Abstract
Carotid-femoral pulse wave velocity (cfPWV) is a measure of arterial stiffness associated with cardiovascular events in the general population and adults with chronic kidney disease (CKD). However, few data exist regarding cfPWV in children with CKD. We compared observed cfPWV assessed via applanation tonometry in children enrolled in the CKiD cohort study to normative data in healthy children and examined risk factors associated with elevated cfPWV. cfPWV Z-score for height/gender and age/gender was calculated from and compared to published pediatric norms. Multivariable linear regression was used to assess the relationship between cfPWV and age, gender, race, body mass index, diagnosis, urine protein-creatinine ratio, mean arterial pressure, heart rate, number of anti-hypertensive medications, uric acid, and serum LDL. Of the 95 participants with measured cfPWV, 60% were male, 19% were Black, 46% had glomerular cause of CKD, 22% had urine protein-creatinine ratio 0.5–2.0 mg/mg, 9% >2.0 mg/mg; mean age was 15.1 years, average mean arterial pressure was 80 mmHg, and median glomerular filtration rate was 63 ml/min/1.73m2. Mean cfPWV was 5.0 m/s (SD 0.8 m/s); mean cfPWV Z-score by height/gender norms was −0.1 (SD 1.1). cfPWV increased significantly with age, mean arterial pressure, and Black race in multivariable analysis; no other variables, including glomerular filtration rate, were independently associated with cfPWV. In this pediatric cohort with mild kidney dysfunction, arterial stiffness was comparable to that of normal children. Future research is needed to examine the impact of CKD progression on arterial stiffness and associated cardiovascular parameters in children.
Keywords: Vascular Stiffness, Chronic Kidney Disease, Pediatrics, Pulse Wave Velocity, Arteriosclerosis
Introduction
Arterial stiffness is associated with cardiovascular (CV) events and mortality in otherwise healthy and hypertensive adult populations.1,2 Models incorporating pulse wave velocity (PWV), a measure of central arterial stiffness, suggest that one standard deviation increment in PWV is equal to about ten years of aging.3 In adults with chronic kidney disease (CKD), arterial stiffness is increased compared to healthy adults4, is associated with CV events and mortality5, and, in many reports, is inversely related with level of kidney function.6–8 There is emerging literature describing arterial stiffness in pediatric populations9 and the relation of arterial stiffness to pediatric CKD, but this research has primarily focused on end-stage renal disease (ESRD).10–14 Recently, in a single-center study of children with pre-dialysis CKD, Sinha et al. demonstrated that PWV did not differ by level of glomerular filtration rate (GFR), nor did it vary from PWV in healthy control children. 15
Assessment of arterial stiffness in children with mild to moderate CKD is important to understand the interactions between early CKD and the CV system, potentially identifying high risk populations that could benefit from targeted intervention. Hence, the aims of this study were to measure carotid-femoral pulse wave velocity (cfPWV) via applanation tonometry in a North American cohort of children with mild to moderate CKD; to compare these values to published reference ranges in healthy children; and to examine risk factors associated with elevated cfPWV in children with CKD, particularly measured GFR.
Methods
Study Population
Participants were children with mild to moderate CKD enrolled in the Chronic Kidney Disease in Children (CKiD) study, a multi-center, prospective, observational cohort study conducted in North America. The design and methods of the CKiD study have been described elsewhere.16 Briefly, eligible participants were 1 – 16 years old at baseline, with an estimated glomerular filtration rate (eGFR) between 30 to 90 ml/min/1.73m2 via the Schwartz formula (Cohort 1) or between 45 to 90 ml/min/1.73m2 via the CKiD formula (Cohort 2). The collection of cfPWV was added to the study protocol in 2013 at a subset of sites with access to the necessary equipment and software. All subjects provided informed consent/assent according to local requirements, and the study received institutional review board approval at participating sites.
Measurements and Data Collection
Arterial stiffness was assessed by cfPWV via applanation tonometry using a SphygmoCor® device (AtCor Medical, Inc., Australia), software version 9. All operators were trained in the collection of cfPWV and were “certified” by a qualification process prior to collecting study data. The qualification process involved the submission of 3 studies each on 3 test patients satisfying quality control parameters built into the software and within-between subject reproducibility statistics (coefficient of variation less than 10%). Qualification was provided after physician review (author KECM). Similarly, all incoming study data was evaluated for satisfaction of quality control parameters, and operators submitting deficient data on two consecutive study patients were required to repeat the qualification process on test patients.
cfPWV was collected three times per participant. Prior to cfPWV, patients were placed in the supine position and rested for five minutes. Three electrocardiographic leads were attached. The right carotid artery was palpated and marked, and the distance between the carotid pulse site and the suprasternal notch was measured twice to the nearest millimeter and recorded in the software (proximal site). This procedure was repeated for the right femoral artery (distal site). For the distal site, distance between the right femoral artery and suprasternal notch was measured directly with the measuring tape against the skin as suggested by Weber et al.17 The pulse was then captured for 10 seconds at the proximal and distal sites using a Millar tonometer. If the pulse acquisition site differed from the palpated site previously marked, the distance measurements were repeated and re-entered into the software. To assist with pulse acquisition, real-time feedback via color-coded visual guidance bars built-in to the software were displayed on the laptop screen, and auto-capture of results occurred after 10 seconds of quality data were obtained.
