Abstract
Background:
Burns are among the most prevalent injuries in humans with high cost in health care and heavy prolonged or permanent physical, psychological and social consequences. Commercial antimicrobial creams and dressing agents are unsuccessful in healing deep burn wounds.
Materials and Methods:
A study was conducted to assess the impact of crude linseed oil (LSO) topical application on burn wounds healing in rabbits in comparison with untreated wounds (NAT) and those treated with Vaseline gel (VAG) and Cicatryl-Bio ointment (CBO). By the 28th day post burning, skin biopsies were analyzed for histological and cytological lesions. The presence of various bioactive phytochemical groups in linseed was also screened.
Results:
Phytochemical screening has resulted in high concentrations of flavonoids and terpenoids, low amounts of catechic tannins and total absence of alkaloids and saponosides. All along the trial, the rate of wounds contraction was found to be significantly higher in burns treated with LSO which had also a significant shorter healing period (26±5.89 days) as compared to the other treatments. LSO healed wounds included less inflammatory cells, complete epithelium regeneration with a reduced thickness of the new formed dermis, discreet fibrosis, enhanced neo-vascularization, increased number of collagen fibers, fibroblasts and many myofibroblasts. Additionally, no adverse effects of LSO on cicatrization process were recorded.
Conclusion:
These findings prove the safety and efficaciousness of linseed oil topical application in the therapy of burn wounds.
Keywords: Linseed oil, phytochemical screening, topical application, burn wounds, healing, rabbits
Introduction
In humans, burns are among the most prevalent injuries which are caused by exposure to heat, electricity, lightening, caustic chemical compounds, radiation, friction or excessive cold (Shan et al., 2010). Frequently, they involve the skin and are categorized according to the extent and depth of tissue injury (Hettiaratchy and Papini, 2004). Deep or widespread burn injuries lead to many physical dysfunctions and subsequent psychological and psychosocial problems (Sousa et al., 2013). Infections are also very common complications which can affect any burn injury, regardless of its depth.
Healing of burns is a very complex process. Antimicrobial creams and other dressing agents used for traumatic wounds are unsuccessful in deep burns with eschar, and only few effective treatments are known to facilitate the burn recovery process (Tiwari, 2012); however, their low accessibility, high cost and several detrimental side effects justify the need for alternative burn dressings and more effective therapeutic drugs (Atiyeh et al., 2007). Thus, an increasing interest has arisen in regard to the potential effects of plants and/or their extracts on burn wounds recovery by augmenting/modulating inflammatory mediators (Murphy and Evans, 2012).
Linseed (Linum usitatissimum) also known as flaxseed has proved several therapeutic effects. Its components have showed antioxidant, antiviral, antibacterial, antifungal, anti-inflammatory and anti-atherosclerosis properties (Prasad, 2000). Daily flaxseed consumption is safe (Beroual et al., 2016) and it protects gastric and urinary tracts membranes, heals scars, protects inflamed skin, improves its elasticity and nourishes and regulates also hair follicles cycle (Halligudi, 2012). These properties have motivated us to investigate the effects of linseed oil topical application on burn wounds healing in adult male New Zealand rabbits.
Material and Methods
Phytochemical Screening and Drugs Composition
Linseeds were purchased from a local herbalist. They were cleaned, washed to remove any impurities present in them, shade dried and powdered for analyses. Common phytochemical tests were carried out on the methanolic extract to ascertain the presence of some major natural chemical groups (Alkaloids, catechic tannins, flavonoids, saponosides and terpenoids) as described by Harborne (1973). The observed test color intensity was used as indicative for each phytochemical quantity. Crude oil was then obtained by cold-pressing of seeds. Specimens of the products (seeds and oil) are deposited at the laboratory of pharmacology-toxicology. Institute of veterinary sciences. University Frère Mentouri of Constantine 1, Algeria.
