Abstract
Molecular markers are helpful diagnostic tools, particularly for cytologically indeterminate thyroid nodules. Preoperative RET/PTC1 rearrangement analysis in BRAF and RAS wild-type indeterminate thyroid nodules would permit the formulation of an unambiguous surgical plan. Cycle threshold values according to the cell count for detection of the RET/PTC1 rearrangement by real-time reverse transcription-polymerase chain reaction (RT-PCR) using fresh and routine air-dried TPC1 cells were evaluated. The correlation of RET/PTC1 rearrangement between fine-needle aspiration (FNA) and paired formalin-fixed paraffin-embedded (FFPE) specimens was analyzed. RET/PTC1 rearrangements of 76 resected BRAF and RAS wild-type classical PTCs were also analyzed. Results of RT-PCR and the Nanostring were compared. When 100 fresh and air-dried TPC1 cells were used, expression of RET/PTC1 rearrangement was detectable after 35 and 33 PCR cycles, respectively. The results of RET/PTC1 rearrangement in 10 FNA and paired FFPE papillary thyroid carcinoma (PTC) specimens showed complete correlation. Twenty-nine (38.2%) of 76 BRAF and RAS wild-type classical PTCs had RET/PTC1 rearrangement. Comparison of RET/PTC1 rearrangement analysis between RT-PCR and the Nanostring showed moderate agreement with a κ value of 0.56 (p = 0.002). The RET/PTC1 rearrangement analysis by RT-PCR using routine air-dried FNA specimen was confirmed to be technically applicable. A significant proportion (38.2%) of the BRAF and RAS wild-type PTCs harbored RET/PTC1 rearrangements.
Keywords: RET/PTC gene rearrangement, air-dried FNA specimen, RT-PCR, Nanostring
1. Introduction
The evaluation of a thyroid nodule is a very common clinical problem. Epidemiologic studies have shown the prevalence of palpable thyroid nodules to be approximately 5% in women and 1% in men living in iodine-sufficient parts of the world [1,2]. In contrast, high-resolution ultrasound (US) can detect thyroid nodules in 19–68% of randomly selected individuals, with higher frequencies in women and the elderly [3,4]. The clinical importance of thyroid nodules rests with the need to exclude thyroid cancer, which occurs in 7–15% of cases depending on age, sex, radiation exposure history, family history, and other factors [5,6]. Differentiated thyroid cancer (DTC) includes papillary and follicular cancer, and comprises the vast majority (90%) of all thyroid cancers [7]. In the United States, approximately 63,000 new cases of thyroid cancer were predicted to be diagnosed in 2014 [8] compared with 37,200 in 2009 when the last ATA guidelines were published. The yearly incidence has nearly tripled from 4.9 per 100,000 in 1975 to 14.3 per 100,000 in 2009 [9].
The most prevalent type of thyroid malignancy in Korea is papillary thyroid carcinoma (PTC), which constitutes more than 97% of the cases, followed by follicular thyroid carcinoma (FTC), comprising 1.5% of the thyroid cancer [10]. Compared to Western countries, the prevalence of PTC is much higher. Therefore, the evaluation of a thyroid nodule in Korea is primarily a search for PTC.
Fine-needle aspiration (FNA) is the safest and most reliable test that can provide a definitive preoperative diagnosis of malignancy [11]. The sensitivity and specificity of FNA are reported to be 68–98% and 56–100%, respectively [12]. However, 15–30% of thyroid FNA diagnoses are “atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS)”, “follicular neoplasm or suspicious for follicular neoplasm (FN/SFN)”, and “suspicious for malignancy” [13]. This leads to an increased rate of unnecessary surgery, as only about 25% of the indeterminate cases will receive a postoperative malignant diagnosis by histological examination [11]. Moreover, patients with a diagnosis of indeterminate category usually undergo hemithyroidectomy, and about 25% of the patients need to have a second stage completion thyroidectomy in most centers [12]. Two-stage surgery has higher morbidity than initial total thyroidectomy undertaken with a definitive malignant diagnosis on FNA. Preoperative molecular analysis using a panel of genetic alterations would overcome the limitation of FNA diagnosis. The most common genetic alteration in thyroid cancer is the activation of the mitogen-activated protein kinase pathway. Activation of this pathway occurs through mutually exclusive mutations of the BRAF and RAS genes and rearrangements of the RET/PTC and NTRK. The overall prevalence of the BRAF mutations is approximately 45% (range, 27.3–87.1%) [14,15], with a significantly higher prevalence in Asia—especially Korea—relative to Western countries [15,16,17]. The mutations of the RAS genes are the second most common genetic alterations in thyroid tumors, and are mostly present in follicular-patterned lesions. The prevalence of RAS mutations in follicular variant of papillary thyroid carcinoma (FVPTC) varies from 26.5% to 33.3% in Korea, where most of the follicular patterned thyroid malignancy is FVPTC [18,19].
RET proto-oncogene rearrangements are commonly seen in PTC. These rearrangements play a role in pathogenesis of PTC, and derive from the fusion of the RET tyrosine kinase domain sequence with 50 sequences of heterologous genes. The resulting chimeric oncogenes are termed RET/PTCs [20,21,22,23,24]. RET/PTC rearrangements are typically common in tumors from patients with a history of radiation exposure (50–80%) and PTC of children and young adults (40–70%) [25,26]. The distribution of RET/PTC rearrangements within this tumor is quite heterogeneous, and varies from the involvement of almost all neoplastic cells to presence in only a small fraction of the tumor cells [27,28]. To date, 13 different types of RET/PTC rearrangements have been reported; RET/PTC1 and RET/PTC3 account for more than 90% of all rearrangements.
