Abstract
Background:
This study aimed to investigate the prevalence of diabetes, impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) in Iranian patients with thalassemia major.
Methods:
The current study has been conducted based on PRISMA guideline. To obtain the documents, Persian and English scientific databases such as Magiran, Iranmedex, SID, Medlib, IranDoc, Scopus, PubMed, ScienceDirect, Cochrane, Web of Science, Springer, Wiley Online Library as well as Google Scholar were searched until December 2015. All steps of the study were conducted by two authors independently. To the high heterogeneity of the studies, the random effect model was used to combine studies. Data were analyzed using STATA Version 11.1 software.
Results:
Thirty-two studies involving 3959 major thalassemia patients with mean age of 16.83 years were included in the meta-analysis. The prevalence of diabetes in Iranian patients with thalassemia major was estimated as 9% (95% CI: 6.8-10.5) and estimated rate was 12.6% (95% CI: 6.1-19.1) for males and 10.8% (95% CI: 8.2-14.5) for females. The prevalence of IFG and IGT were 12.9% (95% CI: 7-18.8) and 9.6% (95% CI: 6.6-12.5) respectively. No relationship between serum ferritin and development of diabetes was noted.
Conclusion:
The prevalence of diabetes, IFG, and IGT in patients with thalassemia major in Iran is high and accordingly requires new management strategies and policies to minimize endocrine disorders in Iranian patients with thalassemia major. Screening of patients for the early diagnosis of endocrine disorders particularly diabetes, IFG, and IGT is recommended.
Key Words: Diabetes, Impaired Fasting Glucose, Impaired Glucose Tolerance, Thalassemia Major, Iran, Meta-Analysis
Thalassemia major is a hereditary hemolytic disease with a severe form of β-thalassemia. It causes severe anemia after a reduced production of β-globin chains (1). Thalassemia belt is expanding in the eastern coast of the Mediterranean region, throughout the Arabian Peninsula, Turkey, Iran, India and the Southeast Asia (2). This disease is one of the most common hereditary diseases in Iran, and the number of patients with thalassemia major is about 18800 people (3). These patients regularly receive blood to prevent complications like chronic anemia and bone changes (4). Over the past 2-3 decades, blood transfusions have significantly increased lifetime and life expectancy in patients with thalassemia major (5). At the same time, the increasing use of this treatment has led to complications of iron overload (6). One of the toxic effects of iron overload occurs in the endocrine glands (7).
Even with careful management of patients, disorders of endocrine glands such as growth retardation, hypogonadism, insulin-dependent diabetes, hypothyroidism, hyporparathyroidism may occur (8-11). To prevent this complication of iron overload, chelation therapy regimens are used (12).
Endocrine gland complications may be due to the unsystematically iron chelation therapy in patients with thalassemia major in the developing countries (10). Diabetes is one of the most common and serious diseases that can be considered as the human’s most important metabolic disease (13).
The most common complications of diabetes are cardiovascular diseases, retinopathy, neuropathy, nephropathy, sexual dysfunction and infection (14). The term impaired glucose tolerance (IGT) was introduced in 1979 by the National Diabetes Data Group (NDDG) as part of the classification and diagnostic criteria. Later on, this term was implemented by the World Health Organization (WHO) criteria (15).
Progress from normal glucose to type-II diabetes has often been an intermediate state associated with change glucose metabolism called IGT or pre-diabetes stage. IGT is a risk factor for type-II diabetes and patients with IGT without lifestyle change may develop to type-II diabetes in ten years (16, 17). Whereas pre-diabetes state was detected at early stage, diabetes can be delayed for several years with appropriate iron chelation therapy and regularly use of desferal (18, 19).
A simple review of the literature showed that the prevalence of diabetes, impaired fasting glucose (IFG) and IGT in patients with thalassemia major in Iran had been reported differently (19-22). Systematic review and meta-analysis study of the review of all literature and combining them can be a comprehensi veview of the problem in a specific population (23-25). Because of no available comprehensive report, this study assesses the prevalence of diabetes, IFG, and IGT in patients with thalassemia major in Iran.
Methods
This review was conducted based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline (24). To avoid bias, all steps of the study including search, selection of studies, quality assessment, and data extraction were conducted by two researchers, independently. Any disagreement was reviewed by third researcher.
Search Strategy: To obtain the related documents in Persian and English, scientific databases such as Magiran, Iranmedex, SID, Medlib, IranDoc, Scopus, PubMed, Science direct, Cochrane, Web of Science, Springer, Wiley Online Library, and Google Scholar were searched until December 2015. Persian and English MeSH keywords were used. Prevalence, diabetes, Glucose Intolerance, prediabetes, endocrine disorders, ferritin, hemosiderosis, iron overload, chelation therapy, endocrine, Iran, thalassemia major and also word combination of and & or operators were used as keywords.
Inclusion Criteria: Related papers about the prevalence of diabetes, IFG, and IGT in thalassemia major patients in both English and Persian Language were considered as inclusion criteria. Diabetes was determined according to World Health Organization (WHO) and American Diabetes Association (ADA). The criterion for the diagnosis of IFG was determined as 100≥FBS<126 mg/dl while the criterion for IGT was determined as two-hour glucose levels of 7.8-11.1 mmol/L(140-200 mg/dl) on the 75 g oral glucose tolerance test (26).
Exclusion Criteria: Studies with non-randomly selected sample size; lack of relevance to the topic; letters to the editor and case report studies.
Evaluation of Quality: Researchers using a STROBE standard checklist (27) including 22 items. The selected studies were appraised in all aspects of methodology including sampling methods, measurement parameters, statistical analysis and objectives of the study. The minimum and maximum scores in this checklist were 16 and 44, respectively. The papers that had reached the minimum score (16) were selected for the meta-analysis stage.
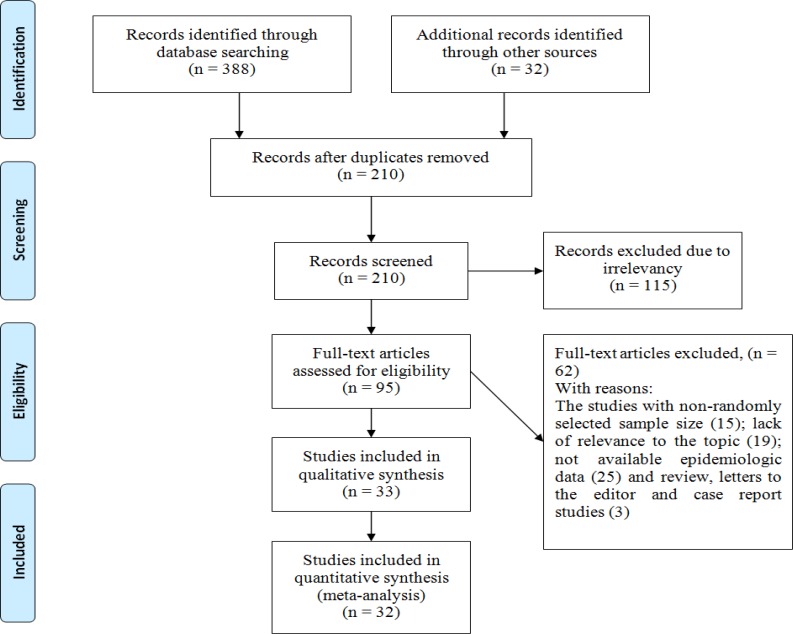
Study Selection: In the initial search, 420 studies probably related to the prevalence of diabetes, IFG, and IGT in patients with thalassemia major were found of which 210 studies were excluded because they were duplicate papers (papers extracted by two researchers with identical titles, authors, and journal). From the remaining 210 studies, 189 cases were excluded after reading the summary and the full text of the paper due to non-relevance of the topic, and the lack of criteria and low quality (figure 1).
