Abstract
The aim of the present study was to investigate the effects of inspiratory muscle exercise (IME) on metabolic and hemodynamic parameters, cardiac autonomic modulation and respiratory function of older women with metabolic syndrome (MS). For this, sixteen older women with MS and 12 aged-matched controls participated of the present study. Two days before and 2 days after the main experiment, fasting blood samples (i.e., total cholesterol, triglycerides and blood glucose), cardiac autonomic modulation (i.e., heart rate variability), and respiratory muscle function were obtained and evaluated. The sessions of physical exercise was based on a IME, which was performed during 7 days. Each session of IME was performed during 20 min, at 30% of maximal static inspiratory pressure. In the results, MS group presented higher levels of triglycerides, blood glucose, and systolic blood pressure when compared to control group. IME was not able to change these variables. However, although MS group showed impaired respiratory muscle strength and function, as well as cardiac autonomic modulation, IME was able to improve these parameters. Thus, the data showed that seven days of IME are capable to improve respiratory function and cardiac autonomic modulation of older women with MS. These results indicate that IME can be a profitable therapy to counteracting the clinical markers of MS, once repeated sessions of acute IME can cause chronical alterations on respiratory function and cardiac autonomic modulation.
Keywords: Elderly, Autonomic nervous system, Breathing exercises, Metabolic syndrome, Respiratory function
INTRODUCTION
Metabolic syndrome (MS) is characterized by a constellation of metabolic disorders, such as: central adiposity, insulin resistance, hyperglycemia, dyslipidemia, hypertension, and a sustained pro-inflammatory profile (Alberti et al., 2006). These conditions are strongly associated with increased risk for the development of atherosclerotic cardiovascular disease (CVD) (Rochlani et al., 2015).
It has been proposed that the increased sympathetic autonomic tone is the possible links between both conditions, since changes on autonomic modulation leads to altered hormonal and immune profile, inducing releasing of bioactive molecules, which are involved on the development of cardiometabolic risk profile (De Angelis et al., 2012). On the clinical setting, cardiovascular changes become evident in obesity individuals only when associated to MS. Evidences have been indicating an exaggerated vasoconstriction in response to metaboreflex activation in MS obesity patients, due to a more elevated sympathetic activity (Milia et al., 2015). Indeed, experimental studies with MS have been demonstrating the susceptibility of this clinical condition to present impaired baroreflex sensitivity (Lindgren et al., 2006), reduced heart rate variability (HRV) (Assoumou et al., 2010), as well as reduced vagal and increased sympathetic cardiac modulation (Lindgren et al., 2006).
In older adults with MS, the autonomic dysfunction seems to be more pronounced in women than in men (Assoumou et al., 2010). Moreover, it has been widely demonstrated that postmenopausal women are at greater risk for CVD development than premenopausal women, thus suggesting a cardiovascular protective role of ovarian hormones (Guthrie et al., 2004). Additionally, the cessation of ovarian hormones collaborates with the development of muscle atrophy and dynapenia (Conceição et al., 2013), while inducing the weakening of respiratory muscles (Bonganha et al., 2013), representing a major age-related functional impairment. Moreover, aging also leads to cardiovascular functional and structural changes that may impact cardiac autonomic function (Hotta and Uchida, 2010).
In turn, respiratory muscle exercises are a nonpharmacological strategy widely used to improve functional capacity in several conditions associated with the impaired respiratory muscle strength, such as chronic obstructive pulmonary disease (Petrovic et al., 2012), chronic heart failure (Mello et al., 2012) and hypertension (Ferreira et al., 2013), along with another aging-associated conditions (Aznar-Lain et al., 2007). Furthermore, studies have demonstrated beneficial effects of inspiratory muscle exercise (IME) on cardiac autonomic function, indicated by reduced sympathetic modulation and increased vagal modulation in young smokers (Rodrigues et al., 2013), as well as hypertensive (Ferreira et al., 2013) and heart failure patients (Mello et al., 2012).
Therefore, in front of the aforementioned information, is possible infer that IME could elicit transient changes on metabolic profile, cardiac autonomic modulation and respiratory muscle function of older women with MS. The present studied is developed to tested this hypothesis.
MATERIALS AND METHODS
Subjects
This is an experimental study with a pre-post protocol of evaluations.
The study protocol was approved by the Ethics Committee of the Sao Judas Tadeu University (São Paulo, Brazil) and all participants signed an informed consent to participate in this study. This study was conducted in accordance with the principles of the Declaration of Helsinki and specific resolution of Conselho Nacional de Saúde (n° 196/96).
