Abstract
Background:
Production of medicinal plants in controlled environments, particularly hydroponic technology, provides opportunities for high quality biomass accumulation and optimizes production of secondary metabolites. Applying special watering regimes in combination with efficient soil draining is an encouraging new tool for the production of pharmaceutical relevant plants. The purpose of this paper was to evaluate the effect of substrate combinations and watering regimes on nutrient uptake, anti-F. oxysporum activity and secondary metabolite profile of S. aethiopicus.
Materials and Methods:
Coir was used as the main component for the preparation of media in different combinations; TI (Coir + vermiculite + perlite + bark), T2 (Coir + bark), T3 (Coir + perlite) and T4 (Coir + vermiculite). Plants in different treatments were grown under two watering regimes: 3 and 5-days watering intervals. At 9 weeks post treatment, plants were harvested, oven dried and tissue nutrient content, anti-F. oxysporum activity and secondary metabolites were analyzed.
Results:
The results showed that there were significant differences (P < 0.05) on the uptake of P, K, N, Mg, Fe, Cu, B and NH4-.The highest mean values for most nutrients were obtained in treatments under 3-days interval. Acetone extracts of S. aethiopicus under 5-days interval were the most bioactive against F. oxysporum. The MIC values obtained are relatively lower for the rhizomes, ranging from 0.078 - 0.3125 mg/ml compared to the higher MIC values (0.375 - 0.75 mg/ml) obtained in the leaves. LC-MS analysis of acetone extracts revealed the presence of phytochemicals such as caffeic acid, quercetin, p-hydroxybenzoic acid, rutin, kaempferol, epicatechin, naringenin, hesperetin and protocatechuic acid.
Conclusion:
The antimicrobial activity and/or the phytochemical profile of the crude extracts were affected by watering regimes.
Keywords: Siphonochilus aethiopicus, secondary metabolites, hydroponics, watering regimes, substrate combinations, nutrient uptake
Introduction
Studies on plant secondary metabolites have been increasing over the last 50 years (Bourgaud et al., 2001). These molecules are known to play a major role in the adaptation of plants to their environment, but also represent an important source of active pharmaceuticals (Ramachandra Rao and Ravishankar, 2002; Pandey et al., 2015; Yadav and Yadav, 2016). Due to their biological activities, plant secondary metabolites have been used in traditional medicine for centuries. Today, they provide valuable compounds such as pharmaceutics, cosmetics, fine chemicals, or more recently nutraceutics (Bourgaud et al., 2001). The three main classes of secondary metabolites include phenolics, terpenes and alkaloids. Horticultural research on medicinal plants has focused on developing the capacity for optimal growth in cultivation (Briskin, 2000).
The production of plant secondary metabolites has for a long time been achieved through wild harvesting of medicinal plants. However, it happens that some plants do not withstand large field cultures due to pathogen sensitiveness (Bourgaud et al., 2001). According to Kirakosyan and Kaufman (2002), the bulk of the market products such as crude extracts from higher plants are obtained from the wild, providing an opportunity for development of production strategies that are environmentally sustainable and economically viable. Wild plants are exposed to a myriad of environmental factors viz; temperature, humidity, the supply of water, nutrients, which vary in space and time and thus result to variation in secondary metabolite production (Ramakrishna and Ravishankar, 2011; Anderson et al., 2014; Liu et al., 2015). This has led scientists, researchers and farmers to consider cultivation and propagation methods such as hydroponics and tissue culture as alternative ways to produce the corresponding secondary metabolites (Bourgaud et al., 2001). Greenhouse production facilities can be maintained throughout the year, leading to increased concentration of biologically active phytochemicals and the medicinal capacity of the plant tissues (Murch et al., 2002). Controlled growth systems make it feasible to contemplate manipulation of phenotypic variation in the concentration of medicinally important compounds present at harvest (Pedneault et al., 2002; Canter et al., 2005). Marapetyan (1984), Gontier et al. (2002) and Manukyan (2005) have used hydroponic cultivation for growth of aromatic and medicinal plants. These studies showed that hydroponic systems could be a promising way for production of aromatic and medicinal plants. Hydroponics may provide a suitable growing system for high quality biomass production while allowing the regulation of secondary metabolism by managing the nutrient solution (Bolonhezi et al., 2010). Furthermore, adoption of hydroponics can increase water-use efficiency (Putra & Yuliando, 2015).
Subjecting plants to controlled stress can result in changes in levels of secondary metabolites production, some of which may be of medicinal interest (Tuomi et al., 1984). Simulated drought stress for quality improvement, such as applying special watering regimes in combination with efficient soil draining is an encouraging new tool for the production of spice and pharmaceutical relevant plants (Selmar, 2008). Limited water supply has a generally negative effect on plant growth and development. However, there are reports on the positive effect of limited water supply on the biosynthesis of secondary metabolites (Singh-Sangwan et al., 2001). It seems necessary to do research related to the relationship between medicinal plants and water deficit for the increasing need of medicinal plants (Jaleel et al., 2007).
Siphonochilus aethiopicus (Schweinf.) B.L. (Zingiberaceae) is a deciduous plant which bears cone-shaped rhizomes; the medicinal value of the plant is associated with these rhizomes (Golding, 2003; FAO, 2008). According to Fouche et al. (2011) S. aethiopicus has anti-inflammatory properties supporting anecdotal accounts of its effectiveness against asthma, sinusitis, colds and flu. Knowles (2005) affirmed that the essential oil of the roots and corresponding essential oil of the rhizomes are virtually identical in composition and are generally used for their decongestant properties, providing more rational for the use of wild ginger in the traditional treatment of flu and coughs. It contains a volatile oil with the antiseptic alpha-terpineol and other monoterpenoids. Herbal extracts obtained from rhizomes of S. aethiopicus have also been shown to exhibit antifungal and antibacterial properties (Lategan et al., 2009; Coopposamy et al., 2010; Igoli et al., 2012; Lall and Kishore, 2014) but little attention has been given to antifungal activities of the leaves. Due to its popular use amongst traditional healers and the method of harvesting (removal of the rhizome) this species has become critically endangered in its natural habitat (Lotter et al., 2006; Viljoen et al., 2008). In the current study, S. aethiopicus was cultivated by means of hydroponics under various watering regimes and substrate combinations. The objective of this study was to evaluate the effect of substrate combinations and watering regimes on macro- and micronutrient uptake, anti-F. oxysporum activity and secondary metabolite profile of S. aethiopicus.
Materials and Methods
Plant material
S. aethiopicus rhizomes were obtained from a commercial nursery, Afro Indigenous in Centurion, South Africa. The rhizomes were originally grown in a tissue culture laboratory as they have literally disappeared from the wild in South Africa. The rhizomes were dipped in Captab (4 g in 1 L) to prevent development of fungi and were imbedded in 49 pots containing a medium mix (2 parts pine bark, 1 part perlite and 1 part vermiculite). The pots were placed in a controlled environment tunnel under the following temperature regimes; day (17 °C - 21 °C) and night (15 °C - 18 °C). Pots were hand irrigated with sterile distilled water as needed until crop emergence was observed. Six weeks old healthy and vigorous seedlings with one to three leaves were each transplanted into separate pots (diameter -12.5 cm) filled with different substrate combinations (Table 1).
Table 1.
Composition of different substrate blends used in the study
| Treatment | Composition |
|---|---|
| T1 | Coir + vermiculite + perlite + bark (1:1:1:1) |
| T2 | Coir + bark (1:1) |
| T3 | Coir + perlite (1:1) |
| T4 | Coir + vermiculite (1:1) |
Substrates preparation
Coconut fibre (coir) was used as the main component for the preparation of media in different proportions and combinations. The combinations used in this study composed of coir, vermiculite, perlite obtained from The Cape Agricultural Suppliers, Cape Town; and bark from the Department of Horticulture, Cape Peninsula University of Technology, South Africa. The coir blocks were then fully expanded by soaking in water and the fibre was sun dried to reduce wetness. The treatments consisted of four different combinations of organic and inorganic substrates in different proportions (Table 1). The quantity of substrate blend in each pot was approximately 200 g. Each combination was thoroughly mixed in a bucket before filling into pots.
Experimental design
The experiment was conducted in a tunnel at Cape Peninsula University of Technology, Bellville campus; Cape Town, South Africa (33° 55’ 48.8” S, 18° 38’ 32.7” E). The structure was made of galvanized steel frame with transparent polycarbonate. Steel tables (2.5 m χ 1 m) were used as a flat surface for white plastic gutters (1.36 m long). Eight plastic gutters (Builders Warehouse, Cape Town) were placed on two steel tables; each steel table had four gutters which were held in place by cable ties. Each gutter had eight plastic pots (diameter - 12.5 cm) containing different substrate combinations. Pots were lined at the bottom with shade cloth to prevent substrates leaving through the drainage holes. Beneath the steel table were four fish tanks (60 L) each containing one submersible water pump, which recirculated water through a 20 ml black plastic pipe to one gutter only. All gutters were wrapped with black plastic polyethylene sheets to avoid algae build-up.
Nutrient solution was supplied to the plants by spaghetti tubing with drippers (1 dripper/plant) at a rate of 2 L/h controlled by a timer. Plants were fertigated with Nutrifeed fertilizer (Starke Ayres, Cape Town) containing 65 g/kg N, 27 g/kg P, 130 g/kg K, 70 mg/kg Ca, 20 mg/kg Cu, 1500 mg/kg Fe, 10 mg/kg Mo, 22 mg/kg Mg, 240 mg/kg Mn, 75 mg/kg S, 240 mg/kg B and mg/kg Zn. Nutrient solutions were prepared by dissolving 60 g of fertilizer in 60 L reservoir with tap water. The pH and electrical conductivity (EC) were maintained at 6.4 and 1.8, respectively. The nutrient solutions were refreshed every 2 weeks to minimize build-up of salts in the substrates. As the water drained out of the pots it drained back into the reservoirs and was reused. The experiment was arranged in a randomized block design and was allowed to run for 9 weeks. The tunnel had the following experimental conditions; average temperature; morning (18 - 33 °C), day (26 - 49 °C) and night (19 - 34 °C), relative humidity; morning (67 - 92%), day (80 - 94%) and night (81 - 92%), and average light intensity; morning (3917 Klux), day (19270 Klux) and night (2685 Klux).