The prevalence of the RET/PTC rearrangements in PTC varies widely in different populations (range, 0–86.8%) [29,30,31], with significant variability in mutational frequency—even within the same geographical regions. Rates of 0–54.5% have been reported in Asia [30,31,32], 2.4–72.0% in the United States [17,33], and 8.1–42.9% in Europe [34,35]. The marked variations may reflect the small size of the studies, geographic variability, or different sensitivities of the detection methods [36,37]. When this variability is considered, the prevalence of RET/PTC rearrangements in Asia is generally low [29,30,31,32,38,39]. The subclonal occurrence of RET/PTC rearrangement in PTC can influence the sensitivity of some methods, and might explain why the reported prevalence of RET/PTC rearrangements in PTCs varies in different studies. Very recent studies demonstrated that RET/PTC rearrangements in benign thyroid nodules are not an uncommon occurrence, and suggested that its presence could be associated with a faster nodular enlargement [40,41,42]. A variety of methods have been used to identify RET/PTC rearrangements. These include real-time reverse transcription-polymerase chain reaction (RT-PCR), Southern blot analysis, fluorescence in situ hybridization, and NanoString nCounter Gene Expression Assay.
Most preoperative detection of these rearrangements has been performed in fresh FNA material. Recently, detection of the PAX8/PPARG and RET/PTC rearrangements in routine air-dried FNA samples was reported [43,44,45,46,47,48]. The FNA approach suffers from the limitation that indeterminate FNA specimens usually contain small numbers of atypical cells, and these cells are often mixed with many inflammatory cells, benign follicular cells, and stromal cells. Therefore, harvesting the cells of interest is the key step in molecular analysis of the FNA specimen.
Preoperative RET/PTC1 rearrangement analysis in BRAF and RAS wild-type indeterminate thyroid nodules would permit the formulation of an unambiguous surgical plan, while foregoing the need for other less-specific diagnostic tests like repeat FNA and intraoperative frozen section evaluation. We have previously reported the value of the preoperative BRAF and RAS mutation analysis in diagnosing PTC in routine air-dried FNA specimens [18,49,50,51]. In our institution, we recommend surgery for BRAF or RAS-positive thyroid nodules with preoperative cytological diagnosis of AUS/FLUS and FN/SFN categories, and have been able to detect considerable numbers of PTCs in cytologically-indeterminate nodules [50]. Considering that 88% of the PTCs harbor either a BRAF or a RAS mutation (Thyroid, 2017, Epub ahead of time), we hypothesized that detection of RET/PTC rearrangements on BRAF and RAS mutation wild-type FNA specimens of the indeterminate thyroid nodules will improve the diagnostic yield of PTC. An algorithmic approach is cost-effective and efficient—especially in BRAF mutation-prevalent populations.
In this study, we investigated the clinical feasibility of preoperative RET/PTC1 rearrangement analysis as an ancillary diagnostic tool in routine air-dried FNA samples. We also evaluated the RET/PTC1 rearrangement status for 76 BRAF and RAS wild-type classical PTC cases.
2. Results
2.1. Detection of the RET/PTC1 Rearrangement in a Fresh TPC1 Cell Line
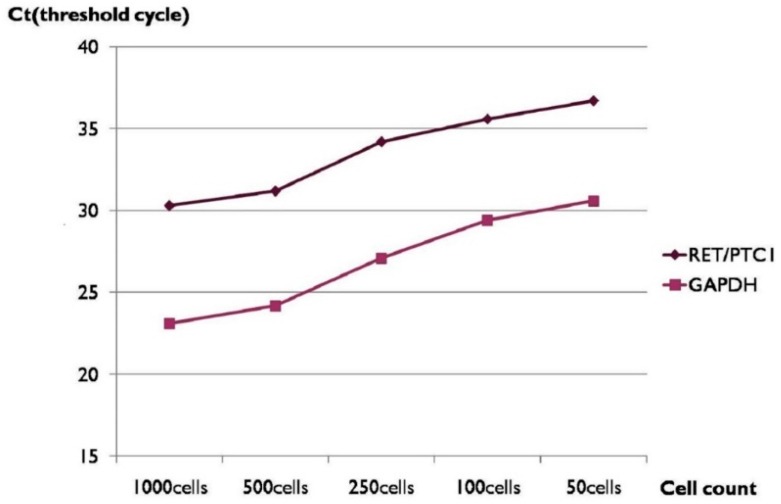
The Ct value was increased when the cell numbers used for analysis were decreased and showed an inverse correlation (Table 1 and Figure 1). RET/PTC1 rearrangement was detectable after 35 PCR cycles when 100 TPC1 cells were used.
Table 1.
Ct values and cell counts of RET/PTC1 rearrangement analysis by RT-PCR using fresh cultured TPC1 cells.
| Cell Number | RET/PTC1 (Ct) | GAPDH (Ct) |
|---|---|---|
| 1000 | 30.3 | 23.1 |
| 500 | 31.2 | 24.2 |
| 250 | 34.1 | 27.1 |
| 100 | 35.6 | 29.4 |
| 50 | 36.7 | 30.6 |
Figure 1.
Threshold cycle (Ct) values and cell counts of RET/PTC1 rearrangement analysis by real-time reverse transcription-polymerase chain reaction (RT-PCR) using fresh cultured TPC1 cells. GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
2.2. Detection of the RET/PTC1 Rearrangement in Routine Air-Dried TPC1 Cell Line
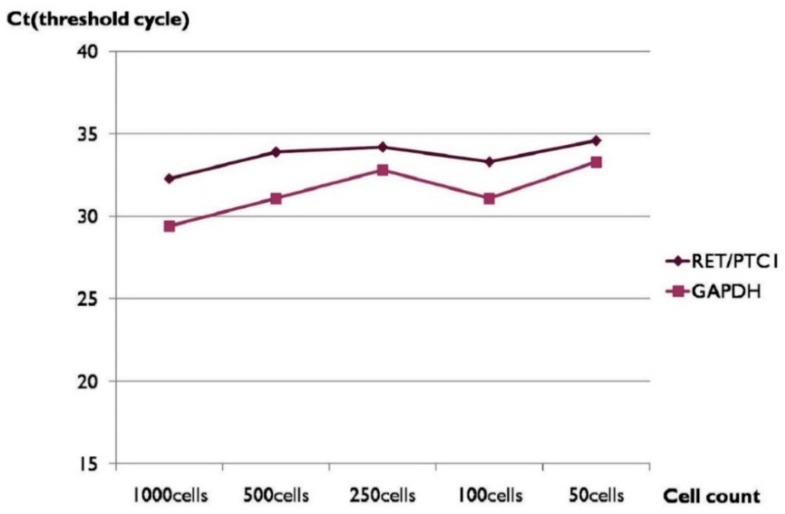
When RET/PTC1 rearrangement was analyzed using various numbers of smeared, alcohol-fixed, and Papanicolaou-stained PTC1 cells, the cell number and the threshold cycle (Ct) value also showed an inverse correlation (Table 2 and Figure 2). The expression of RET/PTC1 rearrangement was detectable after 33 PCR cycles when 100 cells were used.