In the present study, 16 women previously clinically diagnosed with MS were recruited from the Physiotherapy Clinic of Sao Judas Tadeu University, and 12 control women were recruited from the surrounding area. Participants met the following eligibility criteria: (1) 60- to 80-year-olds; (2) post menopause; (3) sedentary, with no changes in physical activity over the previous 3 months; (4) nonalcoholic. In addition, MS participants presented: (5) previous MS diagnosis based on the criteria of the National Cholesterol Education Program (Adult Treatment Panel III, NCEP ATP III) (NCEP, 2002); and (6) clinical stability with no change in medications for at least 2 months preceding the study. Individuals were excluded if they had a recent cardiac event, heart failure or renal failure, if they were current smokers, or in use of beta blocker.
Study design
On the first day of the protocol, personal data, clinical and family history of cardiovascular, pulmonary, metabolic and renal diseases, number and class of medications used, as well as lifestyle habits of the participants were collected through interviews. Anthropometric measurements were then taken, followed by spirometry analysis of lung function. On the second nonconsecutive day of protocol, RR interval (time elapsing between two consecutive R waves) during 20 min, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, triglycerides (TG), and blood glucose [BG] were measured. A manovacuometry was also performed to evaluate the maximal inspiratory (MIP) and maximal expiratory pressures (MEP). One day after these baseline measurements, an IME session was performed. After seven days of IME, baseline evaluations (as records of RR interval, SBP, DBP, MIP, MEP, and metabolic parameters) were performed again within 48 hr. An illustration of the study design is showed in the Fig. 1.
Fig. 1.
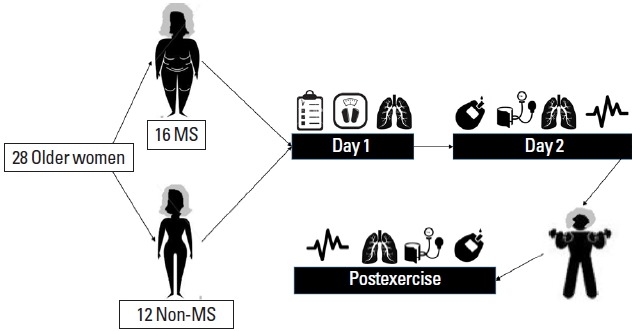
An illustration of the study design. MS, metabolic syndrome.
The subjects were instructed to avoid strenuous exercises, alcohol and caffeinated beverages at least 12 hr before evaluations. All evaluations were performed in the morning (8:00 a.m. to 12:00 p.m.).
Measurements
Anthropometric measurements
Anthropometric measurements included body weight, height, waist-hip ratio, and body mass index (BMI, by the ratio of body weight [kg] and square height [m2]).
A weight scale with stadiometer Filizola (Sao Paulo, Brazil) was used to determine body weight (kg) and height (cm). To waist and hip circumferences measurement, an anthropometric tape (flexible and inextensible) Sanny (São Bernardo do Campo, Brazil) was handling. To evaluation, subjects remained in the standing position, head held erect, eyes forward, with the arms relaxed at the side, foot in parallel (i.e., together), with light clothes. The waist circumference was evaluated at the midpoint between the last floating rib and the highest point of the iliac crest (Conceição et al., 2013). In turn, hip circumference was evaluated at the highest point of the buttocks (Conceição et al., 2013).
Blood pressure and metabolic measurements
The procedures for the measurement of blood pressure (BP) were according to the guidelines of the The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) (Chobanian et al., 2003). In summary, older women remained in the sitting position in a comfortable chair for 20 min. With an automatic and noninvasive BP monitor (BP710, Omron, Tokyo, Japan), three measurements of BP were performed on the right arm, with at least a 2-min interval between each one. After IME, BP values were obtained during the 20 min of the end of the session, and the average of these values is presented in this study.
A fasting blood sample was obtained for cholesterol, TG, and BG quantification, which was performed using a Roche device (Accutrend GCT, Roche, Mannheim, Germany).
Respiratory function
In the morning of the day reserved to baseline measurements, a Spirometry test (Spirocard, QRS diagnostics, Maple Grove, MN, USA) was performed by a qualified technician. The subjects underwent the spirometry test in the sitting position, wearing a nose clip. Each individual underwent a forced spirometry to obtain the following parameters: forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), as well as the ratio of FEV1 to FVC (FEV1/FVC, expressed as a percentage). In addition to the automatic evaluation performed by the software device, the quality of spirometric tests was assessed according some criteria, including: the number of acceptable maneuvers according to American Thoracic Society (ATS), ranging from 0 to 3, the highest kept by the spirometry software; the reproducibility (FEV1 and FVC were considered reproducible according to ATS criteria when the best two trials differed by not more than 200 mL).