Figure1.
The entrance steps of systematic review and meta-analysis
Data Extraction: All final papers imported to study process were extracted by a pre-prepared checklist. The checklist includes the author name, year of study, place of study, type of study, sample size, prevalence of diabetes, IFG and IGT, prevalence of diabetes, IFG and IGT according to gender, diagnostic criteria for diabetes, IFG and IGT and mean of serum ferritin level in diabetes and control groups.
Statistical Analysis: The variance of each study was calculated according to the binomial distribution. According to the sample size and variance, the studies were combined. To assess the heterogeneity of the studies, Cochran test, and I2 index was used. The heterogeneity of the study was 84.8% classified among studies with high heterogeneity (I2 index less than 25%: low heterogeneity, 25%-75%: average heterogeneity and more than 75%: high heterogeneity). Due to the heterogeneity of the studies, the random effects model was used to combine studies. To find the source of heterogeneity among studies, meta-regression model was used for the year of study, sample size, quality of studies and diagnostic method. To investigate propagation bias, Beggs test and draw of funnel plot were used. Data were analyzed using the Stata Version 11.1 software. The significance level was considered as p<0.05.
Results
In a systematic review of studies, 32 studies were included into the meta-analysis process. All participants in the study were 3959 with thalassemia patients in an average age of 16.83 years (95% CI: 15.71-17.94) (table 1).
Table 1.
Detailed characteristics of 32 articles included in the systematic review on the prevalence of diabetes, IFG and IGT in patients with thalassemia major
| Ref. | Author Name | Place of study | Year of study | Sample size |
Age
(Mean±SD) |
Diagnostic criteria for diabetes | Prevalence of Diabetes (%) | Prevalence of IGT (%) | Prevalence of IFG (%) |
|---|---|---|---|---|---|---|---|---|---|
| 19 | Najafipour | Tabriz | 2006 | 65 | 15.6±4.4 | DAD and WHO | 8.9 | 7.1 | 28.8 |
| 20 | Rostami | Bushehr | 2009 | 60 | 20.23±23 | WHO | 18.3 | 6.7 | |
| 21 | Safari | Qazvin | 2006 | 63 | 20.89±5.01 | DAD | 25.4 | ||
| 22 | Rezaei | Kohgiluyeh va boyer ahmad | 2003 | 233 | 13.24±6.1 | DAD | 3.1 | 4 | |
| 28 | Kashanchi Langarod | Karaj | 2010 | 184 | 19.64±7.06 | DAD | 10.22 | 12.4 | |
| 29 | Najafipour | Tabriz | 2005 | 56 | 15.6±4.25 | DAD and WHO | 8.9 | 7.1 | 30 |
| 30 | Company | Ahwaz | 2003 | 195 | 14.9±6.09 | DAD and WHO | 16.4 | 19 | |
| 31 | Kawsarian | Sari | 1996 | 70 | DAD and WHO | 28.5 | 15.7 | 1 | |
| 32 | Mortazavi | Zanjan | 1991 | 146 | DAD and WHO | 2.7 | 6.2 | ||
| 33 | Soheili Kha | Yazd | 1998 | 53 | 10.7 | DAD | 3.7 | ||
| 34 | Keihanian | Tehran | 2010 | 133 | 18.28 | DAD | 6 | ||
| 35 | Azimi | Tehran | 2003 | 45 | DAD and WHO | 11.1 | |||
| 36 | Mahdavi Anari | Tehran | 1999 | 60 | DAD | 8.3 | |||
| 37 | Fotoohi | Tehran | 1999 | 60 | DAD and WHO | 18.3 | 8.3 | ||
| 38 | Younesi | Qazvin | 1998 | 94 | DAD | 5.3 | |||