Watering regime
Two watering regimes were used in this study to evaluate their effect on plant growth. Seedlings in four different treatments were grown under two watering regimes: watering twice in three days and watering twice in five days. One set of 24 plants planted in four treatments (each treatment replicated three times) received 3-days interval watering and another set received 5-days interval watering. All the 24 plants were allocated to each of the watering regimes giving a total population of 48 plants for the experiment. In both watering regimes, treatments were irrigated for 15 minutes twice a day; at 10:00 h and 18:00 h. The volume of nutrient applied was 1.38 L per pot per watering regime. All plants within each watering regime received the same cultivation procedure viz; application of fertilizer, experimental conditions, and treatments.
Determination of water holding capacity (WHC)
Three separate pots for each treatment filled with different substrate combinations but without plants were used to determine WHC as described by Reddy (2002). Water was added to each combination for 15 min. After irrigation, water was left to spread and soak in the substrates and allowed to drain for 30 min. Following this, single samples (50 g) taken from each treatment were placed in containers, weighed and transferred to labeled brown paper bags. The paper bags were placed in a 105 °C oven for 48 hours and were allowed to cool down at room temperature, placed back in containers and reweighed to determine dry mass of the substrate. The test was conducted with three separate samples (replicates) for each treatment. After the tests, substrates were removed and substituted with new substrate combinations for the next test.
Tissue analysis
Leaf samples were analyzed for macro- and microelements by using an inductively coupled mass spectrometry (ICP-MS) and a LECO-nitrogen analyzer with suitable standards (Bemlab (Pty) Ltd laboratory, South Africa). Leaves were washed with Teepol solution, rinsed with de-ionised water and dried at 70 °C overnight in an oven. The dried leaves were then milled, ashed at 480 °C and agitated in a 50:50 HCl (50%) solution for extraction through filter paper (Campell & Plank, 1998; Miller, 1998). The potassium (K), phosphorus (P), calcium (Ca), magnesium (Mg), sodium (Na), manganese (Mn), iron (Fe), copper (Cu), zinc (Z) and boron (B) content of the extracts were analyzed using Ash method. Total nitrogen (N) content of the leaves was determined through total combustion in a Leco N-analyzer. The amounts of N, P, K, Ca and Mg were converted from percentage (%) to mg/kg; 10 000 was used as the conversion factor.
Extraction of plant material
Fresh rhizomes and aerial parts were harvested at 9 weeks post-treatment and air-dried at 28 ± 2 °C. Dried plant material was cut into smaller pieces and ground using a Jankel and Kunkel Model A 10 mill into fine powder. Powdered plant material (3 g) was extracted with 60 ml of acetone in glass beaker and the supernatant filtered through Whatman No.1 filter paper. Acetone is a useful extractant because it is less toxic, highly volatile and dissolves a wide range of hydrophilic and lipophilic compounds (Eloff, 1998). The extracted material was left to dry over night at room temperature (22 ± 2 °C) and the dried acetone extracts were weighed.
Antimicrobial activities of extracts
The microdilution method described by Nchu et al. (2010) was used with minor modifications in determination of the minimum inhibitory concentration (MIC) of the extracts. S. aethiopicus aerial and rhizome extracts were diluted into acetone to obtain a starting concentration of 6 mg/ml. The starting concentration was diluted two fold in each successive serial dilution. The Fusarium oxysporum f sp.glycines strain (UPFC no. 21) was obtained from the Phytomedicine Programme, University of Pretoria. The fungus strain was originally isolated by C. Cronje from roots of a maize plant in Delmas, Gauteng. F. oxysporum was sub-cultured from stock agar plates and grown in Nutrient Broth (Merck, South Africa) for four hours. The fungal culture (100 ml) was added to each well of the 96-well microplates (105 cells/ml). Amphotericin b (160 μg/mL) was prepared as stock solution in acetone and served as a positive control and acetone was used as a negative control. Forty micro litre (40 μl) of 0.2 mg/ml of p-iodonitrotetrazolium chloride (INT) (Sigma) dissolved in sterile distilled water was added to each microplate well, sealed in a plastic bag and incubated at 37 °C and 100% RH. The MIC values were recorded after 12 and 18 hours. The antifungal bioassay (MIC) consisted of three replicates per treatment and per watering regime.
Total activity (TA)
The total activity in ml/g indicates the volume to which the extract derived from one (1) gram of plant material can be diluted and still inhibits the growth of the tested microorganism (Eloff, 2000). The total activity of the acetone extracts of S. aethiopicus was calculated using the following equation: Total activity = total mass (yield) in mg extracted from 1 g of dried plant material divided by the MIC value in mg/ml (Eloff, 2000). The higher the total activity, the more effective is the plant extract.
Liquid Chromatography-Mass Spectrometry (LC-MS)
Due to limited S. aethiopicus plant material (aerial parts and rhizomes), only treatments with the highest antifungal activity for each watering regime were subjected to LC-MS analysis. Three replicates were used for each treatment. In each case, powdered plant material (3 g) was suspended in acetone (60 ml) followed by stirring for 18 hours. Each mixture was then filtered using Whatman No.1 filter paper and the supernatant was evaporated to dryness. Methanol (2 ml) was added to the supplied samples, it was sonicated for 10 min and centrifuged for 5 min at 3000 G and transferred to vials. A Waters Synapt G2 quadrupole time-of-flight mass spectrometer was used for LC-MS analysis. It was fitted with a Waters Ultra pressure liquid chromatograph and photo diode array detection. LC separation was attained on a Waters BEH C18, 2.1 x 100 mm column with1.7 um particles. A gradient was applied using 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B). The gradient started at 100% solvent A for 1 minute and changed to 28% B over 22 minutes in a linear way. It then went to 40% B over 50 seconds and a wash step of 1.5 minutes at 100% B, followed by re-equilibration to initial conditions for 4 minutes. The flow rate was 0.3 ml/min and the column was kept at 55 °C. The injection volume was 2 μL Data was acquired in MSE mode which consisted of a low collision energy scan (6V) from m/z 150 to 1500 and a high collision energy scan from m/z 40 to 1500. The high collision energy scan was done using a collision energy ramp of 30-60V. The photo diode array detector was set to scan from 220-600 nm. The mass spectrometer was optimized for best sensitivity, a cone voltage of 15 V, desolvation gas was nitrogen at 650 L/hr and desolvation temperature 275 °C. The instrument was operated with an electrospray ionization probe in the negative mode. The negative mode was chosen for the purpose of targeting the phenolic class of compounds, because they are amenable to this method of analysis and they occur broadly in the Zingiberaceae family. Sodium formate was used for calibration and leucine encephalin was infused in the background as lock mass for accurate mass determinations. The entire flow from the LC was directed into the mass spectrometer. LC-MS chromatograms obtained for the crude extracts were analyzed by Masslynx to get spectra of various peaks. The initial approach was to extract ion chromatograms of molecular masses corresponding to compounds which had previously been reported for the family Zingiberaceae. Only major peaks in each chromatogram were analyzed for determination of molecular masses and fragmentation data for targeted compounds were compared to known data. Retention time peaks for crude extracts were compared to the peak obtained with the standards (protocatechuic acid, caffeic acid, quercetin, p-hydroxybenzoic acid, rutin, kaempferol, epicatechin, naringenin and hesperetin). From the chromatograms, the observed area under each peak was used to visually estimate the quantity of each of the targeted compounds present in the eluted crude plant extract at a fixed scale.
Statistical analysis
The experimental data collected were analyzed using One-way analysis of variance (ANOVA) and Tukey’s HSD test was used to separate the means at a level of significance, P < 0.05. These computations were performed using STATISTICA software (StatSoft, 2013). Sigma Plot 10.0 package was used for plotting graphs.
Results
Water Holding Capacity
There were significant differences (P < 0.05) detected in WHC among treatments (Figure 1). The WHC of treatments ranged from 174% to 365%. Maximum (average) WHC (337.86%) was found in T3 (50% coconut fiber + 50% perlite) followed by T4 (277.66%) (50% coconut fiber + 50% vermiculite) and T1 (216.33%) (25% coconut fiber + 25% vermiculite + 25% perlite + 25% bark) respectively, while minimum WHC (201.06%) was observed in T2 (50% coconut fiber + 50% bark). T3 was significantly different (df = 3, 56; P < 0.05) from the other three (3) treatments.
Figure 1.
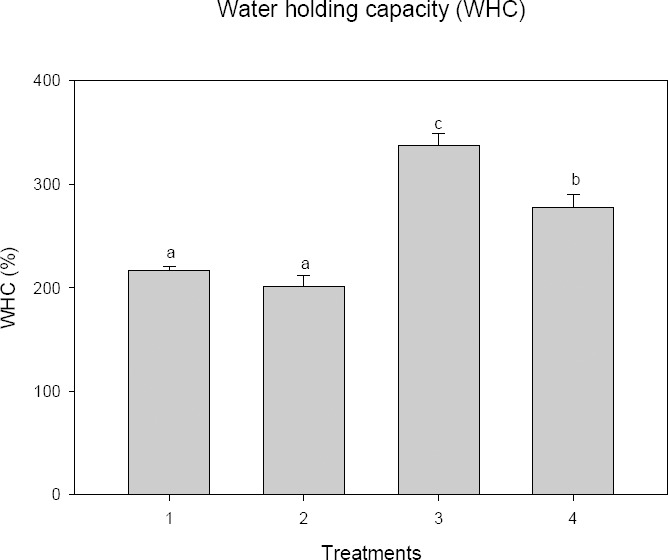
Water holding capacity of four different substrate combinations (see Table 1 for details on treatments). Vertical columns are means and the bars on each column are ± standard errors of mean. Means followed by the same lower case letters on top of the bars means not significant different (P > 0.05) following comparison using Tukey Test.