Table 2.
Ct values and cell counts of RET/PTC1 rearrangement analysis by RT-PCR using RNA extracted from routine air-dried and Papanicolaou-stained TPC1 cells.
| Cell Number | RET/PTC1 (Ct) | GAPDH (Ct) |
|---|---|---|
| 1000 | 32.3 | 29.4 |
| 500 | 33.9 | 31.1 |
| 250 | 34.2 | 32.8 |
| 100 | 33.3 | 31.1 |
| 50 | 34.6 | 33.3 |
Figure 2.
Ct values and cell counts of RET/PTC1 rearrangement analysis by RT-PCR using RNA extracted from routine air-dried and Papanicolaou-stained TPC1 cells.
2.3. Correlation of the RET/PTC1 Rearrangement between Routine Air-Dried FNA and Paired FFPE PTC Tissue Specimens
When RET/PTC1 rearrangement was analyzed using PTC cells from archival air-dried FNA slides aspirated from the patients proven to have a histopathological diagnosis of PTC, RET/PTC1 rearrangement was detected in all 6 cases even though the Ct values of the archival specimen were higher than those of formalin-fixed paraffin-embedded (FFPE) PTC tissue specimens (Table 3). Four cases lacking RET/PTC1 rearrangement in tissue specimen also failed to reveal rearrangement in FNA samples. These results confirmed that RET/PTC1 rearrangement analysis by RT-PCR can be applied in preoperative FNA samples as an ancillary diagnostic tool.
Table 3.
Correlation of RET/PTC1 rearrangement status between routine air-dried fine-needle aspiration (FNA) and paired formalin-fixed paraffin-embedded (FFPE) specimens.
| Ct of FFPE Specimen | Ct of FNA Specimen | ||||
|---|---|---|---|---|---|
| Case | RET/PTC1 | GAPDH | Case | RET/PTC1 | GAPDH |
| 1 | 26.15 | 27.40 | 1 | 35.05 | 35.86 |
| 2 | 26.15 | 29.23 | 2 | 36.41 | 37.54 |
| 3 | 26.32 | 28.96 | 3 | 37.03 | 34.79 |
| 4 | 40 | 34.67 | 4 | 50 | 36.74 |
| 5 | 24.64 | 28.84 | 5 | 37.66 | 35.86 |
| 6 | 31.71 | 29.92 | 6 | 34.26 | 35.47 |
| 7 | 24.71 | 26.98 | 7 | 36.62 | 36.78 |
| 8 | 50.00 | 28.64 | 8 | 50.00 | 31.69 |
| 9 | 50.00 | 27.55 | 9 | 50.00 | 30.34 |
| 10 | 50.00 | 29.31 | 10 | 50.00 | 30.01 |
2.4. Detection of the RET/PTC1 Rearrangement in Resected BRAF and RAS Wild-Type PTC Cases Using FFPE Tissue Specimen
Of 600 surgically resected FFPE specimens histologically diagnosed as PTC, classical type, 518 had BRAF mutations and 6 had RAS mutations. Among 76 BRAF and RAS wild-type PTCs, 29 (38.2%) cases turned out to have RET/PTC1 rearrangement. Considering that alteration of BRAF, RAS, and RET genes are mutually exclusive, 29 (4.8%) of 600 classical PTC cases harbored RET/PTC1 rearrangement.
2.5. Comparative Analysis of RT-PCR with the NanoString nCounter Gene Expression Assay for Detecting RET/PTC1 Rearrangement in FFPE PTC Tissue Specimen
Twenty-six cases showed correlation on both methods (5 positives and 21 negatives), whereas five cases showed discrepancy between the two methods (three cases positive for Nanostring but not for RT-PCR, two cases positive for RT-PCR but not for Nanostring). Two different analysis methods showed moderate agreement with a κ value of 0.56 (p = 0.002).
3. Discussion
The value of molecular markers on preoperative FNA specimens has been described in various thyroid nodules [18,47,49,50,51,52,53]. RET/PTC rearrangements are commonly found in adult sporadic PTCs with a marked variable prevalence in different studies owing to geographic variability or different sensitivity of the detection methods [17,29,30,31,32,33,34,35,36,37]. The reported prevalence rates of RET/PTC rearrangements varied largely among studies. While geographical factors and radiation exposure can partially account for this wide range of prevalence, the methodology applied appears to be the most important factor to explain this variability. Searching for RET/PTC rearrangements by a less sensitive method may have the drawback of leaving some PTCs undiagnosed, but has the advantage of reducing false positive findings. Indeed, while sporadic cells harboring RET/PTC rearrangements can be present in benign nodules, its clonal occurrence is exclusive to PTC. Hence, the less sensitive RT-PCR seems to be more suitable for diagnostic purposes. RET/PTC rearrangements analysis on thyroid tumor has not been extensively performed in Korea, given the prevalence of the BRAF V600E mutation in PTC in Korea.
In this report, we assessed the clinical usability of preoperative RET/PTC1 rearrangement analysis as an ancillary diagnostic tool for BRAF and RAS wild-type indeterminate thyroid nodules, and explored the RET/PTC1 rearrangement status in a large number of PTC cases. These explorations have never been done in Korea, to our knowledge. We routinely use atypical follicular cells marked by the cytopathologists and dissected from routine air-dried FNA samples to increase the sensitivity. Since clinical FNA samples contain limited numbers of cells to perform several steps required for deciding optimum number of cells for successful analysis and cutoff values, we performed same analysis using fresh TPC1 cells which are equivalent to the fresh FNA samples in step 1 and air-dried Papanicolaou-stained TPC1 cells equivalent to the archival FNA slides in step 2.