Vaseline gel and Cicatryl-Bio® ointment are commercial drugs and were purchased from a local private pharmacy. The active substances in Cicatryl-Bio® (to 100 g of ointment) are: Allantoin (1.0 g), guaiazulene (0.007 g), para-chlorometacresol (0.120 g) and α-tocopherol acetate (0.010 g). It contains also methylparaben, propylparaben, light liquid paraffin, vaseline officinale, mixture of cetostearyl alcohol (90%) and sodium cetearyl sulphate (10%), organic ethoxylated fatty acid (20%), glycerol monostearate, macrogol glycol, sorbitol (70% solution) and purified water.
Animals and Husbandry
The experiment has been undertaken on 08 healthy male New Zealand rabbits of the same flock, the same age (06 months) and approximately of the same weight (2.8 Kg). They were individually identified and kept separately in standard cages under the same environmental conditions (temperature, relative humidity, light-dark cycle and hygiene). They were allowed access to water and feed ad-libitum.
The trial was undertaken in compliance with the suggested ethical guidelines described in the Guide for the Care and Use of Laboratory Animals, 8th Edition (Institute of Animal Resources, Commission on Life Sciences, National Research Council; National Academy Press; Washington, DC; 2011).
Experimental Design
The back of each animal was shaved at first with an electric clipper then with a sterilized razor blade 24 hours before the beginning of the trials. On day zero, the animals were sedated by intramuscularly injection of ketamine hydrochloride (1ml/10kg). The four zones to be burned (two dorsal left and right and two lumbar left and right) were disinfected with 70% alcohol and locally anesthetized with xylocain infiltration of 1 ml/zone. Animals were put in prone position and the four burns of the same size (20 mm of diameter) were realized, using a 200 g stainless steel cylinder heated in boiling water for 3 minutes, instantly dried and put for 15 seconds on the skin as described by Hamdi-Pacha et al. (2002). Each animal served as his own control.
To avoid results bias due to body movements, each wound was randomly pre-assigned a substance test so that each drug was applied at different wound location in each rabbit. Immediately after burning, the following substances were topically applied gently and slowly, every day and at the same time until complete epithelialization: Lin seed oil (1ml/wound, LSO group), Cicatryl-bio® ointment (1g/wound, CBO group) and Vaseline gel (1g/wound, VAG group). The fourth wounds group was left to heal naturally with no treatment (Control group, NAT). Wounds were inspected daily (form, color, odor) before treatment application. Each four days and during one month a sheet of flexible calques papers were placed over the wounds and their areas were drawn using a felt pen. Then, the calques papers were scanned and the resulting digital pictures were analyzed through the software ImageJ™ ver. 1.44 (National Institutes of Health, Bethesda, MD, USA, 2011) to determine the precise wounds size (Digital planimetry). All pictures were acquired and analyzed using identical settings. The rate of wound healing was measured as the percentage of wound contraction and calculated as: 100x[(Initial wound size - specific day wound size) / Initial wound size] (Srivastava and Durgaprasad, 2008). For histopathologic studies, 04 rabbits were sacrificed on the 28th day post-burning, and tissue biopsies were taken, fixed in 10% buffered formalin and histology slides were prepared and stained with hematoxylin and eosin. They were qualitatively assessed with respect to epidermis epithelialization, inflammatory cells infiltration, extent of fibrosis as well as report of any observed anomalies.
Statistical Analysis
Measurements were expressed as mean ± SD. To assess the differences between the four treatments, data normality was tested, then one-way ANOVA test (followed by Tukey’s post hoc test for multiple comparisons) was applied using the GraphPad Instat prism 6.04 (GraphPad Software, Inc., San Diego, CA, USA, 2014). The statistical significance was set at a P value<0.05.
Results
Phytochemical Analysis
Phytochemical screening of linseed showed positive results for different groups of secondary metabolites such as flavonoids, terpenoids and catechic tannins which are of medicinal importance. The testing for alkaloids content and saponosides was negative. The results are summarized in Table 1.
Table 1.