Measurements of maximal respiratory pressures were performed by a blinded investigator. A pressure transducer (MVD-300, Globalmed, Porto Alegre, Brazil) connected to a system with two unidirectional valves (DHD Inspiratory Muscle Trainer, Chicago, IL, USA) was used. MIP and MEP were determined in deep inspiration and expiration from residual volume against an occluded airway with a minor air leak (2 mm), following a previously described procedure (Ferreira et al., 2013). The highest pressure of six measurements was defined as MIP and MEP.
Cardiac autonomic modulation
RR interval was continuously recorded during 20 min in individuals sitting, using a Polar S810i (Polar Electro-OY, Kempele, Finland) to power spectral analyses of HRV. The spectrum resulting from the Fast Fourier Transforms modeling is derived from all the data present in the recorded signal; it includes the entire signal variance, regardless of whether its frequency components appear as specific spectral peaks or as nonpeak broadband powers. The RR interval variability was evaluated in the time and frequency domains. Spectral power for low (LF: 0.03–0.15 Hz) and high (HF: 0.15–0.4 Hz) frequency bands was calculated by means of power spectrum density integration within each frequency bandwidth, using a customized routine (MATLAB 6.0, Natick, MA, USA) (Rodrigues et al., 2013).
Inspiratory muscle exercise
All participants were already familiar with IME. During the experimental sessions, older women remained in the sitting position in a comfortable chair. The session of exercise consisted of 3 sets of 15 min (in a clinical setting), with a 3-min interval between the sets, using the Threshold Inspiratory Muscle Training device (Threshold Inspiratory Muscle Trainer, Healthscan Products Inc., Cedar Grove, NJ, USA). The inspiratory load was set at 30% of MIP (Aznar-Lain et al., 2007). During exercise, subjects were instructed to maintain diaphragmatic breathing at a rate of 15 to 20 breaths/min. All the seven sessions was performed in the same time of the day (i.e., 08:00 a.m. to 11:00 a.m.).
Statistical analysis
Data are presented as mean±standard deviation. Normality of data was tested using the Kolmogorov-Smirnov test. Unpaired Student t-test was used to verify differences in the participant characteristics between the groups. Differences in the percentage of medications between the groups were calculated using chi-squre test. Statistical differences between the groups were obtained by 1-way analysis of variance (ANOVA) followed by the Bonferroni posthoc. Baseline vs. post-IME statistical differences was obtained by repeated measures ANOVA. Cohen d was calculated to assess the magnitude of the results. The effect size (ES) was considered to be medium for Cohen d values between 0.2 and 0.5, good for values between 0.5 and 0.8, and large for values ≥0.8. The level of significance was 5% (P<0.05) and all procedures were performed using the IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). The power of the sample size was determined using G*Power ver. 3.1.9.2 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). The ES required for a sample size of 16 volunteers with a level of significance set at 5% and power (β) of 0.80 was 0.80 (larger ES).
RESULTS
Tables 1 and 2 show the characteristics of the sample and the effects of IME on metabolic and hemodynamic variables. No differences were found between control and MS groups for age, time after menopause and BMI (Table 1); however, the waist-hip ratio was increased in MS group. Regarding DBP, heart rate, and total cholesterol, data indicate that these parameters were similar between groups. On the other hand, the MS group presented higher levels of TG, BG, and SBP when compared to control group; IME was not able to change these variables (Table 2). ES evaluation through Cohen d, corroborate with data from hypothesis test, since results show a “medium” classification (0.08–0.49). Furthermore, MS showed higher use of medications, with the exception of angiotensin-converting-enzyme inhibitor, in comparison with control.
Table 1.
Participant characteristics
| Characteristic | Control (n=12) | MS (n=16) |
|---|---|---|
| Age (yr) | 68±3 | 69±4 |
|
| ||
| Time after menopause (yr) | 10±3 | 12±4 |
|
| ||
| MS diagnosis (yr) | - | 10±5 |
|
| ||
| Weight (kg) | 75.3±12 | 78.5±10 |
|
| ||
| Height (cm) | 155±15 | 153±12 |
|
| ||
| Body mass index (kg/m2) | 32.6±5 | 34.5±6 |
|
| ||
| Waist-hip ratio | 0.82±0.06 | 0.93±0.07* |
|
| ||
| Medications (%) | ||
| ACE inhibitor | 9 | 25 |
| HMG-CoA reductase inhibitor | 5 | 78* |
| Diuretic | 18 | 60* |
| Acid acetylsalicylic | 8 | 38* |
| ARBs | 0 | 45* |
| Oral hypoglycemiants | 0 | 95* |
Values are presented as mean±standard deviation unless otherwise indicated.