The Ct values in Table 2 tend to decrease when the cell numbers were increased; however, both Ct values of the RET/PTC1 and GAPDH expression using 250 cells are greater than those using 100 cells. Since Ct values of the housekeeping gene expression also showed the same phenomenon, we assumed that a considerable amount of RNA in 250-cell groups might have been deteriorated. At any rate, we found that RET/PTC1 expression could be measured when 50–100 air-dried Papanicolaou-stained TPC1 cells were used.
When we compared the Ct values of fresh and air-dried Papanicolaou-stained TPC1 cells according to the cell numbers, the Ct values were slightly decreased when air-dried Papanicolaou-stained cells were used. We assumed that this finding might have resulted from the imprecise cell count in step 2. Fresh cells were counted using a hemocytometer, whereas air-dried and Papanicolaou-stained cells were counted on a slide using a square micrometer under the microscope. The expression of RET/PTC1 rearrangement detectable after 33 PCR cycles when routine air-dried 100 TPC1 cells were used suggests that RET/PTC1 expression could be detected in routine air-dried FNA samples containing 100 cells.
When RET/PTC1 rearrangement status from ten FNA and paired FFPE samples were compared, the results showed complete agreement. The higher Ct value of FNA samples compared to the matched FFPE samples could be attributed to the much smaller numbers of cells in FNA samples. The other reason might be the different RNA extraction method used for the two different samples.
Since BRAF mutations, RAS mutations, and RET/PTC rearrangements are mutually exclusive, we analyzed the RET/PTC1 rearrangement status on both BRAF (V600E and K601E) and RAS (NRAS codons 12, 13, 61; HRAS codons 12, 13, 61; KRAS codons 12, 13, 61) wild-type PTC cases to save cost and effort. The main limitation of our experiment is that we only performed RET/PTC1 rearrangement, even though the prevalence for RET/PTC3 arrangement in previous Korean report was 0%. Another reason for analyzing RET/PTC1 is that we were able to secure a cell line harboring only the RET/PTC1 rearrangement.
Among 76 surgically resected both BRAF and RAS wild-type FFPE specimens histopathologically diagnosed as PTC, classical type, 29 (38.2%) cases turned out to have RET/PTC1 rearrangement; this means that RET/PTC1 rearrangement was detected in 29 (4.8%) of 600 classical-type PTCs. Two previous studies reported REP/PTC rearrangements in Korea. One study failed to identify any RET/PTC1, 2, 3 rearrangements in 24 cases of PTC by RT-PCR [31]. The other study detected 2 (6.5%) RET/PTC1, 2 (6.5%) RET/PTC2, and no (0%) RET/PTC3 rearrangements in 31 PTCs by RT-PCR [38]. Both studies used fresh frozen tumor tissue. The slight discrepancy could be explained by the difference of the sample size. The slightly lower prevalence of RET/PTC1 rearrangement compared to the second study might also be attributed to the poor RNA preservation in the FFPE specimens.
The NanoString nCounter Gene Expression Assay is a robust and highly reproducible method for detecting the expression of up to 800 genes in a single reaction with high sensitivity and linearity across a broad range of expression levels. The methodology serves to bridge the gap between genome-wide (microarrays) and targeted (real-time quantitative PCR) expression profiling. The nCounter assay is based on direct digital detection of mRNA molecules of interest using target-specific, color-coded probe pairs. It does not require the conversion of mRNA to cDNA by reverse transcription or the amplification of the resulting cDNA by PCR. The expression level of a gene is measured by counting the number of times the color-coded barcode for that gene is detected, and the barcode counts are then tabulated [54]. Comparative analysis of RT-PCR with the Nanostring method for detecting RET/PTC1 rearrangement in FFPE PTC tissue showed moderate agreement with a k value of 0.56 (p = 0.002). There is a discrepancy between these two methods (three cases positive for Nanostring but not for RT-PCR, two cases positive for RT-PCR but not for Nanostring). The discrepancy might be attributed to the different RNA extraction methods and cut-off values of each method. In three cases with discrepancy, the results were near to the cut-off value. Another reason might be attributed to the difference of tumor portion used in two different methods. Since we did not initially plan to compare RT-PCR with Nanostring, we made the tumor sections only for RT-PCR analysis. Therefore, the tumor portions which were used for Nanostring might have been slightly different from the initial tumor portion. Next generation sequencing (NGS) is being used to study genetic alterations in institutions worldwide. However, it may be a long time until NGS becomes a routine part of thyroid cancer practice in Korea, since only NGS panels relevant for the therapeutic modality have been approved by the Korean government. Furthermore, only large institutions like university hospitals can adopt NGS in practice. Therefore, algorithmic approach of BRAF mutation analysis followed by RAS mutation and RET/PTC1 rearrangement may be of more practical help to refine FNA diagnosis of indeterminate thyroid nodules.
We confirmed the technical applicability of RET/PTC1 rearrangement analysis using routine air-dried FNA samples as an ancillary diagnostic tool through several steps of the experiment. The presence of RET/TPC1 rearrangement in a significant proportion (38.2%) of the patients with BRAF and RAS wild-type PTCs can be used to diagnose and manage patients with BRAF and RAS wild-type indeterminate thyroid nodules. Since the BRAF V600E mutation, NRAS codon 61 mutation, and RET/PTC1 rearrangement comprise more than 90%, 75%, and 50% of the BRAF mutations, RAS mutations, and RET/PTC rearrangements [18,50], an algorithmic approach of BRAF V600E mutation analysis followed by NRAS 61 mutation and RET/PTC1 rearrangement analysis would cost-effectively and efficiently overcome a diagnostic limitation of the thyroid FNA by triaging considerable numbers of PTCs in cytologically indeterminate nodules.
4. Materials and Methods
4.1. Total RNA Extraction and First-Strand Synthesis
Total RNA from fresh and fixed TPC1 cells (derived from human thyroid papillary carcinoma, classic type and harboring RET/PTC1 rearrangement) was extracted using MasterPure Complete DNA and RNA Purification Kit (Epicentre, Madison, WI, USA). Total RNA from formalin-fixed paraffin-embedded (FFPE) specimen was extracted using a High Pure FFPE RNA isolation kit (Roche Diagnostics, Mannheim, Germany). First-strand synthesis was performed on 2 µg of total RNA using a Tetro cDNA synthesis kit (Bioline, London, UK). Tetro reverse transcriptase with diethyl pyrocarbonate water and cDNA reverse transcribed product from the TPC1 cells were used as negative and positive controls, respectively.