| 39 | Yazdi | Yazd | 2005 | 65 | 10.3 | DAD | 8.3 | 18.3 | |
| 40 | Haghverdi | Sari | 1998 | 20 | DAD | 5 | 20 | ||
| 41 | Shiva | Tabriz | 2006 | 71 | 12.9±5.2 | DAD and WHO | 8.5 | 21.1 | |
| 42 | Ishraqi | Babol | 2010 | 280 | 19.6±8.5 | DAD and WHO | 13.9 | ||
| 43 | Jahantigh | Zahedan | 2011 | 346 | 17.7±4.9 | DAD and WHO | 15.9 | 6.6 | |
| 44 | Faalpur | Ardebil | 2002 | 51 | WHO | 3.9 | 3.9 | ||
| 45 | Saffari | Qazvin | 2012 | 77 | 21.26±4.53 | WHO | 16.9 | 13 | |
| 46 | Arjmandi Rafsanjan | Tehran | 2004 | 273 | DAD | 18.3 | |||
| 47 | Eshghi | Zahedan | 2001 | 66 | DAD and WHO | 4.5 | 3 | ||
| 48 | Moayeri | Tehran | 2006 | 158 | 4.8 | DAD | 10.1 | ||
| 49 | Karamifar | Shiraz | 2003 | 150 | DAD | 7.3 | |||
| 50 | Mowla | Sari | 2004 | 98 | 4 | DAD | 8.2 | ||
| 51 | Shams | Tehran | 2009 | 78 | 16±6 | DAD | 5.1 | 12.8 | |
| 52 | Karimi | Shiraz | 2008 | 47 | 19.7±5.3 | DAD and WHO | 19 | ||
| 53 | Raissi | Shahrekord | 2003 | 40 | DAD and WHO | 10 | |||
| 54 | Sadat | Gorgan | 2007 | 185 | DAD | 11.9 | |||
| 55 | Mehrvar | Tehran | 2004 | 437 | DAD | 6.2 |
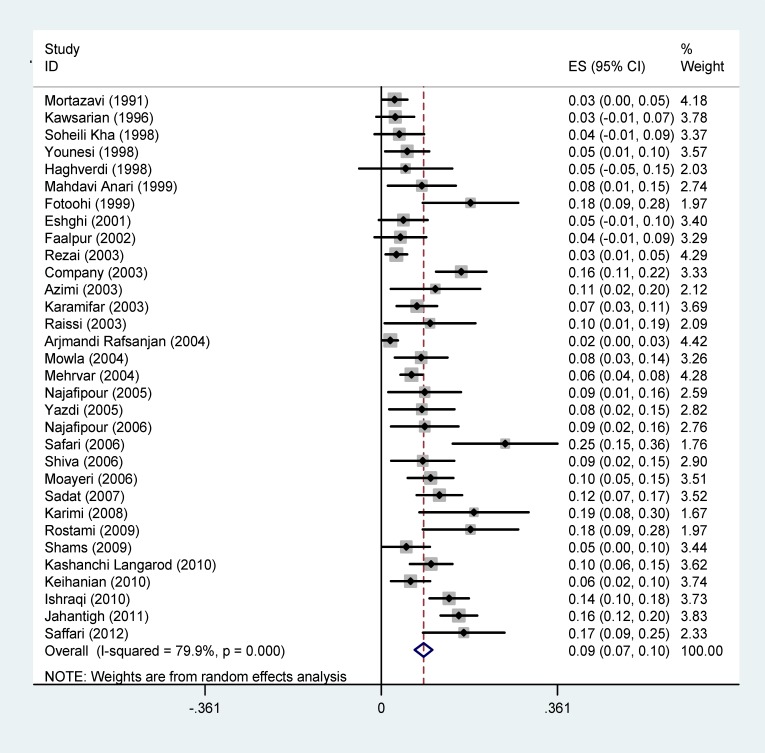
Diabetes: The prevalence of diabetes in patients with thalassemia major in Iran was estimated as 9% (95% CI: 6.8-10.5). The lowest prevalence of diabetes is associated with Arjmandi's (2004) in Tehran (1.8%) and the highest prevalence of diabetes was reported in Safari’s (2006) in Qazvin (25.4%) (figure 2).
Figure 2.
Forest plots presenting the prevalence of diabetes in patients with thalassemia major. Weights are assessed from the random-effects model analysis
The prevalence of diabetes in patients with thalassemia major had been examined in 7 studies according to sex. This rate was estimated as 12.6% (95% CI: 6.1-19.1) in males and 10.8% (95% CI: 8.2-14.5) in females.
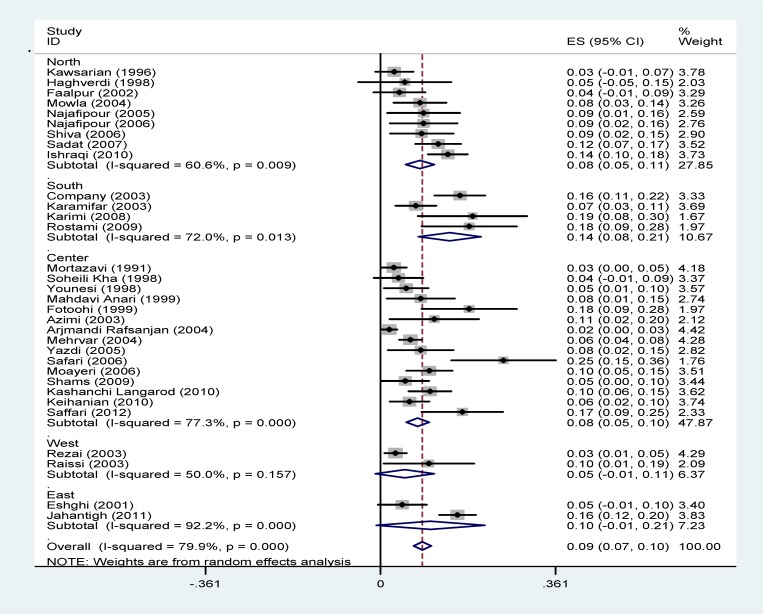
The prevalence of diabetes in patients with thalassemia major was indicated according to the geographical regions in figure 3 and shows that the lowest prevalence was in the West of Iran (5%) in which the highest prevalence rate was in South (14.3%).
Figure 3.
Forest plots presenting the prevalence of diabetes in patients with thalassemia major sub-grouped by geographical regions.
In terms of the diagnostic criteria for diabetes, the lowest and the highest prevalence rates are related to DAD (7%) and the history of insulin therapy (13%) (table 2).
Table 2.
Estimates for prevalence of diabetes in patients with thalassemia major according to diagnostic criteria for diabetes
| Diagnostic criteria | (N) studies | Sample size | I 2 (%) | Confidence interval (%) | Overall prevalence (%) |
|---|---|---|---|---|---|
| DAD | 16 | 2284 | 73.3 | 5-9 | 7 |
| WHO | 3 | 188 | 80.7 | 2-13 | 12 |
| WHO And DAD | 13 | 1487 | 82.7 | 6-13 | 10 |
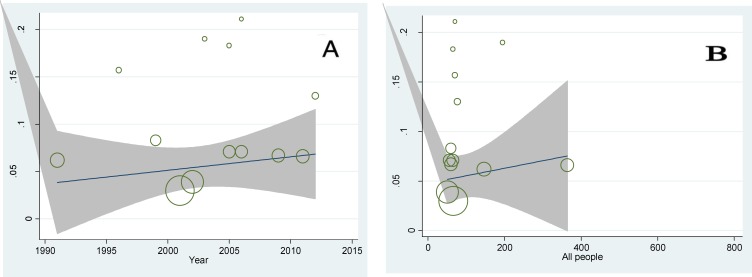
Regression model was used to investigate the relationship between prevalence of diabetes with the year of study and sample size. The p-values were calculated 0.382 and 0.326, respectively and no statistically significant was found (figure 4).
Figure 4.
