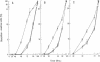
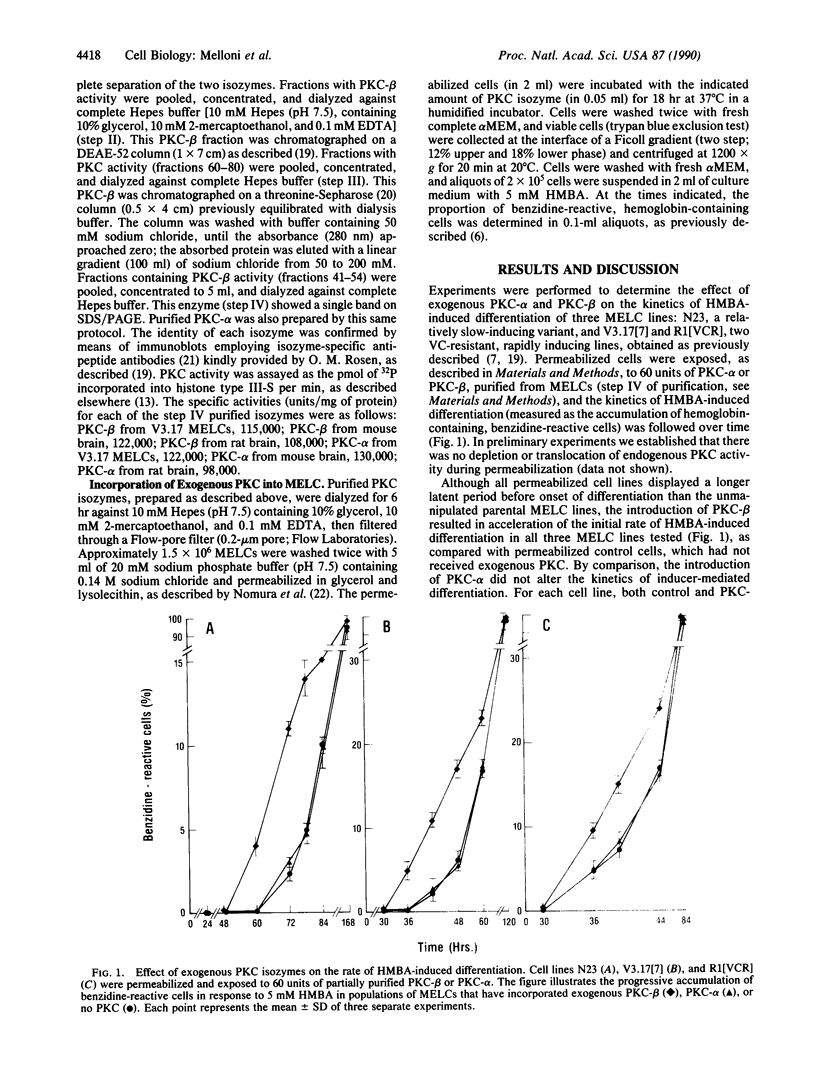
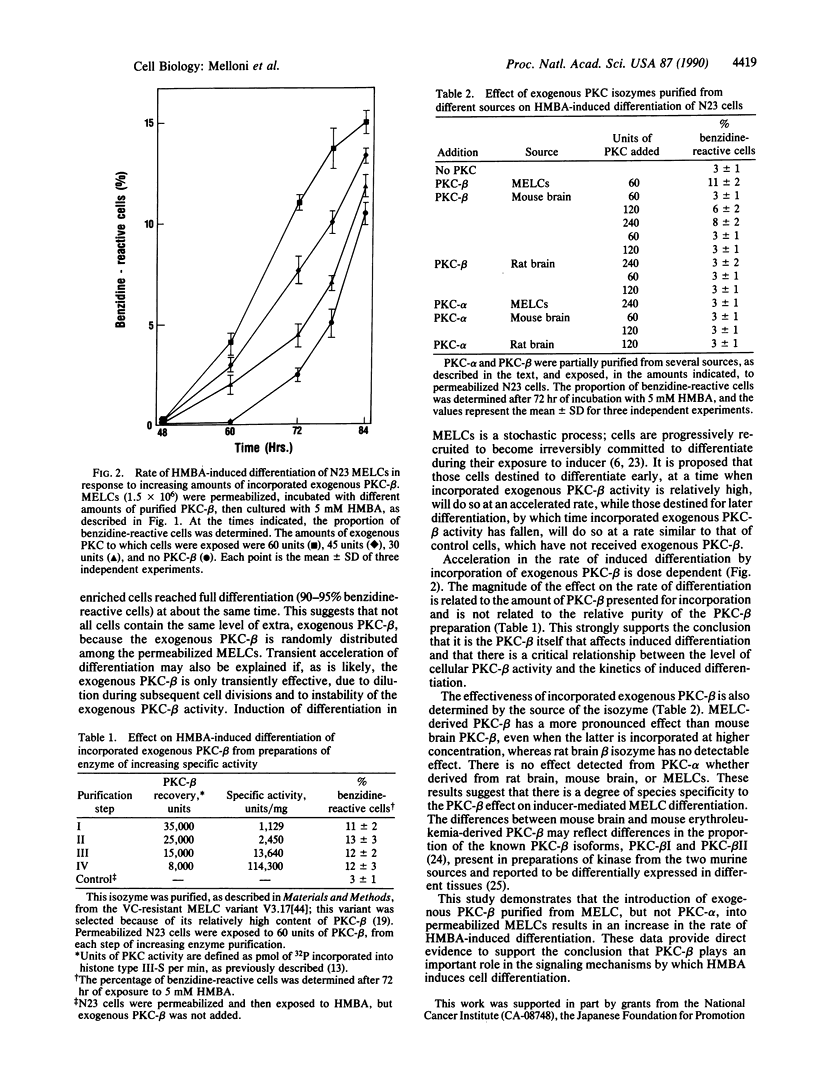
Abstract
Induction of differentiation in murine erythroleukemia cells (MELCs) involves a protein kinase C (PKC)-mediated step. Vincristine-resistant cells respond more rapidly to hybrid polar/apolar inducers than the parental cells. These vincristine-resistant MELCs contain elevated levels of the beta isozyme of PKC (PKC-beta). Exogenous homologous murine PKC-beta, incorporated into permeabilized MELCs, accelerates induced differentiation. Neither rat PKC-beta, nor mouse PKC-alpha, nor rat PKC-alpha, incorporated into permeabilized MELCs, is effective in altering the kinetics of induced differentiation. This provides direct evidence for a rate-limiting role for this PKC isozyme during N,N'-hexamethylenebisacetamide-mediated induced differentiation of a transformed cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balazovich K. J., Portnow D., Boxer L. A., Prochownik E. V. Changes in protein kinase C activity are associated with the differentiation of Friend erythroleukemia cells. Biochim Biophys Acta. 1987 Feb 18;927(2):247–255. doi: 10.1016/0167-4889(87)90141-8. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Hunt D. M., Crichley V., Mak T. W. Induction by ouabain of hemoglobin synthesis in cultured Friend erythroleukemic cells. Cell. 1976 Nov;9(3):375–381. doi: 10.1016/0092-8674(76)90082-9. [DOI] [PubMed] [Google Scholar]
- Bridges K., Levenson R., Housman D., Cantley L. Calcium regulates the commitment of murine erythroleukemia cells to terminal erythroid differentiation. J Cell Biol. 1981 Aug;90(2):542–544. doi: 10.1083/jcb.90.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Banks J., Rifkind R. A., Marks P. A. Inducer-mediated commitment of murine erythroleukemia cells to differentiation: a multistep process. Proc Natl Acad Sci U S A. 1982 Jan;79(2):471–475. doi: 10.1073/pnas.79.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Reuben R. C., Rifkind R. A., Marks P. A. Effect of hexamethylene bisacetamide on the commitment to differentiation of murine erythroleukemia cells. Cancer Res. 1977 Feb;37(2):440–444. [PubMed] [Google Scholar]