MS, metabolic syndrome; ACE, angiotensin-converting-enzyme; HMG-CoA, 3-hydroxy-3-methyl-glutaryl coenzyme A; ARBs, angiotensin II receptor blockers.
P<0.05 vs. C group.
Table 2.
Metabolic and hemodynamic effects of IME in control and MS groups
| Parameter | Control | MS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | After IME | ES | Baseline | After IME | ES | |
| Metabolic | ||||||
|
|
||||||
| Total cholesterol (mg/dL) | 180±20 | 178±25 | 0.08 | 191±22 | 188±22 | 0.13 |
|
|
||||||
| Triglycerides (mg/dL) | 98±15 | 95±12 | 0.22 | 201±25* | 205±18* | −0.18 |
|
|
||||||
| Fasting BG (mg/dL) | 95±5 | 98±7 | −0.49 | 122±9* | 126±10* | −0.42 |
|
| ||||||
| Hemodynamic | ||||||
| Systolic BP (mmHg) | 119±10 | 117±15 | 0.15 | 132±13* | 129±10* | 0.25 |
| Diastolic BP (mmHg) | 74±8 | 77±6 | 0.42 | 80±9 | 82±13 | −0.17 |
| Heart rate (bpm) | 77±8 | 80±10 | 0.33 | 83±9 | 79±12 | 0.37 |
Values are presented as mean±standard deviation.
IME, inspiratory muscle exercise; MS, metabolic syndrome; ES, effect size; BG, blood glucose; BP, blood pressure.
P<0.05 vs. control group at the same evaluation time.
At baseline, reduced values of absolute MIP, MEP, and their percentage of predicted values, as well as FVC and FEV1 were observed in MS participants when compared to control (Table 3). Increased absoulut (cmH2O) and predicted (%) MIP was observed in control individuals after IME when compared to their baseline evaluation. Similarly, the MS group displayed higher MIP, FVC, and FEV1/FVC values after IME when compared to their baseline evaluation (Table 3).
Table 3.
Respiratory effects of IME in control and MS groups
| Parameter | Control | MS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | After IME | ES | Baseline | After IME | ES | |
| Pressures | ||||||
| MIP (cmH2O) | 102.7±25 | 118.3±15† | −0.77 | 69.0±14* | 94.8±11*,‡ | −1.98 |
| % Predict | 129.1±25 | 155.3±10† | −1.36 | 93.1±15* | 121.7±9*‡ | −2.26 |
| MEP (cmH2O) | 102.2±19 | 110.4±14 | −0.47 | 78.7±20* | 86.7±15* | −0.45 |
| % Predict | 144.3±15 | 149.5±16 | −0.32 | 112.8±14* | 122.3±12* | −0.76 |
|
| ||||||
| Pulmonary function | ||||||
| FVC (L) | 3.65±0.3 | 3.90±0.5 | −0.60 | 2.93±0.2* | 3.28±0.6*,‡ | −0.78 |
| FEV1 (L) | 2.74±0.1 | 2.84±0.4 | −0.34 | 2.46±0.1* | 2.58±0.2* | −0.75 |
| FEV1/FVC (%) | 76.7±4 | 80.1±8 | −0.63 | 73.2±5 | 78.9±4‡ | −1.10 |
Values are presented as mean±standard deviation.
IME, inspiratory muscle exercise; MS, metabolic syndrome; ES, effect size; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; FVC/FEV1, ratio of FEV1 to FVC.
P<0.05 vs. control group at the same evaluation time.
P<0.05 vs. baseline in control group.
P<0.05 vs. baseline MS group.
ES seems to corroborate with the hypothesis test in the control and SM. In control, significantly increase on MIP and in predicted values was followed by a Cohen d result of −0.77 (good) and −1.36 (larger), respectively. In turn, ES from MIP, FVC and FEV1/FVC in MS were −1.98 (larger), −2.26 (larger), and −0.78 (good), respectively.