A: Meta-regression plot of the prevalence of diabetes based on the year of study (P=0.382). B: Meta-regression plot of the prevalence of diabetes based on sample size of the study (P=0.362
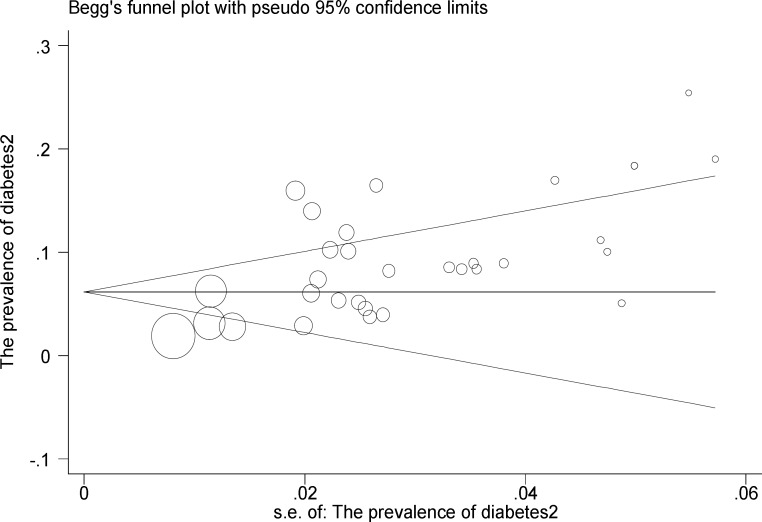
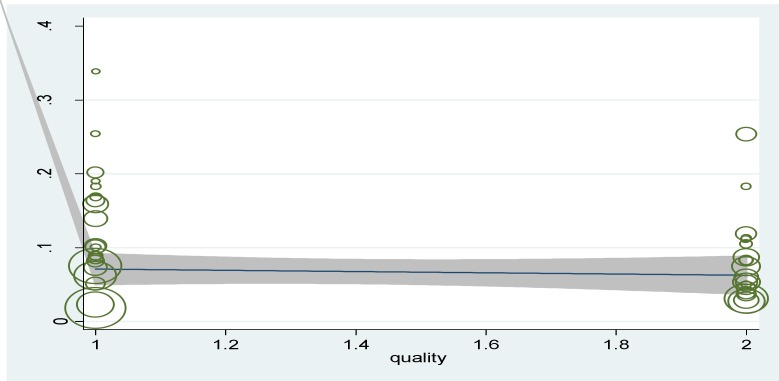
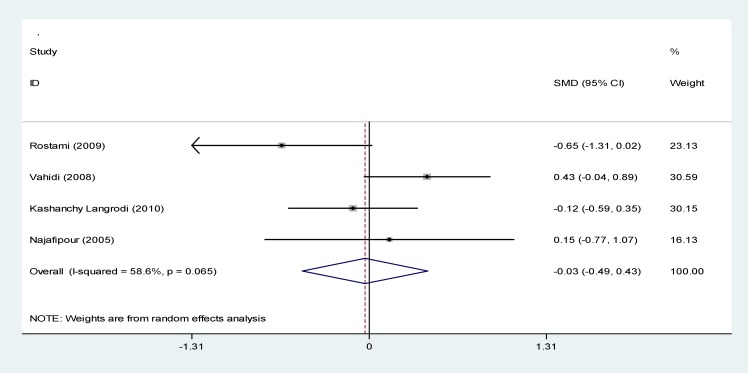
In figure 5 publication bias is shown as symmetry in a funnel plot and bias is not involved in these studies (P=0.345). In figure 6 the relationship between prevalence of diabetes with studies of quality was provided and p-values were calculated 0.187 and no significant relationship was observed in this regard. In 4 studies investigated, between serum ferritin levels and diabetes, no significant relationship was found (p<0.05) (figure 7).
Figure 5.
Publication bias for the prevalence of diabetes in patients with thalassemia major (P=0.435). The size of circles shows the weight of studies (bigger circles represent more samples
Figure 6.
Meta-regression plot of the prevalence of diabetes based on the quality of the study (P=0.187
Figure 7.
Forest plots presenting the relationship between serum ferritin level and diabetes based on a random effects model in the meta-analysis. SMD indicates the standardized mean difference
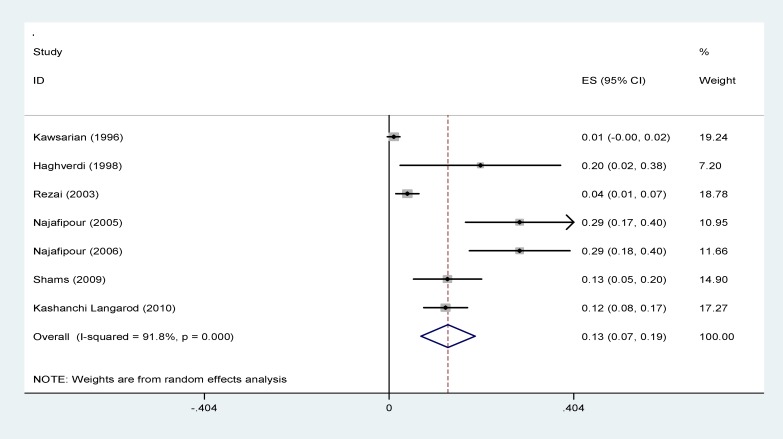
IFG: The prevalence of IFG in patients with thalassemia major in Iran was shown in figure 8 and this rate was estimated as 12.9% (95% CI: 7-18.8).
Figure 8.
Forest plots presenting the prevalence of impaired fasting glucose in patients with thalassemia major
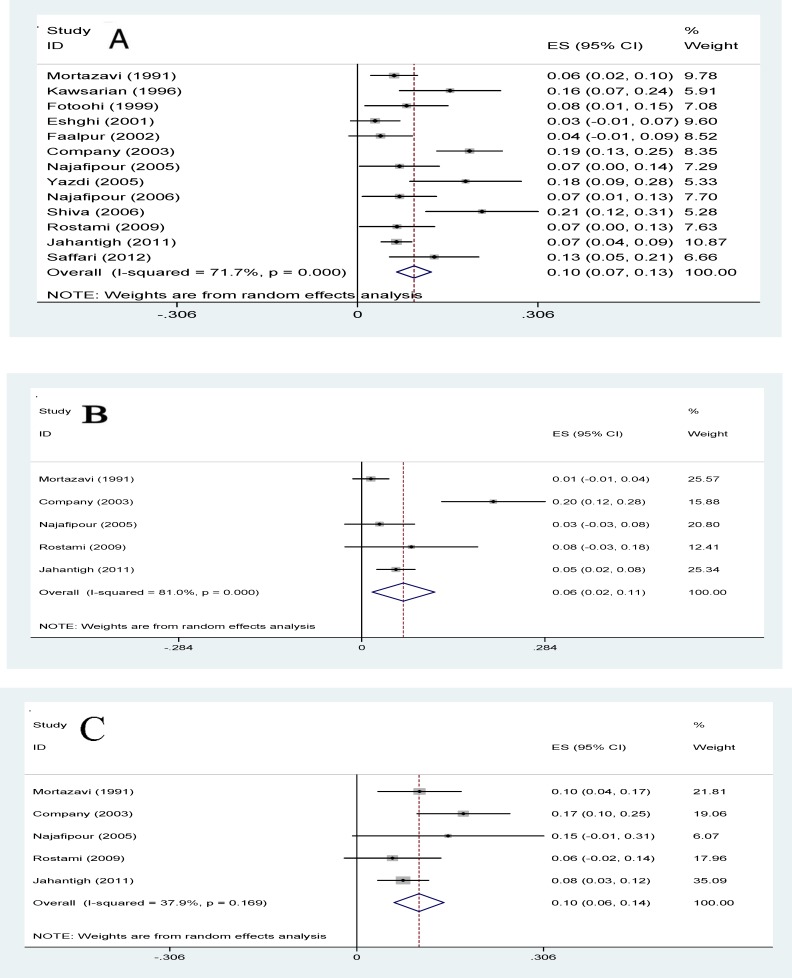
IGT: In 13 studies, the prevalence of IGT in patients with thalassemia major in Iran was estimated 9.6% (95% CI: 6.6-12.5). The lowest prevalence of IGT was related to a study in 2001 in Zahedan (3%) and the highest prevalence of IGT was related to a study in 2006 in Tabriz (21.1%). In 5 studies, the prevalence of IGT in patients with thalassemia major had been investigated according to sex estimated in males & females as 6.5% (CI 95%: 1.6-11.3) and 10.2% (CI 95%: 6.1-14.3), respectively (figure 9). The relationship between IGT in patients with thalassemia major with the year of study and sample size, meta-regression model was used and the p-values were calculated 0.702 and 0.736, respectively and no significant correlation was found (figure 10).