Tissue analysis
Macronutrients
In 3-days interval watering, the levels of N, Ca, Mg and Na did not differ significantly among treatments (df = 3, 8; P > 0.05) (Table 2). However, there were significant differences (P < 0.05) on the uptake of P and K. P increased significantly in aerial parts grown in T3 (6266.6 ± 88.19 mg/kg), while K (63000 ± 763. 76 mg/kg) levels were higher in T2. On the contrary, in 5-days interval watering; levels of P, K, Ca and Na did not show significant differences (P > 0.05) in aerial parts of S. aethiopicus in all treatments (Table 2). Nevertheless, the levels of N and Mg varied significantly (P < 0.05) in different treatments; N uptake (33400 ± 360.55 mg/kg) best result was observed in T4 while Mg (3233.3 ± 66.66 mg/kg) highest uptake was found in T1. The uptake of these macronutrients displayed higher values in watering regime with increased water supply. Generally, the best results in macronutrient uptake were observed in treatments involved in 3-days interval watering.
Table 2.
Effect of different substrate combinations and watering regimes on macronutrient uptake of S. aethiopicus aerial parts
| Watering regimes | |||
|---|---|---|---|
| Nutrient (mg/kg) | Treatment | 3-days interval | 5-days interval |
| N | T1 | 30600 ± 1686.2 a | 29466 ± 751.29 a |
| T2 | 29866.6 ± 1102 a | 30533 ± 218.58 ab | |
| T3 | 30400 ± 901.85 a | 29466 ± 1201.8 a | |
| T4 | 33533.3 ± 788.1 a | 33400 ± 360.55 b | |
| P | T1 | 6200 ± 57.73 a | 5666.6 ± 240.37 a |
| T2 | 6033.3 ± 88.19 ab | 5800 ± 351.18 a | |
| T3 | 6266.6 ± 88.19 a | 5966.6 ± 284.8 a | |
| T4 | 5333.3 ± 284.8 b | 5266.6 ± 375.65 a | |
| K | T1 | 61300 ± 1096.96 ab | 57333.3 ± 1328.3 a |
| T2 | 63000 ± 763.76 b | 63866.6 ± 1922.9 a | |
| T3 | 60366.6 ± 328.29 ab | 59466.6 ± 1003.8 a | |
| T4 | 59033.3 ± 866.66 a | 58633.3 ± 2251.9 a | |
| Ca | T1 | 6033.3 ± 463.08 a | 5233.3 ± 33.33 a |
| T2 | 6166.6 ± 145.29 a | 5466.6 ± 133.33 a | |
| T3 | 6533.3 ± 185.59 a | 5133.3 ± 33.33 a | |
| T4 | 6433.3 ± 296.27 a | 5533.3 ± 366.66 a | |
| Mg | T1 | 3166.6 ± 120.18 a | 3233.3 ± 66.66 b |
| T2 | 2933.3 ± 33.33 a | 2800 ± 0 a | |
| T3 | 3000 ± 152.75 a | 2866.6 ± 88.19 a | |
| T4 | 2933.3 ± 88.19 a | 2933.3 ± 88.19 ab | |
| Na | T1 | 239.66 ± 47.69 a | 313 ± 22.47 a |
| T2 | 249.66 ± 35.07 a | 365.6 ± 28.75 a | |
| T3 | 382.66 ± 86.56 a | 337 ± 18.5 a | |
| T4 | 255.33 ± 29.62 a | 286.6 ± 15.05 a | |
Values presented are means ± SE. Means followed by same lowercase letters in the same column are not significantly different (P > 0.05) following comparison using Tukey test. Grey and white colours are used to differentiate columns with macronutrients.
T1= coir + vermiculite + perlite + bark, T2= coir + bark, T3= coir + perlite, T4= coir + vermiculite.
Values presented are means ± SE. Means followed by same lowercase letters in the same column are not significantly different (P > 0.05) following comparison using Tukey test. Grey and white colours are used to differentiate columns with macronutrients.
T1= coir + vermiculite + perlite + bark, T2= coir + bark, T3= coir + perlite, T4= coir + vermiculite.
Micronutrients
Uptake of Mn, Zn and NO3- in 3-days interval watering did not show significant difference (P > 0.05) in aerial parts of S. aethiopicus between treatments (Table 3). However, uptake of Fe, Cu, B, and NH4- varied significantly (P < 0.05) in different treatments. The best Fe uptake (85.3 ± 2.18 mg/kg) was recorded in T1; Cu (3.3 ± 0.33 mg/kg) and NH4- (4646.6 ± 140.1 mg/kg) highest uptake was recorded in T4 while B (73.33 ± 5.89 mg/kg) levels were higher in T3. On the other hand, in 5-days interval watering different treatments did not significantly (P > 0.05) affect the uptake of Mn, Fe, Cu, Zn, NO3- and NH4- (Table 3). Whereas, only B (82.33 ± 6 mg/kg) levels recorded significant variations in aerial parts when grown in T3. Generally, micronutrients displayed higher values in watering regime with increased water supply. The best results for each were observed in treatments involved in 3-days interval watering except for Mn and B which showed best results in 5-days interval watering.
Table 3.
Effect of different substrate combinations and watering regimes on micronutrient uptake of S. aethiopicus aerial parts.
| Watering regimes | |||
|---|---|---|---|
| Nutrient (mg/kg) | Treatment | 3-days interval | 5-days interval |
| Mn | T1 | 206 ± 19.08 a | 234.6 ± 36.19 a |
| T2 | 158.66 ± 7.12 a | 240 ± 25.02 a | |
| T3 | 161.33 ± 37.22 a | 235.3 ± 39.26 a | |
| T4 | 220 ± 26.62 a | 240.3 ± 49.8 a | |
| Fe | T1 | 85.3 ± 2.18 b | 69 ± 3.21 a |
| T2 | 66 ± 5.68 a | 54 ± 3.05 a | |
| T3 | 61.3 ± 3.33 a | 65.3 ± 6.56 a | |
| T4 | 66.3 ± 4.66 a | 67.6 ± 3.92 a | |
| Cu | T1 | 3 ± 0 ab | 3.33 ± 0.33 a |
| T2 | 2.6 ± 0.33 ab | 3 ± 0 a | |
| T3 | 2 ± 0 a | 3 ± 0 a | |
| T4 | 3.3 ± 0.33 b | 3.33 ± 0.33 a | |
| Zn | T1 | 38.66 ± 1.45 a | 33.66 ± 2.18 a |
| T2 | 38.33 ± 2.02 a | 37.33 ± 3.92 a | |
| T3 | 34.33 ± 1.66 a | 33.66 ± 1.33 a | |
| T4 | 36.33 ± 1.45 a | 31 ± 1.15 a | |
| B | T1 | 62 ± 2.88 ab | 60 ± 4.04 ab |
| T2 | 50.33 ± 2.03 a | 55.66 ± 4.05 a | |
| T3 | 73.33 ± 5.89 b | 82.33 ± 6 b | |
| T4 | 66.66 ± 4.63 ab | 80.33 ± 7.53 ab | |
| NO3- | T1 | 2679.3 ± 167.61 a | 1972.6 ± 183.6 a |
| T2 | 1983.3 ± 265.83 a | 1957 ± 124.85 a | |
| T3 | 1681.3 ± 192.24 a | 1754.6 ± 59.52 a | |
| T4 | 2214.6 ± 443.72 a | 1497.3 ± 208.22 a | |
| NH4- | T1 | 3888 ± 158.75 a | 3549.6 ± 267.12 a |
| T2 | 4511.6 ± 234.9 ab | 3927.6 ± 281.34 a | |
| T3 | 4251 ± 78.07 ab | 3451.6 ± 228.91 a | |
| T4 | 4646.6 ± 140.1 b | 3751 ± 125.48 a | |
Values presented are means ± SE. Means followed by same lowercase letters in the same column are not significantly different (P > 0.05) following comparison using Tukey test. Grey and white colours are used to differentiate columns with micronutrients.
T1= coir + vermiculite + perlite + bark, T2= coir + bark, T3= coir + perlite, T4= coir + vermiculite.
Values presented are means ± SE. Means followed by same lowercase letters in the same column are not significantly different (P > 0.05) following comparison using Tukey test. Grey and white colours are used to differentiate columns with micronutrients.
T1= coir + vermiculite + perlite + bark, T2= coir + bark, T3= coir + perlite, T4= coir + vermiculite.
Yield, Minimum Inhibitory Concentration (MIC) and Total Activity (TA)
The yield following acetone extraction of aerial parts of S. aethiopicus was not statistically different (P > 0.05) among treatments and watering regimes. However, it was observed that in 3-days interval watering (Table 4a) the highest mean yield value was obtained in T3 (179 ± 10.17 mg) followed by T1 (162 ± 19.42 mg), T2 (119 ± 61.27 mg) and T4 (104.66 ± 47.47 mg), respectively. Similarly, in 5-days interval watering T3 gave the highest values compared to other treatments (T1, T4 and T2 respectively). On the other hand, there was no marked difference (P > 0.05) in yield of S. aethiopicus rhizomes among treatments and watering regimes (Table 4b). It was however observed that in 3-days interval watering the highest yield was obtained in T2 (166 ± 33.3 mg) followed by T1 (133 ± 33.3 mg) while T3 and T4 had the lowest mean values (100 ± 0 mg). Conversely, in 5-days interval the highest yield was observed in T1 and T2 (133 ± 33.3 mg) followed by T3 and T4 (100 ± 0 mg). The MIC values of acetone extracts of S. aethiopicus aerial parts and rhizomes ranged from 0.06 ± 0.02 - 0.75 ± 0 mg/ml and were not statistically different (P > 0.05) among treatments and watering regimes (Table 4a and 4b).
Table 4a.