- Gazitt Y., Deitch A. D., Marks P. A., Rifkind R. A. Cell volume changes in relation to the cell cycle of differentiating erythroleukemic cells. Exp Cell Res. 1978 Dec;117(2):413–420. doi: 10.1016/0014-4827(78)90154-4. [DOI] [PubMed] [Google Scholar]
- Gazitt Y., Reuben R. C., Deitch A. D., Marks P. A., Rifkind R. A. Changes in cyclic adenosine 3':5'-monophosphate levels during induction of differentiation in murine erythroleukemia cells. Cancer Res. 1978 Nov;38(11 Pt 1):3779–3783. [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Go M., Koumoto J., Nishizuka Y. Rapid purification of protein kinase C by high performance liquid chromatography. Biochem Biophys Res Commun. 1986 Mar 13;135(2):636–643. doi: 10.1016/0006-291x(86)90040-9. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Mager D., Bernstein A. The program of Friend cell erythroid differentiation: early changes in Na+/K+ ATPase function. J Supramol Struct. 1978;8(4):431–438. doi: 10.1002/jss.400080405. [DOI] [PubMed] [Google Scholar]
- Makowske M., Ballester R., Cayre Y., Rosen O. M. Immunochemical evidence that three protein kinase C isozymes increase in abundance during HL-60 differentiation induced by dimethyl sulfoxide and retinoic acid. J Biol Chem. 1988 Mar 5;263(7):3402–3410. [PubMed] [Google Scholar]
- Marks P. A., Breslow R., Rifkind R. A., Ngo L., Singh R. Polar/apolar chemical inducers of differentiation of transformed cells: strategies to improve therapeutic potential. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6358–6362. doi: 10.1073/pnas.86.16.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Sheffery M., Rifkind R. A. Induction of transformed cells to terminal differentiation and the modulation of gene expression. Cancer Res. 1987 Feb 1;47(3):659–666. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Damiani G., Viotti P., Weich N., Rifkind R. A., Marks P. A. Vincristine-resistant erythroleukemia cell line has marked increased sensitivity to hexamethylenebisacetamide-induced differentiation. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3835–3839. doi: 10.1073/pnas.85.11.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Cakiroglu A. G., Jackson J. F., Rifkind R. A., Marks P. A. Protein kinase C activity and hexamethylenebisacetamide-induced erythroleukemia cell differentiation. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5282–5286. doi: 10.1073/pnas.84.15.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Viotti P. L., Patrone M., Marks P. A., Rifkind R. A. Differential expression of protein kinase C isozymes and erythroleukemia cell differentiation. J Biol Chem. 1989 Nov 5;264(31):18414–18418. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and prospectives of the protein kinase c family for cellular regulation. Cancer. 1989 May 15;63(10):1892–1903. doi: 10.1002/1097-0142(19890515)63:10<1892::aid-cncr2820631005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Nomura S., Kamiya T., Oishi M. A procedure to introduce protein molecules into living mammalian cells. Exp Cell Res. 1986 Apr;163(2):434–444. doi: 10.1016/0014-4827(86)90074-1. [DOI] [PubMed] [Google Scholar]
- Ramsay R. G., Ikeda K., Rifkind R. A., Marks P. A. Changes in gene expression associated with induced differentiation of erythroleukemia: protooncogenes, globin genes, and cell division. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6849–6853. doi: 10.1073/pnas.83.18.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ase K., Kikkawa U., Nishizuka Y. Mode of activation and kinetic properties of three distinct forms of protein kinase C from rat brain. J Biochem. 1988 May;103(5):759–765. doi: 10.1093/oxfordjournals.jbchem.a122343. [DOI] [PubMed] [Google Scholar]
- Todokoro K., Ikawa Y. Sequential expression of proto-oncogenes during a mouse erythroleukemia cell differentiation. Biochem Biophys Res Commun. 1986 Mar 28;135(3):1112–1118. doi: 10.1016/0006-291x(86)91043-0. [DOI] [PubMed] [Google Scholar]