Figure 9.
Forest plots presenting the prevalence of impaired glucose tolerance in total (A) male (B) and female (C) patients with thalassemia major
Figure 10.
A: Meta-regression plot of the prevalence of IGT based on the year of study (P=0.702). B: Meta-regression plot of the prevalence of IGT based on sample size of the study (P=0.736
Discussion
The overall prevalence of diabetes in patients with thalassemia major was estimated 9 percent, the prevalence of diabetes in patients with thalassemia major in other countries was reported 6-27%, (Such as: United Arab Emirates (10.5%), Oman (27%), Taiwan (26.8%), South America (14%) and Italy (6.5%) (56-60). Genetic, geographical, cultural and economic factors as well as the quality of blood transfusion and chelation therapy, especially onset and the rate of the desferal dosage can be causes of different in reporting prevalence in various countries. . A systematic review of Iranian thalassemia patients has reported the regular iron chelation therapy as 54%. Therefore, chelation therapy in many patients with thalassemia major in Iran has been done unsystematically that must be considered (61).
The difference in reporting the prevalence of diabetes in patients with thalassemia major in Iran was 1.8-34% and it seems the most prospective reason for this difference, different diagnostic criteria. Therefore, the subgroup analysis was performed on diagnostic criteria and most studies (16 studies) had used ADA criteria, and this rate was estimated 7% and was not significantly different. Also, in the results of meta-regression model, no significant difference was found based on diagnostic criteria (P=0.343).
In a systematic review study in Iran, the prevalence of diabetes among adults is 3% and 16.8%, respectively for the age range of 25-34 and 55-64-years-old (62). In another review study in Iran, the prevalence of diabetes among the 15-25 years-old (young society) has been reported about 3.6% (63). And both studies indicated the incidence of diabetes significantly increases with age. In the present study, the age range of patients was 10-20 years-old (mean age of 16.8), which the prevalence of diabetes was estimated 9% that is more than the non-thalassemic population with same age. In some studies, the obvious role of iron overload has been proven in the endocrine glands including pancreas in the pathogenesis of diabetes (64,65) and other studies have shown that insulin resistance and lack of insulin are the two reasons of pre-diabetes and diabetes in this patients (66, 67). Endocrine complications in patients with thalassemia major mostly happen in their second decade of life. The highest prevalence of diabetes is reported in Razavi (33.9%) (22), Saffari (25.4%) (21) and Rabbani’s (25.4%) (32), and the lowest prevalence occurred in Arjmandi (1.8%) (41) and Mortazavi (2.7%) (27) and the results were not highly different in terms of average age of subjects participating in studies but the prevalence of diabetes was variable that can indicate different therapeutic follow-ups of these patients in different parts of Iran. The most comprehensive study in terms of sample size and examination areas in Iran was in Mehrvar’s et al. (50) in 2004 with a sample size of 407 thalassemia patients in Shiraz was reported a prevalence of diabetes as 6.6% which was consistent with the present results. The prevalence of diabetes in male patients with thalassemia major (12.6%) is more than female patients (10.8%), but this difference was not significant. A review study in the general population of Iran has estimated the prevalence of diabetes in males and females, 1.7% and 3.8%, respectively (62), which was inconsistent with results of this study. The most obvious reason can be the role of iron overload in thalassemia patients that its pathogenesis has been proven in endocrine disorders (7).
The prevalence of diabetes in patients with thalassemia major in studies of high and moderate quality was estimated as 9% and 11%, respectively and results of, no significant correlation was found between the prevalence of diabetes and quality of studies (P=0.187) in meta-regression model, which poor-quality studies can be the cause of that.
In the present study, in the relationship between serum ferritin level and diabetes, a mean difference of serum ferritin in case and control groups was estimated as -0.3 (95% CI:-49 to 43) and was not a significant. In other studies, different results were reported, in Mula-Abed’s et al. (57) there was not a significant relationship but in Borgna’s (64) and Gamberini’s studies (65) there was a significant relationship. The prevalence of IFG in patients with thalassemia major in Iran was estimated to be 9.6%. In a review study, the prevalence of IFG in the adult population of Iran has been reported as 16.8% (62) and it also showed that with increasing age, the prevalence was increased. Due to the small number of studies, could not do a sub-group analysis on the prevalence of IFG.
The overall prevalence of IGT in Iranian patients with thalassemia major was estimated as 9.6%. Due to the low number of studies, we could not sub-group based on the IGT. The prevalence of this disorder in other countries including Turkey (2.2%), Italy (6.5%), Thailand (12.5%) and Egypt (24.1%) was variable (68-71).
The prevalence of IGT in patients with thalassemia major in 5 studies under review on females (10.2%) was more than males (6.5%). However, this difference was not significant.
Meta-regression model for finding the source of heterogeneity among studies was used, and meta-regression results in a year of studies and sample size for diabetes and IGT prevalence was not statistically significant. During years of studies (1991-2015), the prevalence of diabetes and IGT has been almost constant. Constant prevalence of the diseases over the last 24 years, and also the high prevalence of diabetes in patients with thalassemia major, attention, and follow-up in this patient seems necessary.
No relationship between serum ferritin and development of diabetes was noted. Jiang et al. was found a strong relationship between ferritin and diabetes (72), also high ferritin level is associated with cardiovascular disease, hepatic steatohepatitis and central adiposity (72-74).
Publication bias for studies entering the meta-analysis process has been shown as symmetry in Funnel plot in which the p-value was 0.345, indicating that the possibility of publication bias is not statistically significant.