4.2. RT-PCR
Amplification was performed by RT-PCR using a LightCycler 480 Instrument (Roche Diagnostics), and measurement was performed using LightCycler quantification software version 1.5 (Roche Diagnostics). The RT-PCR reaction mixture was prepared in a Light Cycler® 480 Multiwell Plate 96 containing 0.5 μM of each primer set (RET/PTC1 and glyceraldehyde-3-phosphate isomerase, GAPDH), 0.25 μM of the probes, 2X of LightCycler 480 Probes Master (Roche Diagnostics), and 1–2 μg (1 µg for the cell and 2 µg for the FFPE tissue) of cDNA template in a final reaction volume of 20 μL (Table 4).
Table 4.
Primers and probes sequences for RT-PCR.
| RET/PTC1 | Primers and Probes Sequences |
|---|---|
| Forward primer (5′–3′) | CGC GAC CTG CGC AAA |
| Reverse primer (5′–3′) | CAA GTT CTT CCG AGG GAA TTC C |
| TaqMan Probe (5′–3′) | FAM-CCA GCG TTA CCA TCG AGG ATC CAA AGT-BHQ1 |
| GAPDH | |
| Forward primer (5′–3′) | GTT CGA CAG TCA GCC GCA TC |
| Reverse primer (5′–3′) | GGA ATT TGC CAT GGG TGG A |
| TaqMan Probe (5′–3′) | FAM-ACC AGG CGC CCA ATA CGA CCA A-BHQ1 |
4.3. NanoString nCounter Gene Expression Assay
Tumor portion on the hematoxylin and eosin-stained FFPE tissue slides was marked by the pathologist, and total RNA was isolated from two to three FFPE tissue sections (10 μm thick) using an miRNeasy FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The probe sets were custom designed and synthesized by NanoString Technologies (Seattle, WA, USA), and nCounter assays were performed according to the manufacturer’s protocol. Briefly, 500 ng of total RNA was hybridized to nCounter probe sets for 16 hours at 65 °C. Samples were then processed using an automated nCounter Sample Prep Station (NanoString Technologies, Inc., Seattle, WA, USA). Cartridges containing immobilized and aligned reporter complexes were subsequently imaged on an nCounter Digital Analyzer (NanoString Technologies, Inc.). Reporter counts were collected using the NanoString’s nSolver analysis software version 1, normalized, and analyzed. A total of eight expression probes were designed, four (5′-1 to 5′-4) proximal and four distal (3′-1 to 3′-4) to most commonly-known junction sites for RET fusions. An imbalance between 5′ and 3′ probe signals was indicative of the presence of a RET fusion transcript. We used a cutoff of three-fold for 3′/5′ ratio. Therefore, a case was considered positive for rearrangement if 3′/5′ imbalance was three-fold or more.
We used Cohen’s κ coefficient to measure agreement between RT-PCR and Nanostring method.
4.4. Detection of the RET/PTC1 Rearrangement in Fresh TPC1 Cell Line
Total RNA was extracted by Master Pure Complete DNA and RNA Purification Kit (Epicentre) using a fresh cell colony formed from 1000 cultured TPC1 cells (provided by Nagataki, Nakasaki University, Japan). RT-PCR was performed and the minimum number of cycles (Ct value) needed to detect the expression of RET/PTC1 rearrangement and GAPDH was determined. Similarly, the number of the cells was reduced to 500, 250, 100, and 50, and RT-PCR was performed to evaluate Ct values according to cell count. The whole procedure was performed in triplicate after TPC1 cells were harvested
4.5. Detection of RET/PTC1 Rearrangement in Routine Air-Dried TPC1 Cell Line
To make a condition identical to that in the routine air-dried FNA preparation, cultured TPC1 cells were smeared on a slide and fixed with 95% ethanol according to the routine FNA preparation in our cytology laboratory. The fixed cells were stained by the routine Papanicolaou procedure. After the coverslips were removed from the smeared slides, the atypical cells of interest were dissected with a 26-gauge needle under the light microscope. Approximately 50, 100, 250, 500, and 1000 cells were dissected using a square micrometer under the microscope. A needle tip was carefully submerged in a tube containing extraction buffer supplied by MasterPure Complete DNA and RNA Purification Kit (Epicentre), and total RNA was extracted. RT-PCR was performed, and Ct values for the expression of RET/PTC1 rearrangement and GAPDH were evaluated using 50, 100, 250, 500, and 1000 air-dried and alcohol fixed TPC1 cells, respectively. The whole procedure was performed in triplicate after TPC1 cells were harvested.
4.6. Correlation of RET/PTC1 Rearrangement between Routine Air-Dried FNA and Paired FFPE PTC Tissue Specimens
PTC cells from the archival FNA slides from the Department of Pathology, Konkuk University Medical Center were used. The slides that were selected were from samples aspirated from ten thyroid nodules with histopathological diagnosis of classical-type PTC. Study approval was obtained from the Institutional Review Board (KUH1210043). After the coverslips were removed from the slides, approximately 100 atypical follicular cells were dissected with a 26-gauge needle under the light microscope, and total RNA was extracted using MasterPure Complete DNA and RNA Purification Kit (Epicentre). RT-PCR was performed, and Ct values of the RET/PTC1 rearrangement and GAPDH expression were evaluated. Tumor portion on the hematoxylin and eosin-stained FFPE tissue slides was marked by the pathologist, and total RNA was isolated from two-to-three FFPE tissue sections (10 μm thick) using High Pure FFPE RNA isolation kit (Roche Diagnostics). RT-PCR was performed and Ct values of the RET/PTC1 rearrangement and GAPDH expression were evaluated. The Ct values defining the analysis as positive is greater than 40 cycles.
