Abstract
Background:
Compound Arnebiae radix oil has been clinically applied to treat burns and scalds for a long time. However, it is unstable and inconvenient to use. The aim of this study was to prepare a compound Arnebiae radix microemulsion gel for transdermal delivery system and evaluate its characteristics.
Materials and Methods:
Based on the solubility of Shikonin, the active component of Arnebiae radix and the results of phase studies, adequate ratio of each component in microemulsion was determined. The optimized microemulsion gel was prepared using Carbomer 940. The gels were characterized in terms of appearance, preliminary stability test and the content of Shikonin in the compound Arnebiae radix microemulsion gel with HPLC analysis.
Results:
The optimized conditions for preparing microemulsion were Tween-80, glycerin, isopropyl myristate (IPM) with the ratio of 6:3:2. The optimal microemulsion gel was obtained with Carbomer 940 (1.0%).
Conclusion:
The prepared compound Arnebiae radix microemulsion gel showed good stability over time. It is more convenience in application than the previous used formulations.
Keywords: Compound Arnebiae radix oil, microemulsion gel, pseudo-ternary phase diagram, characterization
Introduction
Compound Arnebiae radix oil is mainly composed of Arnebiae radix, sesame oil, Angelicae sinensis radix, Rhei radix et rhizoma, Glycyrrhizae radix et rhizoma, Phellodendri chinensis cortex and Sanguisorbae radix. It is an empirical prescription for the treatment of burns and scalds, and it has been applied in the clinic for nearly 40 years with significant clinical effects. The active ingredient of Arnebiae radix is Shikonin, which has the effect on cooling blood, detoxification, anti-inflammation, and so on. It is mostly used for the treatment of scalds, burns, ulcers, sores, ulcerated bedsores, and so on. Currently, most of the Arnebiae radix forms in clinical application are oil formulations, and there are very few reports on gel forms.
In the process of preparing compound Arnebiae radix oil, Arnebiae radix, Angelicae sinensis radix, Phellodendri chinensis cortex and other ingredients are directly immersed in sesame oil, which causes incomplete extraction, instability and poor patient compliance. Studies have revealed (Eskandar and Binazir, 2015) that microemulsion as a drug carrier via transdermal administration can enhance solubility of poorly soluble drugs, increase skin permeability and maintain sustained actions (Yao, 2013). However, microemulsion with strong mobility, poor bioadhesive capacity can limit its application.
The microemulsion gel is a new administration system which combines the advantages of microemulsion and gel. It can resolve the problems of microemulsion such as increased surfactant concentration due to long-term storage and evaporation of water. Thus, it is expected to achieve the purposes of stability, strong bioadhesive capacity and sustained release (Liu et al., 2011).
In this study, compound Arnebiae radix microemulsion gel was prepared to enhance stability and patient compliance. Based on the results of solubility and phase studies, adequate ratio of each component in microemulsion was determined (Furlanetto et al., 2011). For preparing microemulsion gel, Carbomer 940 was used. The gels were characterized in terms of appearance, preliminary stability test and the content of Shikonin with HPLC analysis (Wu, 2005).
Materials and Methods
Materials
Shikonin standard was purchased from the National Institute For the Control of Pharmaceutical and Biological Products, China. Oleic acid, tween-80 and span-80 were supplied by Yantai Shuangshuang Chemical Co., Ltd. (Yantai, China). Glycerin was purchased from Xingsha Pharmaceuticals Co., Ltd. (Xiamen, China). Analytical reagent grade methanol was supplied by Guangnuo Chemical Technology Co., Ltd. (Shanghai, China). HPLC grade methanol was purchased from Fisher Scientific (UK). Purified water (Robust, Guangdong, China) was used in the preparation of solutions. All other chemicals were of reagent grade and used without further purification.