Research limitations: 1. The inability of internal databases for searching the combined keywords. Thus, we cannot use the keywords in combination; 2. Because of no the prevalence of diabetes, IFG, and IGT by age reported in studies, we could not calculate the prevalence based on age; 3. Since desferal dosage and intervals of blood transfusion were not reported in most studies, we could not calculate the relationship between these variables with diabetes, IFG and IGT; and 4. Due to the limited number of studies, we could not do a subgroup analysis of studies on the prevalence of IFG and IGT.
In conclusion the prevalence rates of diabetes, IFG, and IGT are high in Iranian patients with thalassemia major. Therefore, more effective protocols and management strategies which include improved protocols, blood transfusion, chelation therapy, educating and enhancing awareness of the parents and patients about iron overload complications seem to be essential to minimize endocrine complications. In addition, screening for the early diagnosis of endocrine complications once every six-month should be done as suggested by the Thalassemia International Federation.
Acknowledgments
Thanks to Ilam University of Medical Sciences for financial support.
Funding:
This present study is the result of an accepted research thesis funded by Ilam University of Medical Sciences (Grant number: 910920).
Conflict of interest
There was no conflict of interest in this study.
References
- 1.Behrman RE, Kligman RM, Jenson HB, eds . Nelson textbook of pediatric. 18th ed. . Philadelphia: Saunders; 2007. pp. 2033–8. [Google Scholar]
- 2.Haghi M, Pouladi N, Hosseinpour Feizi M, Hosseinpour Feizi A. Β-Thalassemia in Iran. JSSU. 2010;18:127–33. [in Persian] [Google Scholar]
- 3.Tabarsi B, Marbaghi A, Safavi M, Afkhami M. Comparative survey of problems in thalassemia major patients with regular and irregular follow ups of therapeutic principles. Sci J Blood Transfus Organ. 2007;4:33–40. [in Persian] [Google Scholar]
- 4.De Sanctis V. Growth and puberty and its management in thalassemia. Horm Res. 2002;58:72–9. doi: 10.1159/000064766. [DOI] [PubMed] [Google Scholar]
- 5.De Sanctis V, Tangerini A, Testa MR, et al. Final height and endocrine function in thalassaemia intermedia. J Pediatr Endocrinol Metab. 1998;11:965–71. [PubMed] [Google Scholar]
- 6.Raiola G, Galati MC, De Sanctis V, et al. Growth and puberty in thalassemia major. J Pediatr Endocrinol Metab. 2003;16:259–66. [PubMed] [Google Scholar]
- 7.Gulati R, Bhatia V, Agarwal SS. Early onset of endocrine abnormalities in beta‐thalassemia major in a developing country. J Pediatr Endocrinol Metab. 2000;13:651–6. doi: 10.1515/jpem.2000.13.6.651. [DOI] [PubMed] [Google Scholar]
- 8.Sayehmiri K, Tardeh Z, Mansouri A, Borji M, Azami M. The prevalence of hypogonadism in patients with thalassemia major in Iran– a systematic review and meta-analysis study. J Shahrekord Univ Med Sci. 2016;18:140–51. [in Persian] [Google Scholar]
- 9.Azami M, Rahmati S, Sayehmiri K. Prevalence of hyperparathyroidism in patients with thalassemia major in Iran. J Babol Univ Med Sci. 2016;18:39–48. [in Persian] [Google Scholar]
- 10.Azami M, Parizad N, Sayehmiri K. Prevalence of hypothyroidism, hypoparathyroidism and the frequency of regular chelation therapy in patients with thalassemia major in Iran: a systematic review and meta-analysis study. IJPHO. 2016;6:260–75. [Google Scholar]
- 11.Azami M, Gheisoori A, Sayehmiri F, Sayehmiri K. The prevalence of hypothyroidism in patients with Beta thalassemia major in Iran- A systematic review and meta-analysis study. Sci J Kurdistan Univ Med Sci. 2016;21:104–16. [Google Scholar]
- 12.Al‐Elq AH, Al‐Saeed HH. Endocrinopathies in patients with thalassemias. Saudi Med J. 2004;25:1347–51. [PubMed] [Google Scholar]
- 13.King H. WHO and the International Diabetes federation: regional Partners. Bull World Health Organization. 1999;77:954. [PMC free article] [PubMed] [Google Scholar]
- 14.Hamman RF, Leroith D, Taylor SI, Olefsky JM. Diabetes mellitus: a fundamental and clinical test. 3rd ed. . USA: Lippincott Williams and Wilkins; 2003. Epidemiology of type 2 diabetes mellitus; pp. 785–96. [Google Scholar]
- 15.Hashemi A, NoraniF , Ayatollah J, Jenabzadeh A, Khir Andish M. J Shahid Sadoughi Univ Med Sci. 2009;17:220–8. Available at: http://jssu.ssu.ac.ir/browse.php?a_code=A-10-1-1058&slc_lang=fa&sid=1&sw [in Persian] [Google Scholar]
- 16.Quirolo K, Vichinsky E, Behrman RE, Kliegman RM, Jenson HB. Nelson text book of pediatrics. 17th ed. . Philadelphia: W.B. Saunders; 2004. Hemoglobin disorder; pp. 1630–4. [Google Scholar]
- 17.Weatheral DJ, Clegg JB. The thalassemia syndromes. 4th ed. . London: 2001. pp. 302–5. [Google Scholar]
- 18.Orkin S, Nathan DG, Nathan DG, Orkin S. Nathan and Oski’s hematology of infancy and childhood. 5th ed. Philadelphia. Philadelphia: W.B. Saunders; 1998. The thalassemia; pp. 811–89. [Google Scholar]
- 19.Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. New Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 20.Najafipour F. Evaluation of Endocrine Disorders in Patients with Thalassemia Major. Int J Endocrinol Metab. 2008;2:104–13. [in Persian] [Google Scholar]
- 21.Rostami P, Hatami G, Shirkani A. Endocrine complications in patients with major β-thalassemia. Iran South Med J. 2011;14:240–5. [in Persian] [Google Scholar]
- 22.Saffari F, Mahyar A, Jalilolgadr S. Endocrine and metabolic disorders in β-thalassemia major patients. Caspian J Intern Med. 2012;3:466–72. [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaffarian Shirazi HR, Rezaei M, Poor Mahmoodi A, Pakbaz F. J Yasuj Unive Med Sci. Vol. 9. Armaghane-Dansh; 2004. Prevalence of diabetes mellitus in thalassemic patients referring to Cooly’s centers of Kohgiloyeh and Boyerahmad; pp. 35–42. Available at: http://health.barakatkns.com/article/20136 [in Persian] [Google Scholar]