4.7. Detection of the RET/PTC1 Rearrangement in Resected BRAF and RAS Wild-Type PTC Cases Using FFPE Tissue Specimen
Archival thyroid neoplasm that had been surgically removed between 2010 and 2014 at Konkuk University Medical Center were blindly re-evaluated according to the 2004 World Health Organization classification of thyroid neoplasm by the two pathologists (Tae Sook Hwang, who is an endocrine pathologist, and Young Sin Ko). In case of a disagreement and to reach a consensus, another endocrine pathologist (Chan-Kwon Jung) independently reviewed the cases. Of the 600 classical PTC cases selected, 518 had BRAF mutation and 6 had RAS mutation. Finally, 76 BRAF and RAS wild-type classical PTC cases were selected. Tumor portion on the hematoxylin and eosin-stained FFPE tissue slides was marked by the pathologist, and total RNA was isolated from two-to-three FFPE tissue sections (10 μm thick) using High Pure FFPE RNA isolation kit (Roche Diagnostics). RT-PCR was performed, and Ct values of the RET/PTC1 rearrangement and GAPDH expression were evaluated. The Ct value defining the analysis as positive is greater than 40 cycles.
4.8. Comparison Analysis of RT-PCR with the NanoString nCounter Gene Expression Assay for Detecting RET/PTC1 Rearrangement
RET/PTC1 rearrangement status was also analyzed by the Nanostring method, using 31 cases having sufficient cancer tissue remaining for the comparative analysis.
5. Conclusions
RET/PTC1 rearrangement analysis by RT-PCR using routine air-dried FNA specimen was confirmed to be technically applicable and significant population (38.2%) of the BRAF and RAS wild type PTCs harbor RET/PTC1 rearrangement. Preoperative RET/PTC1 rearrangement analysis in BRAF and RAS wild type indeterminate thyroid nodules would permit a formulation of unambiguous surgical plan, while foregoing the need for other less specific diagnostic test such as repeat FNA and intraoperative frozen section evaluation. An algorithmic approach is cost-effective and efficient especially in BRAF mutation prevalent populations.
Acknowledgments
This paper was supported by Konkuk University. The authors thank Chan-Kwon Jung (Department of Pathology, College of Medicine, The Catholic University, Seoul, Korea) for reviewing thyroid tissue slides.
Abbreviations
| AUS | Atypia of undetermined significance |
| Ct | Threshold cycle |
| FFPE | Formalin-fixed paraffin-embedded |
| FN | Follicular neoplasm; |
| FNA | Fine needle aspiration |
| FLUS | Follicular lesion of undetermined significance |
| FVPTC | Follicular variant of papillary thyroid carcinoma |
| FTC | Follicular thyroid carcinoma |
| NGS | Next generation sequencing |
| RT-PCR | Real-time reverse transcription-polymerase chain reaction |
| SFN | Suspicious for follicular neoplasm |
| PTC | Papillary thyroid carcinoma |
| TPC1 | Thyroid papillary carcinoma 1 |
Author Contributions
Young Sin Ko designed the experiments, conducted the main experiments and prepared the manuscript; Tae Sook Hwang conceived and designed the experiments and revised the manuscript; Ja Yeon Kim also conducted the main experiments and analyzed the data; Seung Eun Lee conducted Nanostring and analyzed the data; Yoon-La Choi, Hye Seung Han, Wan Seop Kim, Suk Kyeong Kim, and Kyoung Sik Park contributed clarifications and guidance on the manuscript. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vander J.B., Gaston E.A., Dawber T.R. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann. Intern. Med. 1968;69:537–540. doi: 10.7326/0003-4819-69-3-537. [DOI] [PubMed] [Google Scholar]
- 2.Tunbridge W.M., Evered D.C., Hall R., Appleton D., Brewis M., Clark F., Evans J.G., Young E., Bird T., Smith P.A. The spectrum of thyroid disease in a community: The whickham survey. Clin. Endocrinol. 1977;7:481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan G.H., Gharib H. Thyroid incidentalomas: Management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann. Intern. Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Guth S., Theune U., Aberle J., Galach A., Bamberger C.M. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur. J. Clin. Investig. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 5.Hegedus L. Clinical practice. The thyroid nodule. N. Engl. J. Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 6.Mandel S.J. A 64-year-old woman with a thyroid nodule. JAMA. 2004;292:2632–2642. doi: 10.1001/jama.292.21.2632. [DOI] [PubMed] [Google Scholar]
- 7.Sherman S.I. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/S0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 9.Davies L., Welch H.G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Registration and Statistics in Korea 2015. Korea Central Cancer Registry; Goyang, Korea: 2015. [Google Scholar]
- 11.Gharib H., Goellner J.R. Fine-needle aspiration biopsy of the thyroid: An appraisal. Ann. Intern. Med. 1993;118:282–289. doi: 10.7326/0003-4819-118-4-199302150-00007. [DOI] [PubMed] [Google Scholar]
- 12.Udelsman R., Chen H. The current management of thyroid cancer. Adv. Surg. 1999;33:1–27. [PubMed] [Google Scholar]
- 13.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goutas N., Vlachodimitropoulos D., Bouka M., Lazaris A.C., Nasioulas G., Gazouli M. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res. 2008;28:305–308. [PubMed] [Google Scholar]
- 15.Kim S.K., Song K.H., Lim S.D., Lim Y.C., Yoo Y.B., Kim J.S., Hwang T.S. Clinical and pathological features and the BRAFV600E mutation in patients with papillary thyroid carcinoma with and without concurrent hashimoto thyroiditis. Thyroid. 2009;19:137–141. doi: 10.1089/thy.2008.0144. [DOI] [PubMed] [Google Scholar]
