. Prevalence of elevated hepatic transaminases among Jordanian patients with type 2 diabetes mellitus. Ann. Saudi Med. 2010;30:25–32. doi: 10.4103/0256-4947.59369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krentz A.J, Bailey C.J. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kim CS, Subedi L, Park KJ, Kim SY, Choi SU, Kim KH, Lee KR. Salicin derivates from Salix glandulosa and their biological activities. Fitoterapia. 2015;106:147–152. doi: 10.1016/j.fitote.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim MY, Kim EJ, Kim YN, Choi C, Lee BH. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012;6:21–27. doi: 10.4162/nrp.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima L.C, Buss G.D, Ishii-Iwamoto E.L, Salgueiro-Pagadigorria C, Comar JF, Bracht A, Constantin J. Metabolic effects of p -coumaric acid in the perfused rat liver. J. Biochem. Mol. Toxicol. 2006;20:18–26. doi: 10.1002/jbt.20114. [DOI] [PubMed] [Google Scholar]
- 21.Mahdi JG. Biosynthesis and metabolism of β-D-salicin: A novel molecule that exerts biological function in humans and plants. Biotechnology Reports. 2014;4:73–79. doi: 10.1016/j.btre.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuja R, Sachdeva S, Jain A, Chaudhary J. A comprehensive review on biological activities of p-hydroxybenzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013;22:109–115. [Google Scholar]
- 23.Miranda M.E, Escobar M.C, Ortega C, Sanchez F, Almanza J.C, Alarcon F.J. Cucurbita ficifolia Bouchéfruit acts as an insulin secretagogue in RINm5F cells. Int. Biotech. Color J. 2013;3:8–14. [Google Scholar]
- 24.Ou Y, Lin L, Pan Q, Yang X, Cheng X. Preventive effect of phycocyanin from Spirulina platensis on alloxan-injured mice. Env. Toxicol. Pharma. 2012;34:721–726. doi: 10.1016/j.etap.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Passonneau V, Lauderdale R. A comparison of three methods of glycogen measurement in tissues. Analyt. Biochem. 1974;60:405–12. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- 26.Pereira D.M, Vaentao P, Pereira J.A, Andrade P.B. Phenolics: from chemistry to biology. Molecules. 2009;14:2202–2211. [Google Scholar]
- 27.Presnell K, Schreibman P. Humason’s Animal Tissue Techniques. USA: W.H. Freeman and Company; 1997. p. 572. [Google Scholar]
- 28.Pianaro A, Pereira P.A, Trevistan F.D, Kazue I.N, Braz-Filho R. Iridoid glucoside and antifungal phenolic compounds from Spathodea campanulata roots. Semina: Ciencias Agrarias, Londrina. 2007;28:251–256. [Google Scholar]
- 29.Prophet E.B, Mills B, Arrington J.B, Sobin L.H. Laboratory methods in histotechnology. Washington: Armed Forces Institute of Pathology (Eds.), USA; 1992. pp. 151–154. [Google Scholar]
- 30.Roman R, Almanza J.C, Fortis A, Angeles S, Banderas T, Zamilpa A, Diaz M, Jasso I, Blancas G, Gomez J, Alarcon F.J. Antioxidant and anti-inflammatory effects of a hypoglycemic fraction from Cucurbita ficifolia Bouche in streptozotocin-induced diabetes mice. Am. J. Chinese Med. 2012;40:1–14. doi: 10.1142/S0192415X12500085. [DOI] [PubMed] [Google Scholar]
- 31.Sawants P, Dnyanmote V, Warbritton A, Latendresse R, Mehendale M. Type 2 diabetic rats are sensitive to thioacetamide hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;211:221–232. doi: 10.1016/j.taap.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Seo S, Uomori A, Yoshimura Y, Sankawa U, Ebizuka Y. Stereospecificity in the side-chain formation of 24 β-ethylsterols in tissue cultures of Trichosanthes kirilowii. J Chem. Soc. Chem. Commun. 1987;24:1876–1878. [Google Scholar]
- 33.Wang D.C, Xiang H, Li D, Gao H.Y, Cai H, Wu L.J, Deng X.M. Purine-containing cucurbitane triterpenoids from Cucurbita pepo cv dayangua. Phytochemistry. 2008;69:1434–1438. doi: 10.1016/j.phytochem.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Wang S.Y, Huang W.C, Liu C.C, Wang M.F, Ho C.S, Huang W.P, Hou C.C, Chuang H.L, Huang C.C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules. 2012;17:11864–11876. doi: 10.3390/molecules171011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilcox G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 36.Xia T, Wang Q. Antihyperglycemic effect of Cucurbita ficifolia fruit extract in streptozotocin-induced diabetic rats. Fitoterapia. 2006a;77:530–533. doi: 10.1016/j.fitote.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Xia T, Wang Q. D-chiro-inositol found in Cucurbita ficifolia (Cucurbitaceae) fruit plays the hypoglycaemic role in streptozocin-diabetic rats. J. Pharm. Pharmacol. 2006b;58:1527–1532. doi: 10.1211/jpp.58.10.0014. [DOI] [PubMed] [Google Scholar]