Time domain of HRV was presented in Table 4. At baseline, the MS group showed a reduction in HRV parameters in time domain (standard deviation of the RR Interval [SDNN], root-meansquare of differences of successive RR interval [RMSSD] and percent of differences of adjacent RR interval >50 msec [pNN50]) when compared to the control group. However, after IME, MS showed improvement in SDNN, RMSSD, and pNN50. Similarly, control group presents increase in SDNN and RMSSD, except for pNN50, after IME. ES of SDNN and RMSSD showed to be larger in control (−1.35, −2.42) and in MS (−1.54, −1.11). Regarding pNN50, just MS shows a classificatory ES, which was “medium” (−0.76).
Table 4.
Heart rate variability in time domain in control and MS groups
| Parameter | Control | MS | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Baseline | After IME | ES | Baseline | After IME | ES | |
| SDNN | 23.2±11 | 35.3±6† | −1.35 | 12.1±5* | 19.7±4*,‡ | −1.54 |
|
| ||||||
| RMSSD | 20.3±5 | 31.1±4† | −2.42 | 13.3±7* | 19.5±3*,‡ | −1.11 |
|
| ||||||
| pNN50 | 3.4±0.9 | 3.9±1.1 | - | 2.2±0.7* | 3.1±1.5‡ | −0.76 |
Values are presented as mean±standard deviation.
IME, inspiratory muscle exercise; MS, metabolic syndrome; ES, effect size; SDNN, standard deviation of the RR interval (time elapsing between two consecutive R waves); RMSSD, root-meansquare of differences of successive RR interval; pNN50, percent of differences of adjacent RR interval >50 msec.
P<0.05 vs. control group at the same evaluation time.
P<0.05 vs. baseline MS group.
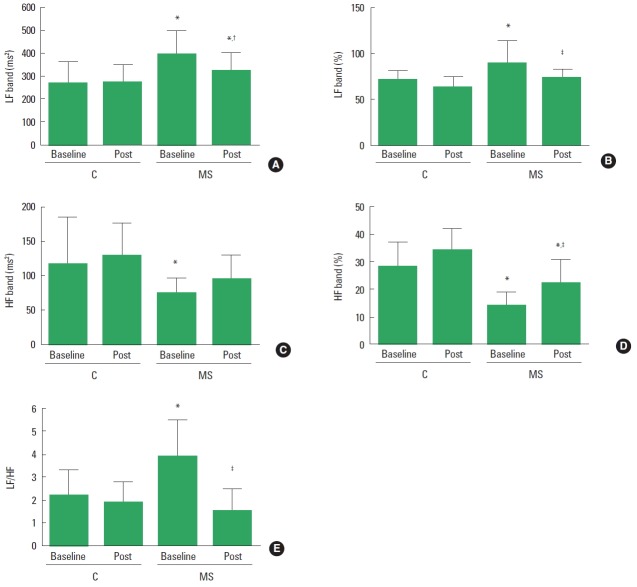
Regarding HRV in frequency domain, at baseline the MS group had impaired LF band (Fig. 2A, B) and HF band (Fig. 2C, D), which resulted in an increased LF/HF (Fig. 2E) ratio when compared to control group. After IME, MS group improved absolute LF and HF (%) bands compared to their baseline evaluation. However, these parameters remained changed in the MS group when compared to control group. In addition, IME improved autonomic balance in MS group compared to their baseline evaluation. In control group, results showed “medium” classification on HF band (ms2) (−0.22) and LF/HF (0.29); and “good” classification on LF band (%) (0.72) and HF band (%) (−0.70). On the other hand, results in MS group seem to corroborate with P-value, since LF band—reported by % (0.77) and ms2 (0.78)—and HF band (ms2) (−0.70) showed “good” classification; and HF band (%) (−1.09) and LH/HF (1.04) presented “large” classification.
Fig. 2.
Heart rate variability in frequency domain in control and metabolic syndrome (MS) groups. (A) Absolute low frequency band. (B) Percent low frequency band. (C) Absolute high frequency band. (D) Percent high frequency band. (E) LF/HF ratio. HF, high frequency band; LF, low frequency band. *P<0.05 vs. control group at the same evaluation time. †P<0.05 vs. baseline in control group. ‡P<0.05 vs. baseline MS group.
DISCUSSION
The aim of this study was evaluate the effects of IME on metabolic parameters and BP, as well as cardiac autonomic modulation and respiratory function of older women with MS. The main finding of our study is that a short-term (i.e., 7 days) protocol of IME was effective to improve cardiac autonomic modulation in MS older women, as demonstrated by the hypothesis test and ES.