Phytochemical screening on the methanolic extract of Linum usitatissimum seeds
| Bioactive compound | Methanolic extract |
|---|---|
| Alkaloids | - |
| Catechic tannins | + |
| Flavonoids | +++ |
| Saponosides | - |
| Terpenoids | +++ |
+: Presence of constituent;–: Absence of constituent
Wound Healing Evaluation
During all the experimental period, rabbits were in good health conditions, neither mortalities nor wound infections were recorded. The rate of wounds contraction was found to increase in all groups during the experiment period (Figure 1, Table 2); however, it was significantly more important in CBO and LSO groups especially since the 12th day.
Figure 1.
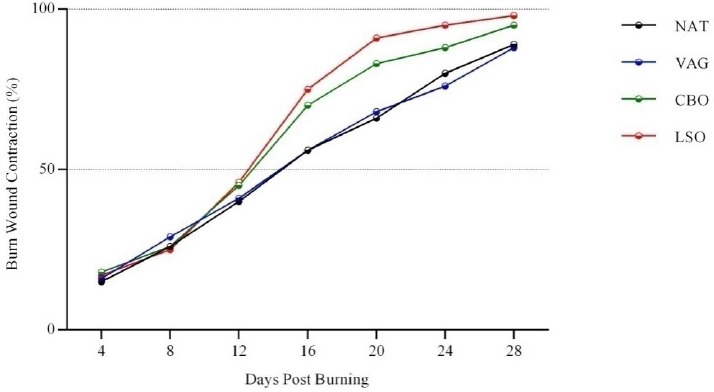
Evolution of wound contraction at different time intervals (NAT: No treatment; VAG: Vaselin Gel; CBO: Cicatryl-Bio Ointment; LSO: Lin Seed Oil)
Table 2.
Burn wound closure per treatment (%)
| Treatment | Burn wound Contraction per treatment (%) Mean ± SD | Healing Mean ± SD duration (days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 4th | 8th | 12th | 16th | 20th | 24th | 28th | ||
| NAT | 15.21±7. | 26.32±6. | 40.30±6. | 56.21±7. | 66.20±7. | 80.22±7. | 89.03±9.6 | 35±1.1 |
| 8 | 7 | 9 | 8 | 9 | 7 | |||
| VAG | 16.07±9. | 29.06±8. | 41.03±9. | 56.09±9. | 68.21±9. | 76.19±9. | 88.11±.9. | 35.6±3.9 |
| 2 | 6 | 4 | 1 | 1 | 5 | 5 | ||
| CBO | 18.14±9. | 26.17±8. | 45.09±8. | 70.22±8. | 83.06±9. | 88.20±9. | 95.13±9.6 | 32.5±2.8 |
| 3 | 4 | 6 | 9 | 2 | 5 | |||
| LSO | 17.22±9. | 25.16±7. | 46.09±9. | 75.03±7. | 91.10±9. | 95.21±9. | 98.07±9.8 | 26±5.8 |
| 4 | 6 | 1 | 2 | 4 | 7 | |||
| Multiple comparisons | ||||||||
| LSO vs NAT (S); LSO vs VAG (S); LSO vs CBO (NS); CBO vs NAT (S); CBO vs VAG (S); VAG vs NAT (NS) | ||||||||
(S): Statistically significant (p<0.05), (NS): Statistically non-significant (p>0.05), NAT: No treatment, VAG: Vaselin Gel, CBO: Cicatryl-Bio Ointment, LSO: Lin Seed Oil
From the 16th day and till the end of the trials, LSO healing activity seemed to be higher than CBO but not in a significant way. Wounds visual inspection showed well-formed granulation tissue in all animals; nevertheless, burns treated with LSO were found to heal significantly faster as compared to the other groups (about 26±5.89 days post-burning) (Table 2, Figure 2). No differences were observed between NAT and VAG treated wounds.
Figure 2.