- 16.Davies L., Welch H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 17.Jung C.K., Little M.P., Lubin J.H., Brenner A.V., Wells S.A., Jr., Sigurdson A.J., Nikiforov Y.E. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J. Clin. Endocrinol. Metab. 2014;99:E276–E285. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J.Y., Kim W.Y., Hwang T.S., Lee S.S., Kim H., Han H.S., Lim S.D., Kim W.S., Yoo Y.B., Park K.S. BRAF and RAS mutations in follicular variants of papillary thyroid carcinoma. Endocr. Pathol. 2013;24:69–76. doi: 10.1007/s12022-013-9244-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.R., Jung C.K., Kim T.E., Bae J.S., Jung S.L., Choi Y.J., Kang C.S. Molecular genotyping of follicular variant of papillary thyroid carcinoma correlates with diagnostic category of fine-needle aspiration cytology: Values of RAS mutation testing. Thyroid. 2013;23:1416–1422. doi: 10.1089/thy.2012.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bongarzone I., Butti M.G., Coronelli S., Borrello M.G., Santoro M., Mondellini P., Pilotti S., Fusco A., Della Porta G., Pierotti M.A. Frequent activation of ret protooncogene by fusion with a new activating gene in papillary thyroid carcinomas. Cancer Res. 1994;54:2979–2985. [PubMed] [Google Scholar]
- 21.Bongarzone I., Monzini N., Borrello M.G., Carcano C., Ferraresi G., Arighi E., Mondellini P., Della Porta G., Pierotti M.A. Molecular characterization of a thyroid tumor-specific transforming sequence formed by the fusion of ret tyrosine kinase and the regulatory subunit RI α of cyclic AMP-dependent protein kinase A. Mol. Cell. Biol. 1993;13:358–366. doi: 10.1128/MCB.13.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grieco M., Santoro M., Berlingieri M.T., Melillo R.M., Donghi R., Bongarzone I., Pierotti M.A., Della Porta G., Fusco A., Vecchio G. PTC is a novel rearranged form of the RET proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60:557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- 23.Jhiang S.M., Smanik P.A., Mazzaferri E.L. Development of a single-step duplex RT-PCR detecting different forms of RET activation, and identification of the third form of in vivo ret activation in human papillary thyroid carcinoma. Cancer Lett. 1994;78:69–76. doi: 10.1016/0304-3835(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 24.Santoro M., Dathan N.A., Berlingieri M.T., Bongarzone I., Paulin C., Grieco M., Pierotti M.A., Vecchio G., Fusco A. Molecular characterization of RET/PTC3; a novel rearranged version of the retproto-oncogene in a human thyroid papillary carcinoma. Oncogene. 1994;9:509–516. [PubMed] [Google Scholar]
- 25.Fenton C.L., Lukes Y., Nicholson D., Dinauer C.A., Francis G.L., Tuttle R.M. The RET/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J. Clin. Endocrinol. Metab. 2000;85:1170–1175. doi: 10.1210/jc.85.3.1170. [DOI] [PubMed] [Google Scholar]
- 26.Rabes H.M., Demidchik E.P., Sidorow J.D., Lengfelder E., Beimfohr C., Hoelzel D., Klugbauer S. Pattern of radiation-induced ret and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: Biological, phenotypic, and clinical implications. Clin. Cancer Res. 2000;6:1093–1103. [PubMed] [Google Scholar]
- 27.Unger K., Zitzelsberger H., Salvatore G., Santoro M., Bogdanova T., Braselmann H., Kastner P., Zurnadzhy L., Tronko N., Hutzler P., et al. Heterogeneity in the distribution of RET/PTC rearrangements within individual post-chernobyl papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2004;89:4272–4279. doi: 10.1210/jc.2003-031870. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Z., Ciampi R., Nikiforova M.N., Gandhi M., Nikiforov Y.E. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: Effects of the detection methods and genetic heterogeneity. J. Clin. Endocrinol. Metab. 2006;91:3603–3610. doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 29.Namba H., Yamashita S., Pei H.C., Ishikawa N., Villadolid M.C., Tominaga T., Kimura H., Tsuruta M., Yokoyama N., Izumi M., et al. Lack of PTC gene (RET proto-oncogene rearrangement) in human thyroid tumors. Endocrinol. Jpn. 1991;38:627–632. doi: 10.1507/endocrj1954.38.627. [DOI] [PubMed] [Google Scholar]
- 30.Nikiforov Y.E., Rowland J.M., Bove K.E., Monforte-Munoz H., Fagin J.A. Distinct pattern of RET oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res. 1997;57:1690–1694. [PubMed] [Google Scholar]
- 31.Park K.Y., Koh J.M., Kim Y.I., Park H.J., Gong G., Hong S.J., Ahn I.M. Prevalences of GS α, ras, p53 mutations and RET/PTC rearrangement in differentiated thyroid tumours in a Korean population. Clin. Endocrinol. 1998;49:317–323. doi: 10.1046/j.1365-2265.1998.00515.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee C.H., Hsu L.S., Chi C.W., Chen G.D., Yang A.H., Chen J.Y. High frequency of rearrangement of the ret protooncogene (RET/PTC) in chinese papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 1998;83:1629–1632. doi: 10.1210/jc.83.5.1629. [DOI] [PubMed] [Google Scholar]
- 33.Rhoden K.J., Johnson C., Brandao G., Howe J.G., Smith B.R., Tallini G. Real-time quantitative RT-PCR identifies distinct C-RET, RET/PTC1 and RET/PTC3 expression patterns in papillary thyroid carcinoma. Lab. Investig. 2004;84:1557–1570. doi: 10.1038/labinvest.3700198. [DOI] [PubMed] [Google Scholar]
- 34.Di Cristofaro J., Vasko V., Savchenko V., Cherenko S., Larin A., Ringel M.D., Saji M., Marcy M., Henry J.F., Carayon P., et al. Ret/PTC1 and RET/PTC3 in thyroid tumors from chernobyl liquidators: Comparison with sporadic tumors from ukrainian and french patients. Endocr. Relat. Cancer. 2005;12:173–183. doi: 10.1677/erc.1.00884. [DOI] [PubMed] [Google Scholar]