Results on yield, minimum inhibitory concentration and total activities of acetone extracts obtained from aerial parts of hydroponically-cultivated S. aethiopicus following exposure to various substrate combinations and watering regimes.
| Treatment | Yield ± SE (mg) | MIC ± SE (mg/ml) | Total Activity (ml/g) | |||
|---|---|---|---|---|---|---|
| 3-days | 5-days | 3-days | 5-days | 3-days | 5-days | |
| T1 (12 H) | 162 ± 19.42 A | 168 ± 21.83 A | 0.625 ± 0.125 A | 0.5 ± 0.125 A | 90.5 ± 9.95 A | 130.76 ± 37.78 A |
| (18 H) | 0.75 ± 0 a | 0.625 ± 0.125 a | 72.13 ± 8.6 a | 103.66 ± 36 a | ||
| T2 (12 H) | 119 ± 61.27 A | 127.3 ± 15.96 A | 0.625 ± 0.125 A | 0.5 ± 0.125 A | 86.64 ± 59.29 A | 97.16 ± 27.05 A |
| (18 H) | 0.75 ± 0 a | 0.625 ± 0.125 a | 52.86 ± 27.2 a | 73.6 ± 15.7 a | ||
| T3 (12 H) | 179 ± 10.17 A | 172.3 ± 13.38 A | 0.5 ± 0.125 A | 0.313 ± 0.06 A | 129.86 ± 20.86 A | 203.2 ± 49.99 A |
| (18 H) | 0.5 ± 0.125 a | 0.375 ± 0 a | 129.86 ± 20.86 a | 153.16 ± 11.89 a | ||
| T4 (12 H) | 104 ± 47.47 A | 165.3 ± 23.88 A | 0.5 ± 0.125 A | 0.375 ± 0 A | 91.4 ± 43.79 A | 120.3 ± 21.2 A |
| (18 H) | 0.625 ± 0.125 a | 0.5 ± 0.125 a | 66.96 ± 34.15 a | 103.4 ± 32.49 a | ||
| Positive control | ||||||
| (12 H) | 0.047 ± 0.01 B | |||||
| (18 H) | 0.047 ± 0.01 b | |||||
| Negative control | ||||||
| (12 H) | No effect | |||||
| (l8 H) | No effect | |||||
Means followed by the same uppercase letters in the same column illustrate no significant difference (P > 0.05) at 12 hours post treatment following comparison using Tukey test
Means followed by same lowercase letters in the same column illustrate no significant difference at 18 hours post treatment.
Table 4b.
Results on yield, minimum inhibitory concentration and total activities of acetone extracts obtained from rhizomes of hydroponically- cultivated S. aethiopicus following exposure to various substrate combinations and watering regimes.
| Treatment | Yield ± SE (mg) | MIC ± SE (mg/ml) | Total Activity (ml/g) | |||
|---|---|---|---|---|---|---|
| 3-days | 5-days | 3-days | 5-days | 3-days | 5-days | |
| T1 (12 H) | 133 ± 33.3 A | 133 ± 33.3 A | 0.156 ± 0.03 A | 0.125 ± 0.03 A | 296 ± 59.1* A | 354.9 ± 0.3* A |
| (18 H) | 0.313 ± 0.06 a | 0.25 ± 0.06 a | 148. 16 ± 29.6* a | 177.8 ± 2* a | ||
| T2 (12 H) | 166 ± 33.3 A | 133 ± 33.3 A | 0.125 ± 0.03 A | 0.125 ± 0.03 A | 532.1 ± 177.1* A | 413.86 ± 156.2* A |
| (18 H) | 0.375 ± 0 a | 0.313 ± 0.06 a | 148.16 ± 29.6* a | 148.16 ± 29.6* a | ||
| T3 (12 H) | 100 ± 0 A | 100 ± 0 A | 0.078 ± 0.02 A | 0.06 ± 0.02 A | 472.8 ± 118.2* A | 591 ± 118.2* A |
| (18 H) | 0.25 ± 0.06 a | 0.1875 ± 0 a | 148.16 ± 29.6* a | 177.8 ± 2 a | ||
| T4 (12 H) | 100 ± 0 A | 100 ± 0 A | 0.125 ± 0.03 A | 0.109 ± 0.04 A | 295.66 ± 58.9* A | 413.86 ± 156.2* A |
| (18 H) | 0.313 ± 0.06 a | 0.1875 ± 0 a | 118.5 ± 29.6* a | 177.8 ± 2* a | ||
| Positive control | ||||||
| (12 H) | 0.047 ± 0.1 B | |||||
| (18 H) | 0.047 ± 0.1 b | |||||
| Negative control | ||||||
| (12 H) | No effect | |||||
| (18 h) | No effect | |||||
Means followed by the same uppercase letters in the same column illustrate no significant difference (P > 0.05) at 12 hours post treatment following comparison using Tukey test.
Means followed by same lowercase letters in the same column illustrate no significant difference at 18 hours post treatment.
Aerial parts: It was observed that in 3-days interval watering (df = 3, 8; F = 1.8; P > 0.05) (Table 4a), the MIC value of acetone extracts of aerial parts that were grown in the different treatments were not significant and ranged from 0.5 ± 0.125 - 0.625 ± 0.125 mg/ml at 12 h and 0.5 ± 0.125 - 0.75 ± 0 mg/ml at 18 h in the anti-F. oxysporum bioassay. Similarly, in 5-days interval watering (F = 1.2; P > 0.05) there was no significant difference among different treatments and ranged from 0.313 ± 0.06 - 0.5 ± 0.125 mg/ml at 12 h and 0.375 ± 0 - 0.625 ± 0.125 mg/ml at 18 h. Generally, T3 MIC values at 12 and 18 h post treatment were lower compared to those obtained with acetone extracts of plants grown in T4, T2 and T1.
Rhizomes: In 3-days interval watering (Table 4b), the MIC value of acetone extracts of rhizomes that were grown in the different treatments were not significant (df = 3, 8; F = 0.88; P > 0.05) and ranged from 0.078 ± 0.02 - 0.156 ± 0.03 mg/ml at 12 h and 0.25 ± 0.06 - 0.375 ± 0 mg/ml at 18 h in the anti-F. oxysporum bioassay. Congruently, in 5-days interval the MIC values of acetone extracts of S. aethiopicus rhizomes ranged from 0.313 ± 0.06 - 0.5 ± 0.125 mg/ml at 12 h and 0.375 ± 0 - 0.625 ± 0.125 mg/ml at 18 h; and were not statistically different (P > 0.05) among treatments. The antifungal activities of the extracts were significantly lower than amphotericin b. Generally, the MIC values of aerial parts and rhizomes in 5-days interval were lower compared to 3-days interval watering. Whereas there were no significant differences in MICs obtained with extracts of aerial parts among treatments within the same watering regimes and between the two watering regimes, there were marked differences (P < 0.05) between corresponding aerial and rhizomes treatments (Tables 4a and 4b).
Means followed by the same uppercase letters in the same column illustrate no significant difference (P > 0.05) at 12 hours post treatment following comparison using Tukey test.
Means followed by same lowercase letters in the same column illustrate no significant difference at 18 hours post treatment.
Means followed by the same uppercase letters in the same column illustrate no significant difference (P > 0.05) at 12 hours post treatment following comparison using Tukey test.
Means followed by same lowercase letters in the same column illustrate no significant difference at 18 hours post treatment.
denotes significant difference (P < 0.05) in total activity between aerial parts (Table 4a) and rhizomes (Table 4b) following One- Way ANOVA.
Total activity demonstrates the quantity at which the extract may be diluted with a solvent and still inhibit growth of a microorganism. In the present study, at 12 hours and 18 hours there was no significant difference in calculated TA among substrate combinations and watering regimes. However, acetone extract of aerial parts grown in T3 under 3-days interval watering (129.86 ± 20.86 ml/g [12 and 18 hours] recorded the highest values of TA against F. oxysporum compared to the other treatments (T1 [90.5 ± 9.95 and 72.13 ± 8.6 ml/g], T4 [91.4 ± 43.79 and 66.96 ± 34.15 ml/g] and T2 [86.64 ± 59.29 and 52.86 ± 27.2 ml/g] at 12 and 18 hours respectively (Table 4a). Similarly, in 5-days interval (Table 4a); T3 (203.2 ± 49.99 and 153.16 ± 11.89 ml/g [12 and 18 hours, respectively] recorded the highest values of the calculated TA compared to the other treatments (T1 [130.76 ± 37.78 and 103.66 ± 36 ml/g], T4 [120.3 ± 21.2 and 103.4 ± 32.49 ml/g] and T2 [97.16 ± 27.05 and 73.6 ± 15.7 ml/g] at 12 and 18 hours respectively.
On the contrary, acetone extract of rhizomes in 3-days interval watering in T2 (532.1 ± 177.1 ml/g) recorded the highest value of TA against F. oxysporum compared to T3 (472.8 ± 118.2 ml/g), T1 (296 ± 59.1 ml/g) and T4 (295.66 ± 58.9 ml/g) at 12 hours respectively (Table 4b). At 18 hours T1, T2 and T3 (148.16 ± 29.6 ml/g) recorded the highest values of TA whereas T4 (118.5 ± 29.6 ml/g) obtained the lowest value. Nonetheless, in 5-days interval (Table 4b) acetone extract of rhizomes grown in T3 (591 ± 118.2 ml/g) recorded the highest value of TA compared to T2 and T4 (413.86 ± 156.2 ml/g) while T1 (354.9 ± 0.33 ml/g) recorded the lowest TA value at 12 hours. At 18 hours, T1, T4 and T3 (177.8 ± 2 ml/g) recorded the highest values of TA against F. oxysporum whereas T2 (148.16 ± 29.6 ml/g) obtained the lowest TA value. Generally, the highest TA values of aerial parts and rhizomes were recorded in 5-days interval compared to 3-days interval watering. There were marked differences (P < 0.05) in total activities between corresponding aerial and rhizomes treatments (Tables 4a and 4b).