- 24.Azami M, Hafezi Ahmadi MR, Sayehmiri K. Hepatitis B Vaccination Efficacy in Iranian Healthcare Workers: A Meta-Analysis Study. Hepat Mon. 2017;17(1):e37781. [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 26.Azami M, Nasirkandy MP, Mansouri A, Darvishi Z, Rahmati S, Abangah G, et al. Global Prevalence of Helicobacter pylori Infection in Pregnant Women: A Systematic Review and Meta-analysis Study. InternationalJournal of Women's Health and Reproduction Sciences. 2017;5(1):30–36. [Google Scholar]
- 27.Roden M. Diabetes mellitus: definition, classification and diagnosis. Wien Klin Wochenschr. 2016;128:S37–40. doi: 10.1007/s00508-015-0931-3. [DOI] [PubMed] [Google Scholar]
- 28.Vandenbroucke JP, Elm EV, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Medicine. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kashanchi Langarodi M, Abdolrahim Poorheravi H. Prevalence of diabetes, hypothyroidism and hypoparathyroidism in thalassemia patients in Shahid Bahonar Hospital, Karaj. Sci J Iran Blood Transfus Organ. 2013;9:422–8. [in Persian] [Google Scholar]
- 30.Najafipour F. Evaluation of endocrine disorders in patients with thalassemia major. Int J Endocrinol Metab. 2008;9:104–13. [Google Scholar]
- 31.Company F, Zandian Kh, Pedram M, Shahbazian H, Rezaei N. Abnormal Glucose tolerance and diabetes mellitus in β thalassemic patients with transfusion dependent at Ahwaz Thalassemia Research Center. Sci Med J Ahwaz Univ Med Sci. 2007;6:199–209. [in Persian] [Google Scholar]
- 32.Kosarian M. Diabetes mellitus and impaired Glucose tolerance in patients with major Thalassemia in Boo Ali Sina educational and treatment center in Sari in 1375-77. J Kashan Univ Med Sci (FEYZ) 1999;3:80–5. [in Persian] [Google Scholar]
- 33.Mortazavi Y. Assessment of diabetes mellitus in patients with beta-thalassemia. J Zanjan Univ Med Sci . 1992;1:20–9. Available at: http://zums.ac.ir/journal/browse.php?a_id=690&slc_lang=fa&sid=1&ftxt=1 [in Persian] [Google Scholar]
- 34.Soheili Khah S, Eslami S. Endocrine disorders in Thalassemia major in Yazd Blood Bank in 1998. J Shahid Sadoughi Univ Med Sci Health Serv. 2000;8:7–11. [in Persian] [Google Scholar]
- 35.Keihanian T, Rabbani A, Khalili I. Frequency of diabetes mellitus and its association with different factors in patients with thalassemia major receiving blood referred to Thalassemia Clinic Children’s Medical Center Hospital in 2011. MD Thesis. Tehran: Tehran University of Medical Sciences; 2012. [in Persian] [Google Scholar]
- 36.Azimi M, Sharbatdar Alai MM. The prevalence of diabetes mellitus patients at Shahid Ashrafi Esfahani. MD Thesis. Tehran: Shahid Beheshti University of Medical Sciences ; 2003. [Google Scholar]
- 37.Mahdavi Anari F, Ahmadian A, Haghshenas Z, Alawi Yazdi Z. Comparison of the frequency of four endocrine disorders in patients with thalassemia major referred to thalassemia clinic of Tehran Imam Khomeini Hospital in 2000. MD Thesis. Tehran: Tehran University of Medical Sciences; 2000. [Google Scholar]
- 38.Rabbani A, Ashtiani S, Fotoohi S. Prevalence of asymptomatic diabetes in patients with thalassemia major in Children's Medical Center. MD Thesis. Tehran: Tehran University of Medical Sciences; 1999. [Google Scholar]
- 39.Younes A, Samii Rad F, Kamali M, Pirzadeh Z. Prevalence of diabetes mellitus in patients with beta-thalassemia in the center Thalassemia Qazvin (Hospital Quds November 1998). MD Thesis. Qazvin: Qazvin University of Medical Sciences; 1998. [Google Scholar]
- 40.Amanat Yazdi M, Hashemi A, Afkhami G, Pour Shamsi F. Evaluation relationship between endocrine disorders in B-thalassemic patients with serum ferritin levels. MD Thesis. Yazd: Shahid Sadoughi University of Medical Sciences; 2004. [Google Scholar]
- 41.Haghverdi A, Shirvani F. Evaluation of blood indices and blood sugar in 20 patients with thalassemia in Boo Ali hospital in 1998. MD Thesis. Tehran: Shahid Beheshti University of Medical Sciences; 1999. [Google Scholar]
- 42.Shiva S, Sari Sorkhabi R. Short stature in patients with beta-thalassemia. J Urmia Univ Med Sci. 2008;19:125–31. Available at: http://umj.umsu.ac.ir/browse.php?a_code=A-10-8-14&slc_lang=fa&sid= [in Persian] [Google Scholar]
- 43.Eshraghi P, Mehrabani Tabari S, Mohseni A. An evaluation of the correlation between short stature and endocrinopathy in thalassemia major patients. J Mashhad Univ Med Sci. 2012;55:7–14. [in Persian] [Google Scholar]
- 44.Jahantigh M, Naderi M, Dorgalaleh A, TabibianS Prevalence of diabetes and impaired glucose tolerance test in patients with thalassemia major. Zahedan JRes Med Sci. 2014;16:86–8. [Google Scholar]
- 45.Faalpour Z, Posti AR. The study of glucose tolerance test (GTT) in patients with thalassemia major who referred to Ali Asghar blood clinic. Iran J Pediatr. 2002;13:1–1. Available at: http://fa.journals.sid.ir/ViewPaper.aspx?ID=43402. [in Persian] [Google Scholar]
- 46.Saffari F, Mahyar A, Jalilolgadr S. Endocrine and metabolic disorders in β-thalassemia major patients. Caspian J Intern Med. 2012;3:466–72. [PMC free article] [PubMed] [Google Scholar]
- 47.Arjmandi Rafsanjani K, Razzaghy-Azar M, Zahedi-Shoolami L, et al. Bone Mineral density in β thalassemia major and intermedia, correlation with biochemical and hormonal profiles. IJBC. 2009;4:121–7. [Google Scholar]
- 48.Eshghi P, Roudbari M, Rzlansry AA. Compare the frequency of impaired glucose tolerance testing in thalassemia major patients with and without hepatitis C virus infection in the city of Zahedan in 2001. J Jundishapur. 2003;38:58–66. Available at: http://fa.journals.sid.ir/ViewPaper.aspx?ID=2396. [in Persian] [Google Scholar]
- 49.Moayeri H, Oloomi Z. Prevalence of growth and puberty failure with respect to growth hormone and gonadotropins secretion in beta-thalassemia major. Arch Iranian Med. 2006;9:329–34. [PubMed] [Google Scholar]
- 50.Karamifar H, Shahriari M, Sadjadian N. Prevalence of endocrine complications in beta-thalassemia major in the Islamic Republic of Iran. East Mediterr Health J. 2003;9:55–60. [PubMed] [Google Scholar]