- 35.Mayr B., Potter E., Goretzki P., Ruschoff J., Dietmaier W., Hoang-Vu C., Dralle H., Brabant G. Expression of RET/PTC1, -2, -3, -Δ3 and -4 in German papillary thyroid carcinoma. Br. J. Cancer. 1998;77:903–906. doi: 10.1038/bjc.1998.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikiforov Y.E. RET/PTC rearrangement in thyroid tumors. Endocr. Pathol. 2002;13:3–16. doi: 10.1385/EP:13:1:03. [DOI] [PubMed] [Google Scholar]
- 37.Tallini G., Asa S.L. Ret oncogene activation in papillary thyroid carcinoma. Adv. Anat. Pathol. 2001;8:345–354. doi: 10.1097/00125480-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Chung J.H., Hahm J.R., Min Y.K., Lee M.S., Lee M.K., Kim K.W., Nam S.J., Yang J.H., Ree H.J. Detection of RET/PTC oncogene rearrangements in Korean papillary thyroid carcinomas. Thyroid. 1999;9:1237–1243. doi: 10.1089/thy.1999.9.1237. [DOI] [PubMed] [Google Scholar]
- 39.Motomura T., Nikiforov Y.E., Namba H., Ashizawa K., Nagataki S., Yamashita S., Fagin J.A. Ret rearrangements in Japanese pediatric and adult papillary thyroid cancers. Thyroid. 1998;8:485–489. doi: 10.1089/thy.1998.8.485. [DOI] [PubMed] [Google Scholar]
- 40.Guerra A., Sapio M.R., Marotta V., Campanile E., Moretti M.I., Deandrea M., Motta M., Limone P.P., Fenzi G., Rossi G., et al. Prevalence of RET/PTC rearrangement in benign and malignant thyroid nodules and its clinical application. Endocr. J. 2011;58:31–38. doi: 10.1507/endocrj.K10E-260. [DOI] [PubMed] [Google Scholar]
- 41.Marotta V., Guerra A., Sapio M.R., Campanile E., Motta M., Fenzi G., Rossi G., Vitale M. Growing thyroid nodules with benign histology and RET rearrangement. Endocr. J. 2010;57:1081–1087. doi: 10.1507/endocrj.K10E-229. [DOI] [PubMed] [Google Scholar]
- 42.Sapio M.R., Guerra A., Marotta V., Campanile E., Formisano R., Deandrea M., Motta M., Limone P.P., Fenzi G., Rossi G., et al. High growth rate of benign thyroid nodules bearing RET/PTC rearrangements. J. Clin. Endocrinol. Metab. 2011;96:E916–E919. doi: 10.1210/jc.2010-1599. [DOI] [PubMed] [Google Scholar]
- 43.Eszlinger M., Krogdahl A., Munz S., Rehfeld C., Precht Jensen E.M., Ferraz C., Bosenberg E., Drieschner N., Scholz M., Hegedus L., et al. Impact of molecular screening for point mutations and rearrangements in routine air-dried fine-needle aspiration samples of thyroid nodules. Thyroid. 2014;24:305–313. doi: 10.1089/thy.2013.0278. [DOI] [PubMed] [Google Scholar]
- 44.Ferraz C., Rehfeld C., Krogdahl A., Precht Jensen E.M., Bosenberg E., Narz F., Hegedus L., Paschke R., Eszlinger M. Detection of PAX8/PPARG and RET/PTC rearrangements is feasible in routine air-dried fine needle aspiration smears. Thyroid. 2012;22:1025–1030. doi: 10.1089/thy.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung C.C., Carydis B., Ezzat S., Bedard Y.C., Asa S.L. Analysis of RET/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J. Clin. Endocrinol. Metab. 2001;86:2187–2190. doi: 10.1210/jcem.86.5.7504. [DOI] [PubMed] [Google Scholar]
- 46.Musholt T.J., Fottner C., Weber M.M., Eichhorn W., Pohlenz J., Musholt P.B., Springer E., Schad A. Detection of papillary thyroid carcinoma by analysis of BRAF and RET/PTC1 mutations in fine-needle aspiration biopsies of thyroid nodules. World J. Surg. 2010;34:2595–2603. doi: 10.1007/s00268-010-0729-4. [DOI] [PubMed] [Google Scholar]
- 47.Nikiforov Y.E., Steward D.L., Robinson-Smith T.M., Haugen B.R., Klopper J.P., Zhu Z., Fagin J.A., Falciglia M., Weber K., Nikiforova M.N. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 48.Salvatore G., Giannini R., Faviana P., Caleo A., Migliaccio I., Fagin J.A., Nikiforov Y.E., Troncone G., Palombini L., Basolo F., et al. Analysis of BRAF point mutation and RET/PTC rearrangement refines the fine-needle aspiration diagnosis of papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2004;89:5175–5180. doi: 10.1210/jc.2003-032221. [DOI] [PubMed] [Google Scholar]
- 49.An J.H., Song K.H., Kim S.K., Park K.S., Yoo Y.B., Yang J.H., Hwang T.S., Kim D.L. Ras mutations in indeterminate thyroid nodules are predictive of the follicular variant of papillary thyroid carcinoma. Clin. Endocrinol. 2015;82:760–766. doi: 10.1111/cen.12579. [DOI] [PubMed] [Google Scholar]
- 50.Hwang T.S., Kim W.Y., Han H.S., Lim S.D., Kim W.S., Yoo Y.B., Park K.S., Oh S.Y., Kim S.K., Yang J.H. Preoperative RAS mutational analysis is of great value in predicting follicular variant of papillary thyroid carcinoma. BioMed Res. Int. 2015;2015:697068. doi: 10.1155/2015/697068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S.K., Kim D.L., Han H.S., Kim W.S., Kim S.J., Moon W.J., Oh S.Y., Hwang T.S. Pyrosequencing analysis for detection of a BRAFV600E mutation in an fnab specimen of thyroid nodules. Diagn. Mol. Pathol. 2008;17:118–125. doi: 10.1097/PDM.0b013e31815d059d. [DOI] [PubMed] [Google Scholar]
- 52.Cantara S., Capezzone M., Marchisotta S., Capuano S., Busonero G., Toti P., Di Santo A., Caruso G., Carli A.F., Brilli L., et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J. Clin. Endocrinol. Metab. 2010;95:1365–1369. doi: 10.1210/jc.2009-2103. [DOI] [PubMed] [Google Scholar]
- 53.Ohori N.P., Nikiforova M.N., Schoedel K.E., LeBeau S.O., Hodak S.P., Seethala R.R., Carty S.E., Ogilvie J.B., Yip L., Nikiforov Y.E. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance”. Cancer Cytopathol. 2010;118:17–23. doi: 10.1002/cncy.20063. [DOI] [PubMed] [Google Scholar]
- 54.Kulkarni M.M. Digital multiplexed gene expression analysis using the nanostring ncounter system. Curr. Protoc. Mol. Biol. 2011 doi: 10.1002/0471142727.mb25b10s94. [DOI] [PubMed] [Google Scholar]