Data presented are in means ± standard error (n = 3)
(-) represents no phenolic compound isolated, TR= retention time
Data presented are in means ± standard error (n = 3)
(-) represents no phenolic compound isolated, TR= retention time
LC-MS analysis was carried out on the acetone extract of aerial parts and rhizomes of S. aethiopicus. Active compounds with their retention time (TR) and concentrations (ppm) are presented in Table 5 (aerial parts) and Table 6 (rhizomes). In the present study, a variety of compounds were detected: protocatechuic acid, caffeic acid, quercetin, p-hydroxybenzoic acid, rutin, kaempferol, epicatechin, naringenin and hesperetin. The results exhibited that the mean concentrations of the compounds detected were not significantly different (P > 0.05) among watering regimes in both aerial parts and rhizome of S. aethiopicus. The chromatograms of 3 and 5-days interval watering demonstrated a similar peak profile in both aerial parts and rhizome of the plant. LC-MS chromatograms showing the peak identities of the compounds are depicted in Figure 2 and 3. However, acetone extracts from the aerial parts showed higher concentration of rutin in 3-days interval [(TR = 13.3 min) 18.23 ± 5.126 ppm] and 5-days interval watering [(TR = 13.27 min) 22.88 ± 18.29 ppm]; followed by caffeic acid (found in 5-days interval, only), p-hydroxybenzoic acid, protocatechuic acid, kaempferol, hesperetin and quercetin, respectively. Naringenin had the lowest concentration in 3-days interval [(TR = 20.75 min) 0.06 ± 0.017 ppm] and 5-days interval watering [(TR = 20.68 min) 0.09 ± 0.02 ppm] (Table 5). Acetone extracts from the rhizomes
Table 5.
Effect of watering regimes on phenolic compounds profile of extracts of S. aethiopicus aerial parts.
| Compounds | Concentration (ppm) | |||
|---|---|---|---|---|
| TR | 3-days interval | TR | 5-days interval | |
| Protocatechuic acid | 4.62 | 2.02 ± 0.44 | 4.61 | 1.59 ± 1.037 |
| Caffeic acid | - | - | 8.12 | 12.33 ± 12.33 |
| Quercetin | 18.76 | 0.23 ± 0.125 | 18.73 | 0.34 ± 0.246 |
| p-hydroxybenzoic acid | 6.32 | 6.09 ± 1.62 | 6.29 | 6.13 ± 4.45 |
| Rutin | 13.3 | 18.23 ± 5.126 | 13.27 | 22.88 ± 18.29 |
| Kaempferol | 21.69 | 0.83 ± 0.236 | 21.67 | 0.66 ± 0.16 |
| Naringenin | 20.75 | 0.06 ± 0.017 | 20.68 | 0.09 ± 0.02 |
| Hesperetin | 18.76 | 0.326 ± 0.06 | 18.73 | 0.33 ± 0.23 |
Data presented are in means ± standard error (n = 3)
(-) represents no phenolic compound isolated, TR= retention time
Table 6.
Effect of watering regimes on phenolic compounds profile of extracts of S. aethiopicus rhizomes
| Compounds | Concentration (ppm) | |||
|---|---|---|---|---|
| TR | 3-days interval | TR | 5-days interval | |
| Protocatechuic acid | 4.62 | 1.11 ± 0.58 | 4.63 | 1.57 ± 0.79 |
| Caffeic acid | 8.8 | 4.47 ± 4.47 | - | - |
| p-hydroxybenzoic acid | 6.21 | 12.03 ± 7.83 | 6.19 | 19.87 ± 10.05 |
| Rutin | - | - | 13.3 | 0.13 ± 0.078 |
| Epicatechin | 9.64 | 0.04 ± 0.02 | 9.63 | 0.046 ± 0.02 |
| Naringenin | 20.74 | 0.19 ± 0.025 | 20.72 | 0.25 ± 0.03 |
Data presented are in means ± standard error (n = 3)
(-) represents no phenolic compound isolated, TR= retention time
Figure 2.
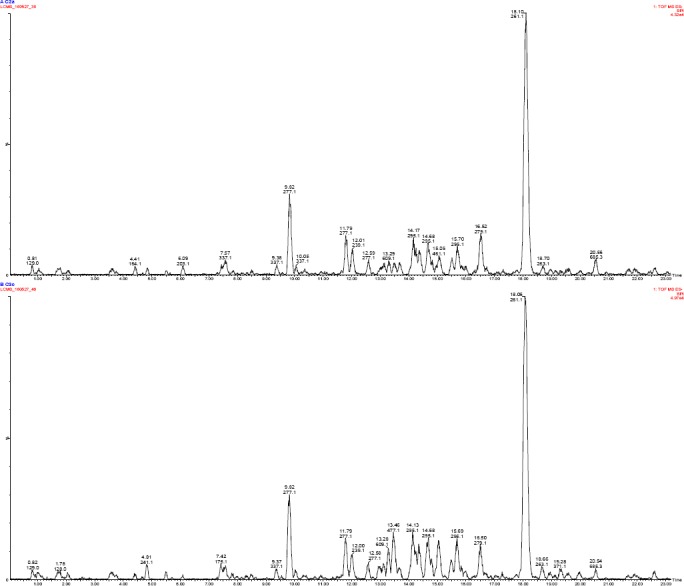
LC-MS profiles (TIC = total ion chromatogram) of acetone extract of S. aethiopicus aerial parts subjected to different watering regimes (3 and 5-days interval).
Figure 3.
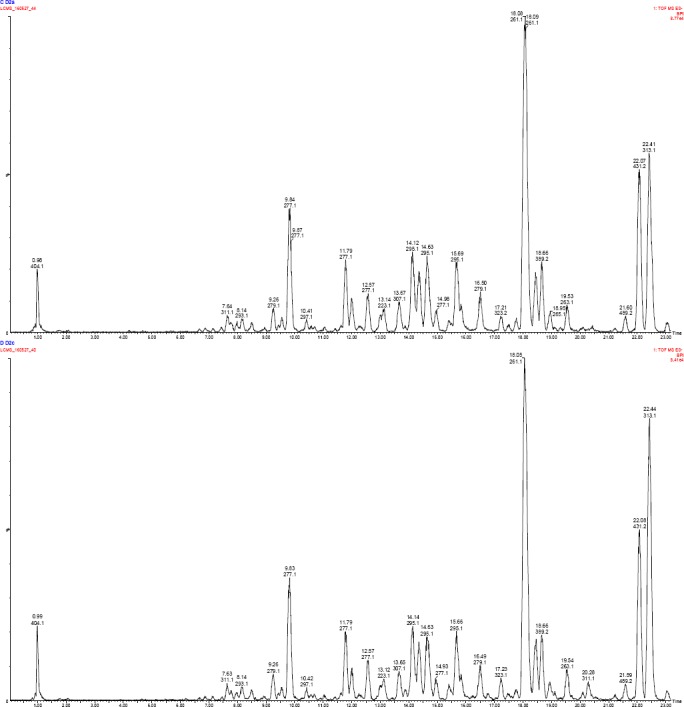
LC-MS profiles (TIC = total ion chromatogram) of acetone extract of S. aethiopicus rhizomes subjected to different watering regimes (3 and 5-days interval).
showed higher concentration of p-hydroxybenzoic acid in 3-days interval [(TR = 6.21 min) 12.03 ± 7.83 ppm] and 5-days interval watering [(TR = 6.19 min) 19.87 ± 10.05 ppm]; followed by caffeic acid, protocatechuic acid, naringenin and rutin, respectively. Whereas, epicatechin had the lowest concentration in 3-days interval [(TR = 9.64 min) 0.04 ± 0.02 ppm] and 5-days interval watering [(TR = 9.63 min) 0.046 ± 0.02 ppm] (Table 6). To further interrogate the LC-MS data, the mean concentrations of protocatechuin acid, caffeic acid and rutin were higher in the aerial parts than in rhizomes; while p-hydroxybenzoic acid and naringenin were significantly (P < 0.05) higher in the rhizome than in the aerial parts. Broadly, 5-days interval watering showed the highest concentrations of phenolic compounds than 3-days interval except for protocatechuic acid and kaempferol (aerial parts); and caffeic acid (rhizomes) which were higher in 3-days interval watering. Compounds such as quercetin, kaempferol and hesperetin were isolated only in aerial parts whereas epicatechin was isolated from the rhizomes, only. Protocatechuin acid, p-hydroxybenzoic acid and epicatechin were isolated from the tissue culture grown rhizome and were higher than hydroponically grown rhizome.
Discussion
In this study, all the substrate combinations had high water holding capacity (WHC) ranging from 174% to 365%; although some were statistically different, they were close to each other. All substrates appeared to have satisfactory WHC. Kukal et al. (2012) showed that coir mixes exhibited significantly higher water holding capacity; the WHC was 6.1 times higher ranging between 283-256%. The high WHC of coir based substrates has also been reported by Evans and Stamps (1996), Prasad (1997), Dombrowsky (2012) and Joshua and Vincent (2015). According to Hernandez-Apaolaza et al. (2005) and Kukal et al. (2012) when coir is used as the main component in substrate combinations, it has a potential to increase WHC (Treder, 2008). The combination of coir and perlite (T3) showed the highest results while coir and bark (T2) combination had the lowest WHC, these findings are in agreement with findings of Torres-Quezada (2012), which showed that pine bark potting mix had lower water retention capacity compared to other mixes due to its lower water-filled pore percentage and reduced capillarity. Differences in water retention capability can be explained by the variation in particle sizes between the substrates. Kukal et al. (2012) who studied water retention characteristics of growing media highlighted that the differences in WHC among the media could be due to diversity in total porosity and pore size distribution. The high WHC of the different substrate combinations indicate that plants grown in coir amended mixes could lessen the frequency of watering, which characterizes coir as a positive property to save water. Tramp et al. (2009) highlighted that high WHC allows the plants growing in the media to withstand longer periods between watering events and reduces the effect of drought.
In controlled hydroponic systems, nutrient availability is one of the fundamental growth factors that may be influenced by the amount of water supplied to the plants. The quantity of water that plants receive has a significant influence on the amount of nutrients it will contain (Sonnenberg, 2012). In this study, substrate combinations had different effects on different nutrient concentrations. According to Cuervo et al. (2012) in systems based on organic substrates it is difficult to track the variation of microelements concentration (Cu, Fe, B, Zn and Mn) due to changes in physical, chemical and microbiological properties of the substrates. On the other hand, significant differences were observed in P, K, N, Mg, Fe, Cu, B and NH4- (Table 2 and 3). These findings may be explained by the difference in chemical and physical properties of substrates and their interaction with nutrient solution composition and plant nutrient uptake (Abdelaziz, 2010). On the other hand, substrate combinations did not have any significant effect on the uptake of the following macro- and micronutrients (Ca, Na, Mn, Zn and NO3-) irrespective of the watering regime. Previously, Alifar and Ghehsareh (2010) showed that organic and inorganic substrates had no significant effect on concentration of macro and micro elements in the leaf of cucumber.