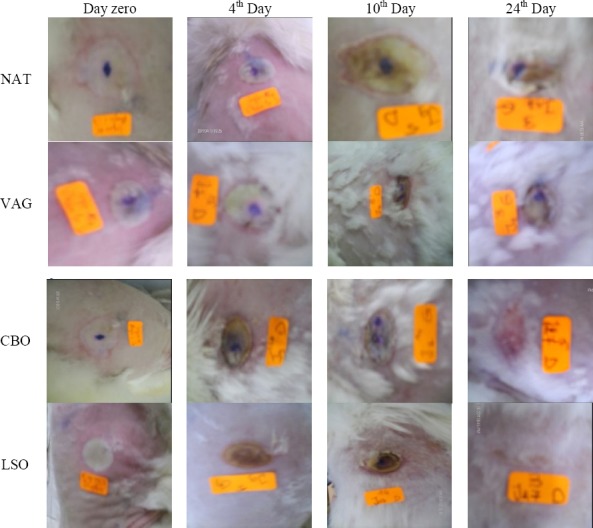
Burn wounds evolution with different treatments (NAT: No treatment; VAG: Vaselin Gel; CBO: Cicatryl-Bio Ointment; LSO: Lin Seed Oil)
Histopathological Study
At the 28th day post-burning, histopathologic analyses showed that NAT, VAG and CBO treated wounds still containing (at different levels) zones of destructed epidermis with bullous lesions (epidermal detachment). An important fibrosis was also observed in association to a massive chronic inflammatory cells infiltration and moderate collagenation, which are characteristic of a prolonged repairing process. On the other hand LSO healed wounds included less inflammatory cells and had a remarkable complete re-epithelialization with a reduced thickness and a discreet fibrosis of the new epidermis in association to an increased number of new capillaries (neovascularization), collagen fibers, fibroblasts and many myofibroblasts (Figure 3).
Figure 3.
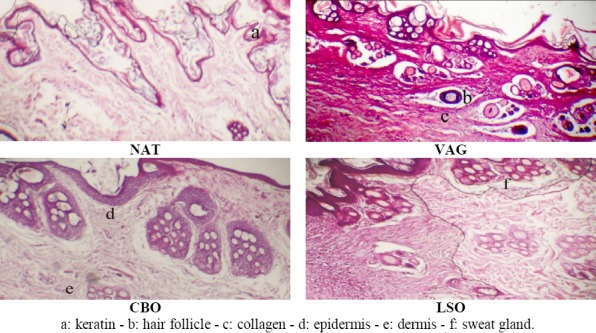
Histopathologic appearance of wounds with different treatments at the 30th day. (NAT: No treatment; VAG: Vaselin Gel; CBO: Cicatryl-Bio Ointment; LSO: Lin Seed Oil)
Discussion
Phytochemicals are biologically active and naturally occurring chemical compounds found in plants. Many studies have investigated the composition of flaxseed, and differences between their results are mostly related to the used extraction methods. For instance, Monica and Joseph (2016) have reported the presence of saponin, quinones, terpenoids, phenols, steroids, coumarins and betacyanin in both fermented and unfermented/aqueous flaxseed extracts. Tannins, flavonoids, glycosides and anthocyanin were absent in both extracts. Bekal et al. (2015) described flaxseed powder aqueous extracts to be positive for glycerides, saponins, alkaloids and flavonoids, and negative for sterols, terpinoids and tannins. It is worth noting that after cold extraction; the majority of bioactive compounds found in the seeds are present in the oil. Thus, linseed oil is rich with fats, flavonoids, glycosides, phenols and tannins which could make it useful in some diseases treatment (Joshi et al., 2014).
Wound healing is a perfectly organized and complicated biological process in cellular and molecular levels that occurs immediately after the disruption of skin integrity. It mainly aims to restore the skin as closely as possible to its normal anatomical and physiological state (Talekar et al., 2012). It depends on reparative capacities of the tissue, type and extent of the damage as well as the general state of health (Singh et al., 2005). This process consists of haemostasis and inflammation, neovascularization, granulation, fibrogenesis, wound contraction, re-epithelization and remodeling of the tissue (Suguna et al., 2002). Inflammation, macrophagia, fibroplasia and collagenation are closely interrelated phases; consequently, intervention at any one of them with drugs might deeply improve or impair one or all steps of healing process (Vinothapooshan and Sundar 2010). In fact, anti-inflammation, antioxidant and antimicrobial are the main needed properties of remedies that accelerate wound healing (Farahpour and Habibi, 2012).