Solubility of Shikonin in various ingredients
Before the solubility test, Arnebiae radix was extracted using 95% ethanol with solid-liquid ratios of 1:10 and 1:8, and the extraction durations were 2.5 hours and 2 hours, respectively. The solubility of Shikonin in various ingredients was determined by adding an excess amount of Arnebiae radix extract to 2 mL selected ingredients in centrifugal tubes with stoppers under ultrasonicating for 15 minutes. The tubes were kept in 37°C water bath for 24 hours and then centrifuged at 3000 rpm for 10 minutes. The supernatant was taken and filtered through a 0.45-μm membrane filter. The filtrate was diluted by 250 times using methanol, and the UV absorption of each solution was determined using UV spectrophotometry at 516 nm. The measurement was repeated five times, and the average value was adopted.
Selection of Km value
For the microemulsion system, the behaviors of the microemulsions at 25°C with the ratios of surfactant to cosurfactant (Km) at 2:1, 1:2 and 1:1 were investigated. Firstly, the Km value was fixed, then the mixture of surfactant and cosurfactant in different ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2 and 9:1 was added to oil phase, and stirred with a magnetic stirrer while adding drops of distilled water to obtain O/W blank microemulsions. The added amounts of water were recorded when the solution became clear or turbid, and the pseudo-ternary phase diagrams of the microemulsion were drawn using Origin8.0 software.
Preparation of compound Arnebiae radix microemulsion
Solubility experiment has revealed that Shikonin is very soluble in oil phase. Thus, the Arnebiae radix extract was first added to the oil phase (IPM) and dissolved under ultrasonicating. Then the mixed emulsifiers were added to the oil phase. Subsequently, the water extracts of the other five ingredients in the prescription were dropped into the mixture followed by agitation with a magnetic stirrer to generate a microemulsion system.
Selection of gel matrix
Blank microemulsion was added in three bottles, then 2.0g Carbomer 940, 2.0g sodium carboxymethyl cellulose and 2.0g sodium alginate were added, respectively in order to swell for 24 hours.
High-performance liquid chromatography
Chromatographic studies were performed on Waters 1525 HPLC instrument (Waters Technologies, USA) equipped with binary gradient pump, a UV detector (2489, USA). Sample solutions were injected onto a Waters Shield C18 column with 25 cmx4.6mm i.d. dimension and 5μm particle size, maintained at 30 °C. The mobile phase consisting of A: 0.025molL-1 phosphate solution, and B: methanol in the ratio of 20 : 80 (v/v) with flow rate of 1ml/min was used, and UV detector wavelength was fixed at 516 nm. The injection volume was 20 μL
Preparation of solutions for HPLC
Stock solution of standard Shikonin in the concentration of 14.25μg/ mL was prepared with methanol. 2.0g of compound Arnebiae radix microemulsion gel was added into a centrifugal tube with 2.0g of sodium sulfate, and the mixture was kept in 60°C water bath for 30 minutes. Then 5mL of methanol was added with sonicating for 30 minutes. The tube was taken out and centrifuged at 3000 rpm for 20 minutes; the supernatant was filtered through a 0.45-μm membrane filter, so that the test sample solution was prepared. The blank microemulsion gel solution was prepared as the same way.
Method validation of HPLC
The method was validated according to Chinese Pharmacopoeia 2015 (Volume IV) covering selectivity, linearity range, accuracy and precision. Selectivity of the method was evaluated by injecting the solutions of test sample and blank microemulsion gel.
Linearity range of the method was established through the calibration curve spiked at six concentration levels of 0.285, 0.7125, 1.425, 2.85 and 3.5625μg/mL. Precision was evaluated by injecting the mid-point of the calibration curve in order to obtain the RSD of the peak area. Stability test of solution was investigated by injecting the test sample solution at 0, 2, 4, 8, 12 and 24 hours to obtain the content of Shikonin in microemulsion gel. Repeatability was evaluated by determining the content of six test sample solutions. Accuracy was estimated by means of recovery experiments. Nine replicate analyses of the samples at three levels (80%, 100% and 120%) were prepared to obtain the recovery (%) of spiked samples.