From the above results, it could be noticed that the uptake of most macro- and micronutrients (Table 2 and 3) was highly enhanced by exposing plants to higher application of water (3-days interval watering) in comparison with 5-days interval; except for Na, Mn, B and Cu were highly enhanced by 5-days interval. These findings are concurred with findings of Singh and Singh (2004) and Sonnenberg (2012) who discovered that nutrient availability increased by exposing plants to higher doses of water. Sonnenberg (2012) emphasized that an increased nutrient uptake of macro-and micronutrients exposed to higher water quantities, was a result of higher moisture availability and improved transpiration. Plants which received the longest interval of 5 days had the lowest results; this agreed with the findings of Singh and Singh (2004); Hussein and El-Dewiny (2011) that total nutrient content of all the nutrient elements decreased with increasing water stress. Drying of the soil and decrease in irrigation level can reduce the availability, uptake and transport of nutrients (Menzel et al., 1986; Singh & Singh, 2009). Decreasing water availability under drought generally results in reduced total nutrient uptake and frequently in reduced concentration of mineral nutrients in plants (Garg, 2003). Despite significant differences in levels of macro- and micronutrients in aerial parts between watering regimes and substrate combinations no symptoms of deficiency were observed in plants.
Results obtained in this study indicated that all the evaluated substrate combinations and watering regimes showed no significant difference on aerial parts and rhizome (Table 4) extracts of S. aethiopicus against F. oxysporum. However, acetone extracts of S. aethiopicus plants that were grown in T3 (coir and perlite) was the most bioactive against F. oxysporum compared to plants grown in T1, T2 and T4. Similarly, Evans (2008) observed that a medium containing 20 percent perlite and 80 percent coconut coir was the most disease suppressive. Generally, acetone extracts of aerial parts and rhizomes of S. aethiopicus were found to be bioactive against F. oxysporum in the antifungal assay. Nevertheless, there was no statistical difference observed in the anti-F. oxysporum activity between coir substrates; this may be due to the presence of phenolic compounds in coir dust. Phenolics in coir may have inhibited disease-causing pathogens (Evans and Stamps, 1996). Phenolic compounds have anti-microbial properties (Zafar et al. 2014) and these compounds play key roles in protecting plants from microorganisms, herbivores (Reidah, 2013). Furthermore, no statistical difference in the antifungal activities of S. aethiopicus was observed among watering regimes. The mean values of MIC due to watering regimes showed that increasing period of irrigation (from 3-days interval to 5-days interval) for both aerial parts and rhizomes of S. aethiopicus increased the antifungal inhibitory effects against F. oxysporum to an extent. Similar results were recorded by Said-Al Ahl et al. (2009). Tissue nutrient content analysis discussed previously showed that manganese, boron, copper and sodium were highly enhanced by 5-days watering interval. There is much scientific support that micronutrients such as Cu, B and Mn can reduce the severity of plant diseases by increasing disease tolerance and the resistance of plants to pathogens (Parker, 2011). Mn and Cu play a key role in synthesis of phenolic compounds and therefore act as an essential component of plant resistance to a wide range of fungal and bacterial pathogens (Cakmak, unpublished; Spectrum Analytic, Inc). Also, B and Mn have been beneficial in the control of Fusarium spp. infections (Walker and Foster, 1946; Dordas, 2008). Spectrum Analytic (unpublished) reported that shortage of the key nutrients such as Mn, Cu, Zn and B reduce the amount of the plants natural antifungal compound at the site of the infection.
In previous studies, S. aethiopicus was shown to have antimicrobial activity against the following pathogens; Aspergillus flavus, Aspergillus glaucus, Candida albicans, Candida tropicalis, Trichophyton mentagrophytes, Trichophyton rubrum, Botrytis cinerea (Knowles, 2005; Coopoosamy et al., 2010; Fielding et al., 2015). The MIC values obtained in the current study are relatively lower for the rhizomes ranged from 0.078-0.3125 mg/ml compared to leaves (0.375-0.75 mg/ml) suggesting rhizomes have better antifungal activity. Correspondingly, Coopoosamy et al. (2010) indicated that an antibacterial activity in the leaf of S. aethiopicus was lesser than that of the rhizomes. This can be explained by the fact that the leaf is predominantly involved as a production center, which in turn through transport mechanisms send the formulated products to the rhizomes (Coopoosamy et al., 2010). Furthermore, F. oxysporum are soil-borne and it is not unexpected for rhizomes, which are found below ground to have better antifungal activity than the aerial parts.
In this study, the extracts of aerial parts grown hydroponically in T3 recorded the highest values of TA against F. oxysporum when compared to the other treatments; for both 3-days (129.86 ml/g [12 and 18 hours]) and 5-days (203.2 and 153.16 ml/g) watering intervals at 12 and 18 hours respectively. T2 recorded the least values of TA in 3 and 5-days watering intervals. On the contrary, the extracts of rhizomes grown in T2 recorded the highest values of TA against F. oxysporum when compared to the other treatments in both 3 and 5-days intervals. T4 recorded the least values of TA in 3-days interval while T1 and T2 recorded the least values of TA activities in 5-days watering interval. Matanzima (2014) stated that total activity is a very good criterion for comparing biological activities among plant species due to its formula which takes into account the yield and antimicrobial activities of the extracts.
According to the LC-MS analysis, in the examined S. aethiopicus acetone extracts nine compounds were identified which matched previously isolated compounds from the family, Zingiberaceae; quercetin, rutin, kaempferol, naringenin, hesperetin and epicatechin (flavonoids), protocatechuic acid, caffeic acid and p-hydroxybenzoic acid (phenolic acids) (Voravuthikunchai, 2007; Ghasemzadeh et al., 2010; Jing et al., 2010; Singh and Gupta, 2013; Taheri et al., 2014; Yashoda et al., 2014; Ghasemzadeh et al., 2016). The results showed that the mean concentrations of the isolated compounds were not significantly different among watering regimes in both aerial parts and rhizome of S. aethiopicus (Table 5 & 6). However, increasing watering interval from 3 to 5-days resulted in enhancement of the identified phenolic compounds. Water deficit is known to increase the secondary metabolites concentration in plant tissues (Ade-Ademilua and Mbah, 2013). In a recent study conducted by Hassan and Ali (2016), it was found that decreasing the irrigation levels increased total phenolic content in cumic (Cuminum cyminum). Aziz et al. (2008) also observed that increasing the period between irrigation gave the highest relative constituents of the most important compounds in Thymus vulgaris. These findings somewhat support the conclusion that the phenolic compound increase under water deficit. Furthermore, most phenolic compounds were present in aerial parts extract in higher concentration than in rhizome, with exception of p-hydroxybenzoic acid and naringenin which were higher in rhizome. It is worth mentioning that quercetin, kaempferol and hesperetin were isolated in aerial parts only. Similar results were also obtained in many other studies. In a study conducted by Chan et al. (2007), it was found that for most ginger species screened; leaf phenolic contents were significantly higher than those of rhizome. Also, Tomovic et al. (2015) observed that flavonoid content of aerial part of Potentilla reptans was higher than flavonoid content of rhizome. Jing et al. (2010) and Singh and Gupta (2013) determined that the antioxidant activity and phenolic contents of the leaves and the amount of phenolics and flavonoids were higher than those in rhizomes. These findings are in disagreement with the findings of Ghasemzadeh et al. (2010) and Yashoda et al. (2014), which showed that levels of flavonoids and phenolic acids were greater in rhizomes compared to leaves.
Phenolic compounds have been reported to defend the plant against microorganisms and herbivores (Hada et al., 2001). Puupponen-Pimia et al. (2001) stated that many plant phenols are known to possess antimicrobial properties. In particular, the nine compounds isolated from S. aethiopicus (quercetin, rutin, kaempferol, naringenin, hesperetin, epicatechin, protocatechuic acid, caffeic acid and p-hydroxybenzoic acid) have been described to possess antimicrobial activity against several bacterial and fungal species (Cowan, 1999; Cetin-Karaca, 2011; Dogasaki et al., 2002; Rauha et al., 2002; Hayek et al., 2013). Since these compounds are categorized as antimicrobials, the effects of antifungal activity of aerial parts and rhizome of S. aethiopicus may be attributed to the content of these compounds. Acetone extracts of S. aethiopicus possess antimicrobial activity and this potential may be due to the presence of flavonoids and phenolic acids of the plant. It is worth mentioning that catechin has been previously detected in rhizomes of this species (Noudogbessi et al., 2013) while the above mentioned compounds were isolated from the plant for the first time however, they were previously detected in Zingiberaceae species. Furthermore, rutin was the major flavonoid presented in aerial parts, while p-hydroxybenzoic acid was the major compound in rhizome.
Conclusion
In conclusion, the uptake of most macro- and micronutrients was highly enhanced by exposing plants to higher application of water (3-days interval watering). Antifungal activities were maintained among S. aethiopicus plants grown hydroponically and plants exposed to coir and perlite mix under 5-days interval watering were the most bioactive against F. oxysporum. Increasing the period between watering (5-days interval) displayed the highest antifungal inhibitory effects. The total activity demonstrates that there is no difference between plant extracts obtained from plants grown in different substrate combinations and watering regimes. The results show that 5-days interval watering boosts S. aethiopicus microbial activity and the accumulation of the phytochemicals, while saving water. Marked antimicrobial potential of S. aethiopicus extracts can be attributed to the presence of flavonoids and phenolic compounds. The potential medicinal uses of S. aethiopicus are supported by the presence of the above mentioned phenolics and flavonoids activities.
Acknowledgements
The Department of Horticultural Sciences, Cape Peninsula University of Technology and National Research Foundation of South Africa (SFH14072177952) provided financial support for the research project.