Linseed oil is a source of diverse biologically active compounds that have been found to act on one or several phases of wound repair. Its richness in flavonoids may promote the viability of collagen fibrils conducting to an increase in the strength of collagen fibers and a reduction of cell damage by improving DNA synthesis (Pradhan et al., 2009). In association to some polysaccharides found in linseed, flavonoids also stimulate fibroblast cells proliferation (Tatli, 2010) as well as their differentiation into specialized myofibroblasts in the granulated tissue which conducts to wound contraction and enclosure (Hinz, 2010). This explains the important number of these cells in LSO healed wounds as compared to the other treatments. Fibroblasts produce collagen and fibronectin to form a new extracellular matrix needed for the adhesion and migration of endothelial and epithelial cells towards the wound bed (neovascularization and epithelialization). Flavonoids are also known to reduce lipid peroxidation by preventing or slowing the onset of cell necrosis and improving angiogenesis (Getie et al., 2002), which is conducive to convey vital nutrients for tissues regeneration by improving the blood flow during the process of wound-healing. This has been verified in the LSO group of our study.
A number of bioactive terpenoids are also abundant in linseed, and in the same way as flavonoids and tannins, they promote the wound healing process mainly through their astringent activity responsible for wound contraction and increased epithelialization rate (Tsuchyia et al., 1996; Karodi et al., 2009; James and Friday, 2010).
Fatty acids and triglycerides in flax are able to increase skin hydration through reducing trans-epidermal moisture loss (Dweck, 2002). Flaxseed oil contains about 73% of polyunsaturated fatty acids (Cunnane et al. 1993) among which, Ω-3 polyunsaturated fatty acids have been reported to increase pro-inflammatory cytokine production at wound sites and accordingly promote cutaneous wound healing (McDaniel et al., 2008). Furthermore, linseed is rich in fatty acids such as α-linolenic acid (the major one 39-60%), oleic, linoleic, palmitic and stearic acids (Pellizzon et al., 2007). Oleic, linoleic and α-linolenic acids supply cells with lipids necessary for their membrane repair and their respiration (Loden and Andersson, 1996).
β-Sitosterol accounted for more than 50% of the total linseed sterols (Teneva et al., 2014). This compound was shown to be angiogenic by stimulating neo-vascularization and promoting endothelial cells motility (Choi et al., 2002).
Flax proteins are rich sources of glutamic acid, glutamine, arginine, branched-chain amino acids BCAA (valine and leucine) and aromatic amino acids (tyrosine and phenylalanine) (Oomah and Mazza, 1993). Arginine and combinations of BCAA and glutamine are important for restoring dermal collagen protein synthesis and enhancing wound healing (Stechmiller et al., 2005; Murakami et al., 2012). Furthermore bioactive peptides mixtures from flaxseed with high levels of BCAA, and low levels of aromatic amino acids have revealed antioxidant activity (Udenigwe and Aluko, 2010) necessary for cells membrane preservation and repair.
The presence in linseed of tocopherols (vitamin E), β-carotene, phenolic compounds (such as lignans, flavonoids, phenolic acids, tannins and phenylpropanoids) are responsible for its high antioxidant activity. These constituents prevent and protect cells against oxidative damage from free radicals (Kasote, 2013) and stimulate natural process of wound clarity by macrophages (Farahpour et al., 2011). Moreover, tocopherol enhances protein synthesis, cells proliferation, and migration in the wound tissue. It possesses also anti-inflammatory effects by attenuating pro-inflammatory cytokines and chemokines production (Salinthone et al., 2013). This explains the low number of macrophages in LSO treated wounds.