Preliminary stability test of the microemulsion gel
The compound Arnebiae radix microemulsion gel was added into a centrifugal tube and centrifuged at 3000 rpm for 30 minutes to record whether there was stratification or not. The microemulsion gel was sealed in a bottle and kept at 25°C for 6 months to observe the properties.
Results
Solubility of Shikonin in various ingredients
In order to improve the solubility of the active ingredient (Committee, 2010), the solubility of Shikonin in different oil phases (oleic acid, isopropyl myristate), surfactants (Tween-80, Span-80) and co-surfactants (glycerin, absolute ethanol) was tested.
The results showed that the solubility of Shikonin was the highest in absolute ethanol followed by oil phase IPM, surfactant Tween-80 and cosurfactant absolute ethanol (Table 1). However, absolute ethanol was not selected as the cosurfactant for the microemulsion since its volatility and skin irritation. Thus, Tween-80-glycerinum-IPM-water were selected for the development of the formulation.
Table 1.
Solubility of Shikonin in various solvents
| Solvent | Oleic acid | IPM | Tween-80 | Span-80 | Glycerin | Absolute ethanol |
|---|---|---|---|---|---|---|
| Absorbance(A) | 0.518 | 0.933 | 0.761 | 0.115 | 0.804 | 1.866 |
| Solubility(g/mL) | 0.0268 | 0.0482 | 0.0393 | 0.00594 | 0.042 | 0.0964 |
Selection of Km value
The pseudoternary phase diagram was constructed based on the observations noted and the results are shown in Figure 1, which revealed that the maximum area of the microemulsion can be obtained when the Km was 2:1.
Figure 1.
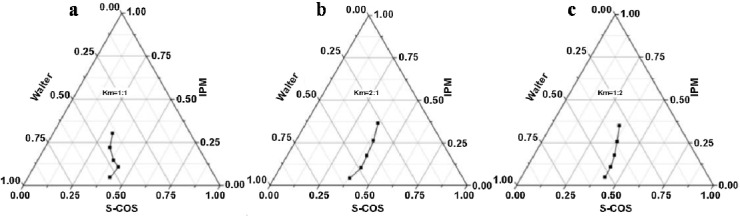
Pseudo-ternary phase diagram of Tween-80/glycerin with different Km values (a: Km=1:1, b: Km=1:2, c: Km=2:1).
Determination of moisture content of the microemulsion
Conductance behavior is one of the important properties of the microemulsion. During the preparation of the microemulsion, the conductivity was changed simultaneously with the changes in the structure and phase of the microemulsion liquid, with which the moisture content was increased. In this experiment, the moisture content was determined so that the maximum conductivity was achieved in the O/W microemulsion based on the changes of conductivity in the preparation of blank microemulsion.
The results demonstrated that the microemulsion system was very stable when the volume ratio of mixed emulsifiers and IPM was between 9:1 and 8:2 (Table 2). In this experiment, the blank microemulsion was prepared and conductivity was tested for several times. Thus, the maximum (optimal) moisture content that produced stable microemulsions was 49.5%, at which the content of mixed emulsifiers and IPM was 40.4% and 10.1%, respectively.
Table 2.
Moisture content of prescription based on the conductivity
| Prescription number | Mixed emulsifiers/IPM | Moisture content% | Centrifugal stability | Short-term shelf stability (25±2 °C) |
|---|---|---|---|---|
| 1 | 9:1 | — | Not laminated, clear and transparent | Not laminated, clear and transparent |
| 2 | 8:2 | 47.6 | Not laminated, clear and transparent | Not laminated, clear and transparent |
| 3 | 7:3 | 31.2 | Not laminated, clear and transparent | Laminated |
| 4 | 6:4 | 15.4 | Laminated | Laminated |
| 5 | 5:5 | 1.3 | — | — |
| 6 | 4:6 | — | — | — |
| 7 | 3:7 2:8 | — | — | — |
| 9 | 1:9 | — | — | — |
Note: “—”: Uncertain; Centrifuge: 3000r/min20min; Short-term observation: 30 days.