Studies have shown that inspiratory muscle training improves inspiratory muscle strength in clinical conditions such as type 2 diabetes (Corrêa et al., 2011), heart failure (Mello et al., 2012) and hypertension Tenorio (Ferreira et al., 2013). However, the protocol of training in these studies ranged between 8 and 12 weeks. In the present study, 7 days of IME elicited increase on respiratory function in MS older women; however, with a smaller magnitude, based on ES results, than in previous studies. Is important to mention, that the increased muscle strength detected in this study may be a product of the technical learning of the maneuvers, so that it might not represent an actual gain in the respiratory muscle strength, since the short period of training would probably not be able to cause morphological and functional adaptations in respiratory muscles. In fact, results from experiments that underwent type 2 diabetes (Corrêa et al., 2011) and obesity (Tenório et al., 2013) patients to IME, did not observe improve on respiratory function.
In the present study, in addition to the reduced pulmonary function and respiratory muscle strength, MS individuals presented impaired cardiac autonomic modulation. In fact, it has been shown that HRV is reduced in subjects with MS (Assoumou et al., 2010), while lung function is often impaired in individuals with type 2 diabetes (Davis et al., 2004).
Results of the present study demonstrated that IME caused significant decrease on sympathetic cardiac modulation (LF band) and increase on cardiac vagal modulation (HF band), accompanied by large classification on ES, consequently improving autonomic balance. These findings may be explained by the fact that IME performance is based on the control of respiratory frequency and alterations on pulmonary volume, mainly pulmonary expansion, which activates pulmonary vagal afferents, inhibiting sympathetic activity and, consequently, changing HF band of HRV (Goso et al., 2001; Malik et al., 1996). Moreover, IME may improve oxygen supply due to augmentation of tidal volume, reducing chemoreflex activity, which is associated with a decreased sympathetic nerve discharge (Rodrigues et al., 2013; Bernardi et al., 2001).
Archiza et al. (2013), for example, showed that lower inspiratory resistive loading intensities promoted marked improvement in parasympathetic sinus node modulation. Researchers observed that one session of IME at 30% of MIP involving healthy older men promoted a greater increase in vagal indexes (RMSSD and HF component of HRV) than IME performed at 60% and 80% of MIP. According to Callegaro et al. (2011), inspiratory loads lower than 60% of MIP does not lead diaphragm fatigue, metaboreflex of inspiratory muscles is not activated, as well as sympathetic tone is not increased. In this sense, a lower inspiratory resistive loading such as the used in our study can promote a vagal response, which may be associated with cardioprotective effects on several disorders.
Moreover, as expected, the MS group presented higher TG, glycaemia and SBP when compared to control group; however, IME was not able to reverse these parameters. Although our data did not show an improvement in metabolic and hemodynamic parameters, probably due to their short-term effects, evidences have showing that chronic protocols of IME (i.e., performed during more than 1 week) are capable to elicit improve on insulin sensitivity and reduce BP values in elderly (Ferreira et al., 2013; Silva et al., 2012).
It has been demonstrated that autonomic changes can modulates hormonal and immune function (De Angelis et al., 2012). In fact, autonomic imbalance was associated with severe insulin resistance (Miller et al., 1999), and preceding the development of type 2 diabetes mellitus (Carnethon et al., 2003). Furthermore, the impairment of the parasympathetic nervous system has been considered an etiological factor in the pathological process of diabetes (Carnethon et al., 2003). Thus, taking into consideration that changes on autonomic function may precede metabolic alterations in different conditions, the opposite must also be considered. In the present study, it is possible that a longer period of IME, probably associated with sustained alterations in autonomic function, could trigger a pronounced effect on lipid profile and BP in MS elderly women.
Therefore, in front of the data presented in our study, it is possible to infer the necessity to design chronic IME protocols based on the procedures performed in the present study. Since the effects observed after short-term protocols of physical exercise have been demonstrating to be positively associate with chronic adaptations (Moreira et al., 2016), MS patients underwent to chronic IME composed by three sets of 15 min with 15 to 20 breaths/min at 30% of MIP can present important autonomic changes, increase on parasympathetic modulation and decrease on sympathetic modulation, as well as increase on inspiratory muscle strength, decreasing exponentially the cardiovascular risk in this population.