- 51.Mowla A, Karimi M, Afrasiabi A, De Sanctis V. Prevalence of diabetes mellitus and impaired glucose tolerance in beta-thalassemia patients with and without hepatitis C virus infection. Pediatr Endocrinol Rev. 2004;2:282–4. [PubMed] [Google Scholar]
- 52.Shams Sh, Haghi Ashtiani MT, Monajemzadeh M, et al. Evaluation of serum insulin, glucose, lipid profile, and liver function in b-thalassemia major patients and their correlation with iron overload. Lab Med. 2010;41:486–9. [Google Scholar]
- 53.Karimi M, Rasekhi AR, Rasekh M, et al. Hypoparathyroidism and intracerebral calcification in patients with beta-thalassemia major. Eur J Radiol. 2009;70:481–4. doi: 10.1016/j.ejrad.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Raeisi N, Mirhosseini M. Prevalence of glucose metabolism disorder among over 10 years old thalassemic patients, Hajar hospital of Shahrekord, 2003. Shahrekord Univ Med Sci J. 2005;6:51–5. [in Persian] [Google Scholar]
- 55.Sadat Zendebad A, Mirbehbahani N, Behnampour N. The relationship between diabetes mellitus, hypothyroidism, hypocalcemia, adrenal insufficiency and serum ferritin in patients with beta thalassemia major-center. MD Thesis. Gorgan: Golestan University of Medical Sciences; 2007. [Google Scholar]
- 56.Mehrvar A, Azarkeivan A, Saberi Nejad J, et al. Prevalence of diabetes mellitus in patients with transfusion dependent ß thalassemia. IJBC. 2008;1:23–7. [Google Scholar]
- 57.Belhoul KM, Bakir ML, Kadhim AM, et al. Prevalence of iron overload complications among patients with b-thalassemia major treated at Dubai Thalassemia Centre. Ann Saudi Med. 2013;33:18–21. doi: 10.5144/0256-4947.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mula-Abed W, Al Hashmi H, Al Muslahi M, et al. Prevalence of endocrinopathies in patients with beta-thalassaemia Major -A Cross-Sectional Study in Oman. Oman Med J. 2008;23:257–62. [PMC free article] [PubMed] [Google Scholar]
- 59.Li MJ, Peng SS, Lu MY, et al. Diabetes mellitus in patients with thalassemia major. Pediatr Blood Cancer. 2014;61:20–4. doi: 10.1002/pbc.24754. [DOI] [PubMed] [Google Scholar]
- 60.Vogiatzi MG, Macklin EA, Trachtenberg FL, et al. Differences in the prevalence of growth, endocrine and vitamin D abnormalities among the various thalassaemia syndromes in North America. Br J Haematol. 2009;146:546–56. doi: 10.1111/j.1365-2141.2009.07793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–93. [PubMed] [Google Scholar]
- 62.Azami M, Nikpay S, Abangah G, Sayehmiri K. Evaluation of the incidence of splenectomy and frequency of regular iron chelation therapy in patients with thalassemia Major in Iran: a meta-analysis. Sci J Iran Blood Transfus Organ. 2016;13:146–55. [Google Scholar]
- 63.Esteghamati A, Gouya MM, Abbasi M, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran. Diabetes Care. 2008;31:96–8. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 64.Haghdoost AA, Rezazadeh-Kermani M, Sadghirad B, Baradaran HR. Prevalence of type 2 diabetes in the Islamic Republic of Iran: systematic review and meta-analysis. East Mediterr Health J. 2009;15:591–9. [PubMed] [Google Scholar]
- 65.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and disease complications in thalassemia major. Ann N Y Acad Sci. 1998;850:227–31. doi: 10.1111/j.1749-6632.1998.tb10479.x. [DOI] [PubMed] [Google Scholar]
- 66.Gamberini MR, De Sanctis V, Gilli G. Hypogonadism, diabetes mellitus, hypothyroidism, hypoparathyroidism: incidence and prevalence related to iron overload and chelation therapy in patients with thalassaemia major followed from 1980 to 2007 in the Ferrara Centre. Pediatr Endocrinol Rev. 2008;6:158–69. [PubMed] [Google Scholar]
- 67.Dmochowski K, Finegood DT, Francombe W, Tyler B, Zinman B. Factor’s determining glucose tolerance in patients with thalassemia major. J Clin Endocrinol Metab. 1993;77:478–83. doi: 10.1210/jcem.77.2.8345055. [DOI] [PubMed] [Google Scholar]
- 68.Messina MF, Lombardo F, Meo A, et al. Three-year prospective evaluation of glucose tolerance, beta-cell function and peripheral insulin sensitivity in non-diabetic patients with thalassemia major. J Endocrinol Invest. 2002;25:497–501. doi: 10.1007/BF03345490. [DOI] [PubMed] [Google Scholar]
- 69.Isik P, Yarali N, Tavil B, et al. Endocrinopathies in Turkish children with Beta thalassemia major: results from a single center study. Pediatr Hematol Oncol. 2014;31:607–15. doi: 10.3109/08880018.2014.898724. [DOI] [PubMed] [Google Scholar]
- 70.De Sanctis V, Eleftheriou A, Malaventura C. Prevalence of endocrine complications and short stature in patients with thalassaemia major: a multicenter study by the Thalassaemia International Federation (TIF) Pediatr Endocrinol Rev. 2004;2:249–55. [PubMed] [Google Scholar]
- 71.Jaruratanasirikul S, Chareonmuang R, Wongcharnchailert M, et al. Prevalence of impaired glucose metabolism in beta-thalassemic children receiving hypertrans fusions with a suboptimal dosage of iron-chelating therapy. Eur J Pediatr. 2008;167:873–6. doi: 10.1007/s00431-007-0602-0. [DOI] [PubMed] [Google Scholar]
- 72.Hafez M, Youssry I, El-Hamed FA, Ibrahim A. Abnormal glucose tolerance in beta-thalassemia: assessment of risk factors. Hemoglobin. 2009;33:101–8. doi: 10.1080/03630260902817131. [DOI] [PubMed] [Google Scholar]
- 73.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291(6):711–7. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 74.Iwasaki T, Nakajima A, Yoneda M, Yamada Y, Mukasa K, Fujita K, Fujisawa N, Wada K, Terauchi Y. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care. 2005;28:2486–2491. doi: 10.2337/diacare.28.10.2486. [DOI] [PubMed] [Google Scholar]
- 75.Dongiovanni P, Fracanzani AL, Fargion S, Valenti L. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol. 2011;55:920–932. doi: 10.1016/j.jhep.2011.05.008. [DOI] [PubMed] [Google Scholar]