Preparation of compound Arnebiae radix microemulsion
The optimal microemulsion was 0.7g Arnebiae radix extract, 2.0g IPM, 9g T-80/glycerin (Km=2:1) and 11.0g water extract. The prepared microemulsion showed no separation of phase for one month, indicating that the preparation has a reasonable physical stability.
Selection of gel matrix
The results showed that sodium alginate was precipitated at the bottom, sodium carboxymethyl cellulose showed uneven swelling, while Carbomer 940 showed complete and uniform swelling. Thus, Carbomer 940 was selected as the gel matrix in this experiment.
0.5%, 1.0%, 1.5% and 2.0% of Carbomer 940 was added to four shares of 2.0g blank microemulsion, respectively. Then sodium hydroxide solution (pH=10) was used to neutralize, and the statuses were changed. The results demonstrated that the viscosity and swelling time of the system were increased with increasing amount of Carbomer 940. So Carbomer 940 weighing 1.0% of the microemulsion was selected to obtain smaller amount of matrix and appropriate viscosity.
According to the Pharmacopoeia (Wu, 2005), the pH value of the gel should be tested. In this experiment, it is found that the viscosity was increased with increasing pH value. When the pH value was neutral, the Carbomer 940 was dissolved evenly and the microemulsion gel was stable as well as skin irritation was relieved when it was applied to a wound.
Preparation of compound Arnebiae radix microemulsion gel
1.0% of Carbomer 940 was added to 2.0g of drug-loaded microemulsion to swell completely. pH value of the mixture was adjusted to 7.0 using sodium hydroxide solution (pH=10) with stirring in order to obtain compound Arnebiae radix microemulsion gel. The prepared microemulsion gel was light purple smooth semisolid mixture.
Method validation of HPLC
Typical HPLC chromatogram of Shikonin in compound Arnebiae radix microemulsion gel showed that there was no interference in the determination of Shikonin (Figure 2). The method had good linear relationship within the range of 0.285~3.5625μg/mL (r=0.9992). The linear equation was Y=27255X-2284. The RSD value of peak area for precision was 0.02% and the content of the test sample was stable for 24 hours. The RSD value of the content of the test sample for repeatability was 0.75% and Table 3 showed that the average recovery of spiked samples was 97.11% (RSD=0.01%).
Figure 2.
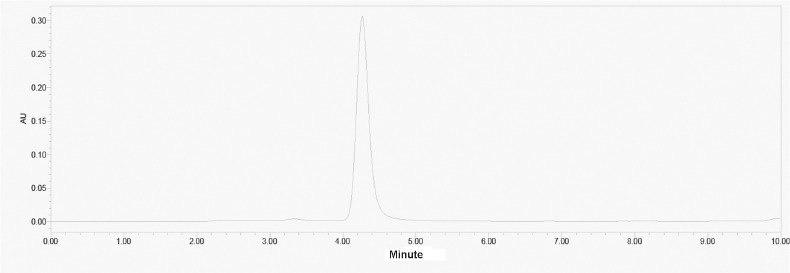
HPLC chromatogram of Shikonin in compound Arnebiae radix microemulsion gel.
Table 3.
Recovery test results
| Addition μg/ml) | Measurement μg/ml) | Recovery (%) | Average recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1.151 | 1.095 | 95.13 | ||
| 1.151 | 1.128 | 98.00 | ||
| 1.151 | 1.111 | 96.52 | ||
| 1.439 | 1.395 | 96.94 | ||
| 1.439 | 1.426 | 99.10 | 97.11 | 0.01 |
| 1.439 | 1.410 | 97.98 | ||
| 1.727 | 1.687 | 97.68 | ||
| 1.727 | 1.656 | 95.89 | ||
| 1.727 | 1.671 | 96.76 |
Determination of Shikonin in microemulsion gel
Three batches of compound Arnebiae radix microemulsion gel were determined and the results revealed that the average concentration of Shikonin in microemulsion gels was 0.02%.