In addition, flaxseed contains high amounts of magnesium (Mg) and calcium (Ca) as well as manganese (Mn) and copper (Cu) but in lower quantities (Mekebo and Chandravanshi, 2014). It has been demonstrated that early increase of Cu, Mg and Mn leads to more stable and solid collagen (Vaxman et al., 1996). Ca is known to regulate inflammatory cells infiltration, fibroblasts multiplication and keratinocytes proliferation, differentiation and migration (Bikle et al., 2001; Lansdown, 2002). Elevated Ca level also improves healing through enhancing blood clotting and aggregation of platelets at the wound site during haemostatic phase of wound healing (Lansdown, 2002).
Finally, antimicrobial and antifungal properties of many constituents of linseed make it effective against many bacteria, fungi and yeasts such as Escherichia coli, Salmonella paratyphii, Lactobacillus spp, Staphylococcus aureus, Proteus vulgaris, Klebsiella pneumoniae and Saccharomyces cerevisiae (Raja-Narender et al., 2016) that may infect the wound and slow down its recovery. These are further properties which could explain the accelerated repair and regeneration of burn wounds treated with LSO.
Conclusion
The present experiment has confirmed that linseed oil compounds have bioactive properties that render it effective in promoting wound healing activity compared with well known commercial drugs. Moreover, they do not produce any adverse effects which make this oil a promising treatment of skin wounds or ulcers. Therefore, there is an urgent need for further bioassays and analyses to isolate and characterize the structure and activity of each component alone or in combination with others to provide successful formulation of products for wound healing.
Declaration of Interest
None
References
- 1.Atiyeh B.S, Costagliola M, Hayek S.N, Dibo S.A. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33(2):139–48. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Bekal M, Kumari S, Sharmila K.P. Preliminary phytochemical screening of flax seed and assesment of its in vitro antioxidant activity. World J. Pharma. Pharma. Sci. 2015;4(8):952–958. [Google Scholar]
- 3.Beroual K, Agabou A, Bachtarzi K, Haouam S, Hamdi-Pacha Y. Safety assessment of Linum usitatissimum (Linn.) ingestion in New Zealand rabbits. Afr. J. Tradit. Complement. Altern. Med. 2016;13(2):151–155. [Google Scholar]
- 4.Bikle D.D, Tu C.L, Oda Y, Xie Z. Calcium and vitamin D regulated keratinocyte differentiation. Mol. Cel. Endocrinol. 2001;177(1-2):161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 5.Choi S, Kim K.W, Choi J.S, Han S.T, Part Y.I, Lee S.K, Kim J.S, Chung M.H. Angiogenic activity of β-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian Gerbil. Planta Med. 2002;68(4):330–335. doi: 10.1055/s-2002-26750. [DOI] [PubMed] [Google Scholar]
- 6.Cunnane S.C, Hamadeh M.J, Liede A.C, Thompson L.U, Wolever T.M.S. Nutritional attributes of traditional flaxseed in healthy young adults. Am. J. Clin. Nutr. 1995;61(1):62–68. doi: 10.1093/ajcn/61.1.62. [DOI] [PubMed] [Google Scholar]
- 7.Dweck A.C. Herbal medicine for the skin. Their chemistry and effects on skin and mucous membranes. Pers. Care Mag. 2002;3(2):19–21. [Google Scholar]
- 8.Farahpour M.R, Habibi M. Evaluation of the wound healing activity of an ethanolic extract of ceylon cinnamon in mice. Vet. Med. 2012;57(1):53–57. [Google Scholar]
- 9.Farahpour M.R, Taghikhani H, Habibi M, Zandieh M.A. Wound healing activity of flaxseed Linum usitatissimum L in rats. Afr. J. Pharm. Pharmacol. 2011;5(21):2386–2389. [Google Scholar]