This study has some limitations that deserve comments. Certainly, an important limitation of our study is the time of intervention; however, as the vast majority of exercise protocols (i.e., aerobic and resistance exercises), understandings the “acute” effects of IME in older women with MS, may ensure better programming chronic protocols, and increase our understanding of the possible beneficial and side effects. The lack of accurate measurements of plasma lipid profile, levels of catecholamine, lactate and gases analyses are also limitations of this study. In addition, our study has been conducted only in women with MS. It is well known that gender can affect the cardiovascular response to exercise (Marongiu and Crisafulli, 2015), not allowing that our findings can be extrapolated to the general population. Finally, we evaluated cardiovascular autonomic changes through HRV, and it is known that this is not the gold standard for evaluation of autonomic function. However, several studies have pointed out the efficacy of this method as a predictor of mortality rate in different clinical populations.
In summary, seven days of IME improves respiratory muscle function and cardiac autonomic modulation in MS older women, despite the changes in BP and metabolic parameters. Therefore, we suggest that IME may be an effective nonpharmacological strategy to be added to exercise training programs in order to counteract deleterious effects of respiratory muscle function and autonomic modulation in older women with MS. However, further studies are needed to confirm the long-term effects of inspiratory muscle training, or their effects in addition to classical exercise training protocols, like aerobic, resistance or combined training.
ACKNOWLEDGMENTS
BR received financial support from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq-BPQ). The authors are also grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarships to DJF and HJCJ.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Archiza B, Simões RP, Mendes RG, Fregonezi GA, Catai AM, Borghi-Silva A. Acute effects of different inspiratory resistive loading on heart rate variability in healthy elderly patients. Braz J Phys Ther. 2013;17:401–408. doi: 10.1590/S1413-35552012005000100. [DOI] [PubMed] [Google Scholar]
- Assoumou HG, Pichot V, Barthelemy JC, Dauphinot V, Celle S, Gosse P, Kossovsky M, Gaspoz JM, Roche F. Metabolic syndrome and short-term and long-term heart rate variability in elderly free of clinical cardiovascular disease: the PROOF study. Rejuvenation Res. 2010;13:653–663. doi: 10.1089/rej.2010.1019. [DOI] [PubMed] [Google Scholar]
- Aznar-Lain S, Webster AL, Cañete S, San Juan AF, López Mojares LM, Pérez M, Lucia A, Chicharro JL. Effects of inspiratory muscle training on exercise capacity and spontaneous physical activity in elderly subjects: a randomized controlled pilot trial. Int J Sports Med. 2007;28:1025–1029. doi: 10.1055/s-2007-965077. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Porta C, Gabutti A, Spicuzza L, Sleight P. Modulatory effects of respiration. Auton Neurosci. 2001;90:47–56. doi: 10.1016/S1566-0702(01)00267-3. [DOI] [PubMed] [Google Scholar]
- Bonganha V, Libardi CA, Santos CF, De Souza GV, Conceição MS, Chacon-Mikahil MP, Madruga VA. Predictive equations overestimate the resting metabolic rate in postmenopausal women. J Nutr Health Aging. 2013;17:211–214. doi: 10.1007/s12603-012-0395-3. [DOI] [PubMed] [Google Scholar]
- Callegaro CC, Ribeiro JP, Tan CO, Taylor JA. Attenuated inspiratory muscle metaboreflex in endurance-trained individuals. Respir Physiol Neurobiol. 2011;177:24–29. doi: 10.1016/j.resp.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Jacobs DR, Jr, Sidney S, Liu K CARDIA study. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care. 2003;26:3035–3041. doi: 10.2337/diacare.26.11.3035. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Conceição MS, Bonganha V, Vechin FC, Berton RP, Lixandrão ME, Nogueira FR, de Souza GV, Chacon-Mikahil MP, Libardi CA. Sixteen weeks of resistance training can decrease the risk of metabolic syndrome in healthy postmenopausal women. Clin Interv Aging. 2013;8:1221–1228. doi: 10.2147/CIA.S44245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa AP, Ribeiro JP, Balzan FM, Mundstock L, Ferlin EL, Moraes RS. Inspiratory muscle training in type 2 diabetes with inspiratory muscle weakness. Med Sci Sports Exerc. 2011;43:1135–1141. doi: 10.1249/MSS.0b013e31820a7c12. [DOI] [PubMed] [Google Scholar]