References
- 1.Abdelaziz I.M.E. Effect of different microorganisms and substrates on yield and fruit quality of cucumber grown in hydroponic system. Dissertation in partial fulfillment of the requirements of the degree of Doctor (Ph.D.). Mendel University in Brno. 2010 [Google Scholar]
- 2.Ade-Ademilua O.E, Mbah G.C. Effect of different water regimes on the growth and phytochemical constituents of Acalypha wilkesiana harvested at 3am and 3pm. Int. J. Sci. Nat. 2013;4:619–623. [Google Scholar]
- 3.Alifar N, Ghehsareh M.A. Ondokuz Mayis University; Samsun-Turkey: 2010. May, The effect of Coco peat, Perlite and Peat moss on some greenhouse cucumber’s growth indices in soilless culture. International Soil Sci Congress on “management of natural resources to sustain soil health and quality”; pp. 26–28. [Google Scholar]
- 4.Anderson T.T, Wagner M.R, Rushworth C.A, Prasad K.S.V.S.K, Mitchell-Olds T. The evolution of quantitative traits in complex environments. Heredity. 2014;112:4–12. doi: 10.1038/hdy.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz E.E, Hendawi S.T, Din E.E, Omer E.A. Effect of soil and irrigation intervals on plant growth, essential oil yield and constituents of Thymus vulgaris plant. Am Eurasian. J. Agric. Environ. Sci. 2008;4(4):443–450. [Google Scholar]
- 6.Bolonhezi D, Khan I.A, Moraes R.M. Biomass yield of Stevia rebaudiana grown on hydroponic systems using different nitrogen rates. Planta Med. 2010;76:2. [Google Scholar]
- 7.Bourgaud F, Gravot A, Gontier M.E. Production of plant secondary metabolites: a historical perspective. Plant Sc. 2001;161:839–851. [Google Scholar]
- 8.Briskin D.P. Medicinal plants and Phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000;124:507–514. doi: 10.1104/pp.124.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cakmak I.M. Effect of micronutrients on plant disease resistance. Turkey: Sabanci University, Faculty of Engineering and Natural Sciences; unpublished. pp. 1–6. [Google Scholar]
- 10.Campbell C.R, Plank C.O. Kalra Y.P. Handbook of Reference Methods for plant. Boca Raton, FL: Analysis CRC Press; 1998. Preparation of plant tissue for laboratory analysis; pp. 37–49. [Google Scholar]
- 11.Canter P.H, Thomas H, Ernst E. Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends. Biotechnol. 2005;23(4):180–185. doi: 10.1016/j.tibtech.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Cetin-Karaca H. Evaluation of natural antimicrobial phenolic compounds against food borne pathogens. Masters theses. Lexington, Kentucky: University of Kentucky; 2011. p. 652. [Google Scholar]
- 13.Chan E.W.C, Lim Y.Y, Lim T.Y. Total phenolic content and antioxidant activity of leaves and rhizomes of some ginger species in Peninsular Malaysia. Gard. Bull. Singapore. 2007;59(1&2):47–56. [Google Scholar]
- 14.Coopposamy R.M, Naidoo K.K, Buwa L, Mayekiso B. Screening of Siphonochilus aethiopicus (Schweinf.) B.L. Burtt for antibacterial and antifungal properties. J. Med. Plant. Res. 2010;4(12):1228–1231. [Google Scholar]
- 15.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuervo B.W.J, Florez R.V.J, Gonzalez M.C.A. Aspects to consider for optimizing a substrate culture system with drainage recycling. Agron Colomb. 2012;30(3):379–387. [Google Scholar]
- 17.Dogasaki L, Shindo T, Furuhata K, Fukuyama M. Identification of chemical structure of antibacterial components against Legionella pneumophila in a coffee beverage. J. Pharm. Soc. Japan. 2002;122:487. doi: 10.1248/yakushi.122.487. [DOI] [PubMed] [Google Scholar]
- 18.Dombrowsky M.P. Guelph, Ontario, Canada: 2012. Growing substrates comprised of composted materials and reduced peat moss for production of greenhouse potted Gerbera (Gerbera jamesonii) Thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Environmental Biology. [Google Scholar]
- 19.Dordas C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008;28(1):33–46. [Google Scholar]
- 20.Eloff J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 21.Eloff J.N. A proposal on expressing the antibacterial activity of plant extracts - a small first step in applying scientific knowledge to rural primary health care in South Africa. S. Afri. J. Sci. 2000;96:116–118. [Google Scholar]
- 22.Evans M.R, Stamps R.H. Growth of bedding plants in sphagnum peat and coir dust-based substrates. J. Environ Hortic. 1996;14(4):187–190. [Google Scholar]
- 23.Evans M.R. Root media play a role in plant health. Researchers work to design better disease suppressiveness. 2008 www.GreenBeanPro.com .
- 24.FAO. Siphonochilus aethiopicus crop plant. Unassigned Eco Port Record. [Date accessed:15/01/2016];2008 http://ecoport.org/ep?Plant=17664&entityType=PLCR**&entityDisplayCategory=full&menuStyle=text.
- 25.Fielding B.C, Knowles C.L, Vries F.A, Klaasen J.A. Testing of eight medicinal plant extracts in combination with Kresoxim- Methyl for integrated control of Botrytis cinerea in apples. Agriculture. 2015;5:400–111. [Google Scholar]
- 26.Fouche G, Nieuwenhuizen N, Maharaj V, van Rooyen S, Harding N, Nthambeleni R, Jayakumar J, Kirstein F, Emedi B, Meoni P. Investigation of in vitro and in vivo anti-asthmatic properties of Siphonochilus aethiopicus. J. Ethnopharmacol. 2011;133:843–849. doi: 10.1016/j.jep.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Garg B.K. Nutrient uptake and management under drought: nutrient-moisture interaction. Curr. Agric. 2003;27(1/2):1–8. [Google Scholar]
- 28.Ghasemzadeh A, Jaafar H.Z.E, Rahmat A. Elevated carbon dioxide increases content of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiberofficinale Roscoe) varieties. Molecules. 2010;15(11):7907–7922. doi: 10.3390/molecules15117907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghasemzadeh A, Jaafar H.Z.E, Ashkani S, Rahmat A, Juraima A.S, Puteh A, Mohamed M.TM. Variation in secondary metabolite production as well as antioxidant and antibacterial activities of Zingiber zerumbet (L.) at different stages of growth. BMC Complement Altern Med. 2016;16(1):104. doi: 10.1186/s12906-016-1072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golding J.S. Tales of plants and people in southern Africa. Environmental Change Institute; UK: 2003. [Date accessed: 15/01/2016]. http://www.myristica.it/current/talesSAfrica.html . [Google Scholar]
- 31.Gontier E, Clement A, Tran T.L.M, Gravot A, Lievre K, Guckert A, Bourgaud F. Hydroponic combined with natural or forced root permeabilization: a promising technique for plant secondary metabolite production. Plant Sci. 2002;163:723–732. [Google Scholar]
- 32.Hada M, Hino K, Takeuchi Y. Development of UV defense mechanisms during growth of spinach seedlings. Plant Cell Physiol. 2001;42(7):784–787. doi: 10.1093/pcp/pce100. [DOI] [PubMed] [Google Scholar]
- 33.Hassan F.A.S, Ali E.F. Water requirements of drip irrigated Cumin and their effects on growth, yield and some physiological as well as biochemical parameters. Res. J. Pharm, Biol. Chem. Sci. 2016;7(3):178–191. [Google Scholar]
- 34.Hayek S.A, Gyawali R, Ibrahim S.A. Antimicrobial natural products. Microbial pathogens and strategies for combating them: science, technology and education. North Carolina. 2013 [Google Scholar]
- 35.Hernandez-Apaolaza L, Gasco A.M, Gasco J.M, Guerrero F. Reuse of waste materials as growing media for ornamental plants. Bioresource Technol. 2005;96(1):125–131. doi: 10.1016/j.biortech.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Hussein M.M, El-Dewiny C.Y. Mineral constituents of fenugreek varieties grown under water stress condition. Aust. J. Basic. Appl. Sci. 2011;5(12):2904–2909. [Google Scholar]
- 37.Igoli N.P, Obamu Z.A, Gray I.A, Clements C. Bioactive Diterpenes and Sesquiterpenes from the rhizomes of wild ginger (Siphonochilus aethiopicus (Schweinf) B.L Burtt) Afr. J. Tradit. Complement. Altern. Med. 2012;9(1):88–93. doi: 10.4314/ajtcam.v9i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaleel C.A, Manivannan P, Sankar B, Kishorekumar Gopi R, Somasundaram R, Panneerselvam R. Water deficit stress mitigation by calcium chloride in Catharanthus roseus Effects on oxidative stress, proline metabolism and indole alkaloid accumulation. Colloids Surf B Biointerfaces. 2007;60:110–116. doi: 10.1016/j.colsurfb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Jing J.L, Mohamed M, Rahmat A, Bakar M.F.A. Phytochemicals, antioxidants properties and anticancer investigations of the different parts of several gingers species (Boesenbergia rotunda, Boesenbergia pulchella var. attenuata and Boesenbergia armeniaca) J. Med. Plant Res. 2010;4(1):27–32. [Google Scholar]
- 40.Joshua R, Vincent P. Cost effective technology for waste management. The Int. J. Sci. Technol. 2015;3(6):252–261. [Google Scholar]
- 41.Kirakosyan A, Kaufman P. New strategies to produce high value secondary plant metabolites from shoot cultures involving a sustainable photobioreactor system. Natural Products in the New Millennium: Prospects and Industrial Application. 2002:375–388. [Google Scholar]
- 42.Knowles C.L. Botrytis cinerea Master’s Thesis. Western Cape: University of the Western Cape; 2005. Synergistic effects of mixtures of the kresoxim-methyl fungicide and medicinal plant extracts in vitro and in vivo against. [Google Scholar]