- 10.Getie M, Gebre M.T, Reitz R, Neubert R.H. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae) Pharmazie. 2002;57(5):320–322. [PubMed] [Google Scholar]
- 11.Halligudi N. Pharmacological properties of flax seeds: a Review. Hygeia. J. D. Med. 2012;4(2):70–77. [Google Scholar]
- 12.Hamdi-Pacha Y, Belkhiri A, Benazzouz M, Benhamza L, Bensegueni L. Evaluation de l’activitécicatrisante suite àdes brûlures expérimentales de quelques plantes algériennes. Rev. Méd. Pharm. Afri. 2002;16:1, 7. (Fr) [Google Scholar]
- 13.Harborne J.B. Phytochemical Methods. A guide to modern techniques of plant analysis. 2nd Ed. London New York: 1973. pp. 34–59. 89-119;183-192. [Google Scholar]
- 14.Hettiaratchy S, Papini R. ABC of burns;Initial management of a major burn: II: assessment and resuscitation. A clinical review. BMJ. 2004;329(7457):101–103. doi: 10.1136/bmj.329.7457.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinz B. The myofibroblast: Paradigm for a mechanically active cell. J. Biomechanics. 2010;43(1):146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 16.James O, Friday E.T. Phytochemical composition, bioactivity and wound healing potential of Euphorbia heterophylla (Euphorbiaceae) leaf extract. International Journal on Pharmaceutical and Biomedical Research. 2010;1(1):54–63. [Google Scholar]
- 17.Joshi Y, Garg R, Juyal D. Evaluation of synergistic antimicrobial activity of Gemifloxacin with Linum usitatissimum seed oil. J. Phytopharmacol. 2014;3(6):384–388. [Google Scholar]
- 18.Karodi R, Jadhav M, Rub R, Bafna A. Evaluation of the wound healing activity of a crude extract of Rubia cordifolia L (Indian madder) in mice. Int. J. Appl. Res. Nat. Prod. 2009;2(2):12–18. [Google Scholar]
- 19.Kasote D.M. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013;20(1):27–34. [Google Scholar]
- 20.Lansdown A.B. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10(5):271–285. doi: 10.1046/j.1524-475x.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- 21.Loden M, Andersson A.C. Effect of topically applied lipids on surfactant-irritated skin. Brit. J. Dermatol. 1996;134(2):215–220. [PubMed] [Google Scholar]
- 22.McDaniel J.C, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound repair regen. 2008;16(3):337–345. doi: 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mekebo D, Chandravanshi B.S. Levels of essential and non-essential metals in linseed (Linum usitatissimum) cultivated in Ethiopia. Bull. Chem. Soc. Ethiop. 2014;28(3):349–362. [Google Scholar]
- 24.Monica S. J, Joseph M. Phytochemical screening of flaxseed (Linum usitatissimum L.) Int. J. Sci. Res. 2016;5(3):218–220. [Google Scholar]
- 25.Murakami H, Shimbo K, Inoue Y, Takino Y, Kobayashi H. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids. 2012;42(6):2481–2489. doi: 10.1007/s00726-011-1059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy P. S, Evans G.R.D. Advances in wound healing: a review of current wound healing products. Plast. Surg. Int. 2012;2012:1–8. doi: 10.1155/2012/190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oomah B.D, Mazza G. Flaxseed proteins. A review. Food Chem. 1993;48(2):109–114. [Google Scholar]
- 28.Pellizzon M.A, Billheimer J.T, Bloedon L.T, Szapary P.O, Rader D.J. Flaxseed reduces plasma cholesterol levels in Hypercholesterolemic mouse models. J. Am. Coll. Nutr. 2007;26(1):66–75. doi: 10.1080/07315724.2007.10719587. [DOI] [PubMed] [Google Scholar]
- 29.Prasad K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: Effect of secoisolariciresinol diglucoside (SDG) isolated from flaxseed. Mol. Cell. Biochem. 2000;209(1-2):89–96. doi: 10.1023/a:1007079802459. [DOI] [PubMed] [Google Scholar]