Preliminary stability test of the microemulsion gel
There was no stratification under the conditions of centrifuging for the compound Arnebiae radix microemulsion gel, and the microemulsion gel was stable kept at 25°C for 6 months without any significant changes.
Discussion
The oil phase is an important component in the formation of microemulsion. In this experiment, isopropyl myristate was selected as the oil phase since it is the best solvent for Shikonin, the active ingredient of Arnebiae radix.
Pior to the preparation of microemulsion, phase diagram of oil-water-surfactent was constructed, in which the main case is to obtain the largest microemulsion region area. When Km was equal to 2:1, there was the largest microemulsion region than that Km was equal to 1:1 or 1:2, and it was likely to achieve the optimal emulsification.
The compound Arnebiae radix microemulsion gel can be used to treat burns and scalds. The pH value of the microemulsion gel was adjusted to 7.0 in order to guarantee the viscosity of the gel and relieve the skin irritation.
Since the carbomer used in this study is likely to adhere to the separate column, demulsification must be performed to remove carbomer before the determination (Bastos et al., 1995). The effects of anhydrous sodium sulfate, calcium chloride, sodium chloride as well as the impact of water bath on the demulsification of microemulsion gel were investigated. The results revealed that the complete demulsification can be obtained using anhydrous sodium sulfate in 60°C water bath for 30 minutes in order to achieve the accurate quantification of Shikonin and extend the useful life of the column.
The compound Arnebiae radix oil with lipophilic character has the problems of poor skin penetration and inconvenience for using. The high stability and strong bioadhesive capacity of microemulsion gel make them suitable for incorporation of lipophilic active ingredients in aqueous-based matrix. In this study, the prepared compound Arnebiae radix microemulsion gel showed good stability over the time and it was convenient to be coated on the skin without gauze.
Acknowledgement
The financial support of the 2011 Scientific and Technological Project of Ningxia Hui Autonomous Region is gratefully acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Bastos J. K. of podophyllotoxin and related compounds in Podophyllum species by reverse phase high performance liquid chromatography. Phytochemical Analysis. 6:101–105. [Google Scholar]
- 2.Committee C. P. Pharmacopoeia. Second edition. Beijing: The Medicine Science and Technology Press of China; 2010. [Google Scholar]
- 3.Eskandar M, Binazir B. Formulation and evaluation of transdermal celecoxib matrix patches. European Journal of Biomedical and Pharmaceutical Sciences. 2015;2:34–43. [Google Scholar]
- 4.Furlanetto S, Cirri M, Piepel G, Mennini N, Mura P. Mixture experiment methods in the development and optimization of microemulsion formulations. J Pharm Biomed Anal. 2011;55:610–617. doi: 10.1016/j.jpba.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Zhai C. M, Chu L. J. Application progress of Chinese medicine gel in Cervicitis. In: Burandt W. J, Nanayakkara C. L, McChesney N., J. D, editors. Acta Chinese Medicine and Phar, Kopycki 1995. Quantitative determination macology; 2011. p. 05. [Google Scholar]
- 6.Parag P, Mansi A. M. S., N. M. Formulation and evaluation of microemulsion based gel of Itraconazole. Pharmagene. 2013;1:32–36. [Google Scholar]
- 7.Wu Y. Determination of Shikonin in Lithospermum oil with HPLC. Traditional Chinese Drugs Research and Clinical Pharmacology. 2005;16:66–68. [Google Scholar]
- 8.Yao N. Application and study of microemulsion gel in pharmaceutics of TCM. Journal of Zhejiang Chinese Medical University. 2013;37:217–219. [Google Scholar]