- Davis WA, Knuiman M, Kendall P, Grange V, Davis TM Fremantle Diabetes Study. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27:752–757. doi: 10.2337/diacare.27.3.752. [DOI] [PubMed] [Google Scholar]
- De Angelis K, Senador DD, Mostarda C, Irigoyen MC, Morris M. Sympathetic overactivity precedes metabolic dysfunction in a fructose model of glucose intolerance in mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R950–957. doi: 10.1152/ajpregu.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JB, Plentz RD, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int J Cardiol. 2013;166:61–67. doi: 10.1016/j.ijcard.2011.09.069. [DOI] [PubMed] [Google Scholar]
- Goso Y, Asanoi H, Ishise H, Kameyama T, Hirai T, Nozawa T, Takashima S, Umeno K, Inoue H. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation. 2001;104:418–423. doi: 10.1161/hc2901.093111. [DOI] [PubMed] [Google Scholar]
- Guthrie JR, Taffe JR, Lehert P, Burger HG, Dennerstein L. Association between hormonal changes at menopause and the risk of a coronary event: a longitudinal study. Menopause. 2004;11:315–322. doi: 10.1097/01.gme.0000094208.15096.62. [DOI] [PubMed] [Google Scholar]
- Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr Gerontol Int. 2010;10( Suppl 1):S127–136. doi: 10.1111/j.1447-0594.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- Lindgren K, Hagelin E, Hansén N, Lind L. Baroreceptor sensitivity is impaired in elderly subjects with metabolic syndrome and insulin resistance. J Hypertens. 2006;24:143–150. doi: 10.1097/01.hjh.0000198024.91976.c2. [DOI] [PubMed] [Google Scholar]
- Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Marongiu E, Crisafulli A. Gender differences in cardiovascular functions during exercise: a brief review. Sport Sci Health. 2015;11:235–241. [Google Scholar]
- Mello PR, Guerra GM, Borile S, Rondon MU, Alves MJ, Negrão CE, Dal Lago P, Mostarda C, Irigoyen MC, Consolim-Colombo FM. Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. J Cardiopulm Rehabil Prev. 2012;32:255–261. doi: 10.1097/HCR.0b013e31825828da. [DOI] [PubMed] [Google Scholar]
- Milia R, Velluzzi F, Roberto S, Palazzolo G, Sanna I, Sainas G, Pusceddu M, Mulliri G, Loviselli A, Crisafulli A. Differences in hemodynamic response to metaboreflex activation between obese patients with metabolic syndrome and healthy subjects with obese phenotype. Am J Physiol Heart Circ Physiol. 2015;309:H779–789. doi: 10.1152/ajpheart.00250.2015. [DOI] [PubMed] [Google Scholar]
- Miller AW, Sims JJ, Canavan A, Hsu T, Ujhelyi MR. Impaired vagal reflex activity in insulin-resistant rats. J Cardiovasc Pharmacol. 1999;33:698–702. doi: 10.1097/00005344-199905000-00004. [DOI] [PubMed] [Google Scholar]
- Moreira SR, Cucato GG, Terra DF, Ritti-Dias RM. Acute blood pressure changes are related to chronic effects of resistance exercise in medicated hypertensives elderly women. Clin Physiol Funct Imaging. 2016;36:242–248. doi: 10.1111/cpf.12221. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Petrovic M, Reiter M, Zipko H, Pohl W, Wanke T. Effects of inspiratory muscle training on dynamic hyperinflation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:797–805. doi: 10.2147/COPD.S23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlani Y, Pothineni NV, Mehta JL. Metabolic syndrome: does it differ between women and men? Cardiovasc Drugs Ther. 2015;29:329–338. doi: 10.1007/s10557-015-6593-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues F, Araujo AA, Mostarda CT, Ferreira J, de Barros Silva MC, Nascimento AM, Lira FS, De Angelis K, Irigoyen MC, Rodrigues B. Autonomic changes in young smokers: acute effects of inspiratory exercise. Clin Auton Res. 2013;23:201–207. doi: 10.1007/s10286-013-0202-1. [DOI] [PubMed] [Google Scholar]
- Silva Mdos S, Martins AC, Cipriano G, Jr, Ramos LR, Lopes GS. Inspiratory training increases insulin sensitivity in elderly patients. Geriatr Gerontol Int. 2012;12:345–351. doi: 10.1111/j.1447-0594.2011.00755.x. [DOI] [PubMed] [Google Scholar]
- Tenório LH, Santos AC, Câmara Neto JB, Amaral FJ, Passos VM, Lima AM, Brasileiro-Santos Mdo S. The influence of inspiratory muscle training on diaphragmatic mobility, pulmonary function and maximum respiratory pressures in morbidly obese individuals: a pilot study. Disabil Rehabil. 2013;35:1915–1920. doi: 10.3109/09638288.2013.769635. [DOI] [PubMed] [Google Scholar]