- 30.Pradhan D, Panda P, Tripathy G. Wound healing activity of aqueous and methanolic bark extract of Vernonia arboreus in wistar rat. Nat. Prod. Radiance. 2009;8(1):6–11. [Google Scholar]
- 31.Raja-Narender B, Tejaswini S, Sarika M, Karuna N, Shirisha R, Priyanka S. Antibacterial and antifungal activities of Linum usitatissimum (Flax seeds) Int. J. Pharma. Educ. Res. 2016;3(2):4–8. [Google Scholar]
- 32.Salinthone S, Kerns A.R, Tsang V, Carr D.W. α-Tocopherol (vitamin E) stimulates cyclic AMP production in human peripheral mononuclear cells and alters immune function. Mol. Immunol. 2013;53(3):173–178. doi: 10.1016/j.molimm.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Shan M, Hafeez S, Mehmood K.T. Management of Burns. J. Pharm. Sci. & Res. 2010;2(8):492–498. [Google Scholar]
- 34.Singh S.D, Krishna V, Mankani K.L, Manjunatha B.K, Vidya S.M, Manohara Y.N. Wound healing activity of the leaf extracts and deoxyelephantopin isolated from Elephantopus scaber Linn. Indian J. Pharmacol. 2005;37(4):238–42. [Google Scholar]
- 35.Sousa A.D, Sonavanet S, Kurver A. Psychological issues in adult burn patients. Review article. Delhi Psych. J. 2013;16(1):24–33. [Google Scholar]
- 36.Srivastava P, Durgaprasad S. Burn wound healing property of Cocos nucifera : An appraisal. Indian J. Pharmacol. 2008;40(4):144–146. doi: 10.4103/0253-7613.43159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stechmiller J.K, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr. Clin. Pract. 20(1):52–61. doi: 10.1177/011542650502000152. [DOI] [PubMed] [Google Scholar]
- 38.Suguna L, Singh S, Sivakumar P, Sampath P, Chandrakasan G. Influence of Terminalia chebula on dermal wound healing in rats. Phytother. Res. 2002;16(3):227–231. doi: 10.1002/ptr.827. [DOI] [PubMed] [Google Scholar]
- 39.Talekar Y.P, Das B, Paul T, Talekar D.Y, Apte K.G, Parab P.B. Wound healing potential of Vitex negundo Linn in experimental animals. Int. J. Pharm. Sci. 2012;4(4):543–546. [Google Scholar]
- 40.Tatli I.I, Akkol E.K, Yesilada E, Akdemir Z.S. Antinociceptive and anti-inflammatory activities of seven endemic Verbascum species growing in Turkey. Pharm. Biol. 2008;46(10-11):781–788. [Google Scholar]
- 41.Teneva O, Zlatanov M, Antova G, Angelova-Romova M, Dimitrova R, Marcheva M. Composition of biologically active substances of flaxseed. Discourse J. Agr. Food. 2014;2(2):59–69. [Google Scholar]
- 42.Tiwari V.K. Burn wound: How it differs from other wounds? Indian J. Plast. Surg. 2012;45(2):364–373. doi: 10.4103/0970-0358.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuchyia H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanka T, Linuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996;50(1):27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]
- 44.Udenigwe C.C, Aluko R.E. Antioxidant and angiotensin converting enzyme-inhibitory properties of a flaxseed protein-derived high Fischer ratio peptide mixture. J. Agric. Food Chem. 2010;58(8):4762–4768. doi: 10.1021/jf100149w. [DOI] [PubMed] [Google Scholar]
- 45.Vaxman F, Olender S, Lambert A, Nisand G, Grenier J.F. Can the wound healing process be improved by vitamin supplementation? Experimental study on humans. Eur. Surg. Res. 1996;28(4):306–314. doi: 10.1159/000129471. [DOI] [PubMed] [Google Scholar]
- 46.Vinothapooshan G, Sundar K. Wound healing effect of various extracts of Adhatoda vasica. Int. J. Pharma. Biosci. 2010;1(4):530–536. [Google Scholar]