- 43.Kukal S.S, Saha D, Bhowmik A, Dubey R.K. Water retention characteristics of soil bio-amendments used as growing media in pot culture. J. Appl. Hortic. 2012;14(2):92–97. [Google Scholar]
- 44.Lall N, Kishore N. Are plants used for skin care in South Africa fully explored? J. Ethnopharmacol. 2014;153:61–84. doi: 10.1016/j.jep.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 45.Menzel C.A, William E.C, Seaman T, Smith P.J. The bioactivity of novel furanoterpenoids isolated from Siphonochilus aethiopicus. J. Ethnopharmacol. 2009;112(1):92–97. doi: 10.1016/j.jep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Liu J, Yin D, Zhao X. Influence of ecological factors on the production of active substances in the anti-cancer plant Sinopodophyllum hexandrum (Royle) T.S Ying. Plos One. 2015;10(4):e0122981. doi: 10.1371/journal.pone.0122981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lotter M, Burrows J.E, von Staden L. Siphonochilus aethiopicus (Schweinf.) B.L. Burtt. [Accessed on 2016/10/20];National Assessment: Red List of South African Plants version 2015.1. 2006 [Google Scholar]
- 48.Manukyan A. Optimum nutrition for biosynthesis of pharmaceutical compounds in celandine and catmit under outside hydroponic conditions. J. Plant Nutr. 2005;28:751–761. [Google Scholar]
- 49.Marapetyan S.K. Wees E, Stewart K.A, editors. Efficiency and perspectives of growing valuable essential-oil bearing plants in open-air hydroponics. ISOSC. Proc. 6thInternational Congress on Soilless Culture. The potential of NFT for the production of six herb species. Soilless Culture 1986. 1984;2(2):61–70. [Google Scholar]
- 50.Matanzima Y. Quantitative and qualitative optimization of antimicrobial bioactive constituents of Helichrysum cymosum using hydroponic technology. Thesis submitted in fulfillment of the requirements for the degree Master of Technology: Horticulture, Cape Peninsula University of Technology. 2014 [Google Scholar]
- 51.Menzel C.M, Simpson D.R, Dowling A.J. Water relations in passion fruit: effect of moisture stress on growth, flowering and nutrient uptake. Sci. Hortic. 1986;29:239–249. [Google Scholar]
- 52.Miller R.O. High-temperature oxidation: dry ashing. In: Kalra Y.P, editor. Handbook of Reference Methods for Plant Analysis. Boca Raton, FL: CRC Press; 1998. pp. 53–56. [Google Scholar]
- 53.Murch S.J, Rupasinghe H.P.V, Saxena P.K. An in vitro and hydroponic growing system for Hypericin, Pseudohypericin, and Hyperforin production of St. John Wort (Hypericum perforatum CV New Stem) Planta Med. 2002;68:1108–1112. doi: 10.1055/s-2002-36352. [DOI] [PubMed] [Google Scholar]
- 54.Nchu F, Aderoga M.A, Mdee L.K, Ellof J.N. Candida albicans compounds from Markhamia obtusifolia (Baker) Sprague (Bignoniaceae) S. Afr. J. Bot. 2010;76:54–57. [Google Scholar]
- 55.Noudogbessi J.P.A, Tchobo P.F, Alitonou G.A, Avlessi F, Soumanou M, Chalard P, Figueredo G, Chalchat J.C, Sohounhloue D.C.K. Chemical study of extracts of Siphonochilus aethiopicus (Schweinf.) B.L. Burtt (Zingiberaceae) from Benin. Asian J. Chem. 2013;25(15):8489–8492. [Google Scholar]
- 56.Pandey A, Sharma A, Lodha P. Isolation of an anti-carcinogenic compound: myricetin from Cochlospermum religiosum. Int. J. Pharm. Sci. Res. 2015;6(5):2146–2152. [Google Scholar]
- 57.Parker L. The importance of micronutrients in reducing Botrytis and other diseases in vines. Central Coast Vineyard, Westbridge Agriculture Products. 2011:2–3. [Google Scholar]
- 58.Pedneault K, Leonhart S, Gosselin A, Papadopoulos A.P, Dorais M, Angers P. Variations in concentration of active compounds in four hydroponically and field grown medicinal plant species. Acta Hortic. 2002;580:255–262. [Google Scholar]
- 59.Prasad M. Physical, chemical and biological properties of coir dust. Acta Hortic. 1997;450:21–29. [Google Scholar]
- 60.Putra P.A, Yuliando H. Soilless culture system to support water use efficiency and product quality: a Review. Agric. Agric. Sci. Procedía. 2015;3:283–288. [Google Scholar]
- 61.Puupponen-Pimia R, Nohynek L, Meier C, Kahkonen M, Heinonen M, Hopia A, Oksman-Caldentey K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001;90(4):494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 62.Ramachandra Rao S, Ravishankar G.A. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv. 2002;20:101–153. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 63.Ramakrishna A, Ravishankar G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011;6(11):1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rauha J.P, Remes S, Heinonen M, Hopia A, Kahkonen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2002;56(1):3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 65.Reddy K.R. Engineering properties of soils based on laboratory testing. Department of Civil and Materials Engineering. University of Illinois; Chicago: 2002. [Google Scholar]
- 66.Reidah I.M.A. Characterization of phenolic compounds in highly consumed vegetable matrices by using advanced analytical techniques. Doctoral Thesis submitted for a Doctoral degree in Chemistry, Granada. 2013 [Google Scholar]
- 67.Said-Al Ahl H.A.H, Hasnaa S.A, Hendawy S.F. Effect of potassium humate and nitrogen fertilizer on herb and essential oil of Oregano under different irrigation intervals. Ozean J. Appl. Sci. 2009;2(3):319–323. [Google Scholar]
- 68.Selmar D. Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Landbauforsch Volk. 2008;58(1/2):139. [Google Scholar]
- 69.Singh-Sangwan N, Farooqi A.H.A, Shabih F, Sangwan R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001;34:3–21. [Google Scholar]
- 70.Singh S, Gupta A.K. Evaluation of phenolics content, flavonoids and antioxidant activity of Curcuma amada (mango ginger) and Zingiber officinale (ginger) J. Chem. 2013;2(1):32–35. [Google Scholar]
- 71.Singh B, Singh G. Influence of soil water regime on nutrient mobility and uptake by Dalbergia sissoo seedlings. Trop. Ecol. 2004;45(2):337–340. [Google Scholar]
- 72.Singh G, Singh B. Effect of varying soil water stress regimes on nutrient uptake and biomass production in Dalbergia sissoo seedlings in India desert. J. For. Res. 2009;20(4):307–313. [Google Scholar]
- 73.Sonnenberg D.M. Thesis submitted in fulfillment of the requirements for the degree Master of Technology, Horticulture. Cape Town: Cape Peninsula University of Technology; 2012. The effects of various drip fertigated water quantities on hydroponically cultivated Cucumis sativa L. [Google Scholar]
- 74.Spectrum Analytic. (Unpublished) The relationship between nutrients and other elements to plant diseases. Washington: [Date accessed: 04/05/2016]. pp. 2–27. http://www.spectrumanalytic.com/support/library/pdf/relationshipbetweennutrientsandotherelementstoplantdiseases.pdf . [Google Scholar]
- 75.StatSoft Inc. STATISTICA (data analysis software system), version 12. 2013 www.statsoft.com .
- 76.Taheri S, Abdullah T.L, Ebrahimi M. Antioxidant capacities and total phenolic contents enhancement with acute Gamma irradiation in Curcuma alismatifolia (Zingiberaceae) leaves. Int. J. Mol. Sci. 2014;15(7):13077–13090. doi: 10.3390/ijms150713077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomovic M.T, Cupara M.S, Popovic-Milenkovic M.T, Ljujic B.T, Kostic M.J, Jankovic S.M. Antioxidant and anti-inflammatory activity of Potentilla reptans. L. Acta Pol. Pharm. 2015;72(1):137–145. [PubMed] [Google Scholar]
- 78.Torres-Quezada E. Evaluation of soilless media, container types and in-row distances on Bell pepper growth and yield. A thesis presented to the graduate school of the University of Florida in partial fulfillment of the requirements for the degree of Master of Science. University of Florida. 2012 [Google Scholar]
- 79.Tramp C, Chard J, Bugbee B. Optimization of soilless media for alkaline irrigation water. Utah State University. 2009 [Google Scholar]
- 80.Treder J. The effect of cocopeat and fertilization on the growth and flowering of oriental Lily ’Star Gazer’. J. Fruit Ornam. Plant Res. 2008;16:361–370. [Google Scholar]
- 81.Tuomi J, Niemela P, Haukioja E, Siren S, Neuvonen S. Nutrient stress: an explanation for plant anti-herbivore responses to defoliation. Oecologia. 1984;61:208–210. doi: 10.1007/BF00396762. [DOI] [PubMed] [Google Scholar]
- 82.Viljoen A.M, Demirci B, Baser K.H.S, van Wyk B.E. The essential oil composition of the roots and rhizomes of Siphonochilus aethiopicus. S. Afr. J. Bot. 2002;68:115–116. [Google Scholar]
- 83.Voravuthikunchai S.P. Family Zingiberaceae compounds as functional antimicrobials, antioxidants and antiradicals. Food. 2007;1(2):227–240. [Google Scholar]
- 84.Walker J.C, Foster R.E. Plant nutrition in relation to disease development III Fusarium wilt of tomato. Am. J. Bot. 1946;33(4):259–264. [Google Scholar]
- 85.Yadav R, Yadav N. Secondary metabolites production in plant tissue culture: A Review. Int. Educ Sci. Res. J. 2016;2(6):63–64. [Google Scholar]
- 86.Yashoda K, Vivek M.M, Prashith K.T.R, Raghavendra H.L. Antimicrobial and radical scavenging activity of leaf and rhizome extract of Alpinia galanga (L.) Willd (Zingiberaceae) Int. J. Drug Dev. Res. 2014;6(1):239–247. [Google Scholar]
- 87.Zafar F, Jahan N, Rahman K.U, Zafar W, Aslam S. Comparative evaluation of phytochemical, mineral and vitamin contents of gemmomodified extracts and leaves of two indigenous medicinal plants. Int. J. Agric. Biol. 2014;16:911–916. [Google Scholar]