A cfPWV Z-score normalized separately by height and gender and by age and gender was calculated using published pediatric reference data.18 Reusz et al., assessed cfPWV via applanation tonometry in over 1000 children and adolescents and provided formulas using the least mean squares (LMS) method to calculate Z-score. While the published LMS formulas were provided for age ranges 7 – 19 years, inclusive, and for height ranges 120–195 cm (males) and 115 – 180 cm (females), several of our participants were outside of these age (N=11) or height (N=4) ranges. In order to calculate Z-scores for all of our participants, we assumed that participants less than 7 were comparable to a 7 year old and that those above 19 were comparable to a 19 year old. Similarly, we assumed that males shorter than 120 cm were comparable to those with a height of 120 cm and that females taller than 180 cm were comparable to those with a height of 180 cm. To see if these assumptions impacted our results, we also calculated cfPWV Z-score excluding those outside the age or height ranges provided by Reusz et al.
Casual blood pressure (BP) was reported as the average of three measurements collected in the sitting position at 30 second intervals by trained and annually re-certified operators via auscultation using an aneroid sphygmomanometer, as previously published.19 Given that both BP and cfPWV were collected by trained and certified operators using a standard protocol, these measurements may not always have been collected by the same person or in immediate succession. Systolic BP (SBP) and Diastolic (DBP) percentiles were standardized by age, sex, and height following the “Fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents”.20
Mean arterial pressure (MAP) was determined using the SphygmoCor device during an assessment of pulse wave analysis, performed immediately before or after cfPWV, using applanation tonometry of the radial pulse. MAP is determined by measuring the area under the radial pressure waveform curve taking into account the length of the cardiac cycle calibrated by the SBP and DBP measurements. For participants lacking an available radial waveform (N=5), MAP was estimated from the casual BP using the formula (DBP + ((SBP − DBP)/3).
Heart rate (HR) was recorded by the SphygmoCor device during tonometric acquisition of the carotid pulse during cfPWV. Height and weight were collected using a calibrated stadiometer and scale, respectively, and reported as the average of two measurements. Age-sex-specific height Z-score was calculated using the 2000 Centers for Disease Control standard growth charts for U.S. children.21
GFR was measured by plasma disappearance of iohexol.22 Where a measured GFR was not available (N=4), GFR was estimated using equations developed by the CKiD study.23 Urine protein and creatinine were analyzed by standard laboratory methods from a first morning urine sample and reported as a urine protein-to-creatinine ratio (UP/C). Other laboratory measures included in the analysis were triglycerides, LDL cholesterol, HDL cholesterol, calcium, phosphate, uric acid, and serum glucose. Where a fasting serum glucose was not available (N=9), a non-fasting serum glucose was used.
Demographic information was collected at study entry. Participants of Hispanic ethnicity were categorized as “Hispanic”, otherwise, participants were categorized as “White”, “Black”, or “Other”. Length of time with CKD was collected by self-report. Specific CKD diagnosis was collected at baseline to confirm study eligibility. As done previously, participants were categorized as having a CKD diagnosis of a glomerular origin (for example, chronic glomerulonephritis or focal segmental glomerulosclerosis) or non-glomerular origin (for example, obstructive uropathy or dysplastic kidney).
Statistical Analyses
Clinical and demographic characteristics of the study participants were summarized using mean ± SD or median and interquartile range (IQR) for skewed continuous data among included and excluded participants; differences were tested using Student t test or Wilcoxon rank sum test. Categorical variables were expressed as frequencies and percentages and compared using Chi-square or Fisher exact tests, as appropriate. The main outcome, mean cfPWV, was used as a continuous variable. Univariable regression models for cfPWV were used to assess the relation between cfPWV and various demographic and clinical variables previously described in the literature as related to PWV (age and MAP) or to CKD. Along with age and gender, all covariates with a p-value <0.20 were then included in the adjusted model, except where determined to be highly collinear. The final multivariable linear regression model included age, gender, race, body mass index (BMI), diagnosis, urine protein-creatinine ratio, MAP, heart rate, number of anti-hypertensive medications, uric acid, and serum LDL. All analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC). A p-value <0.05 was considered statistically significant.
Results
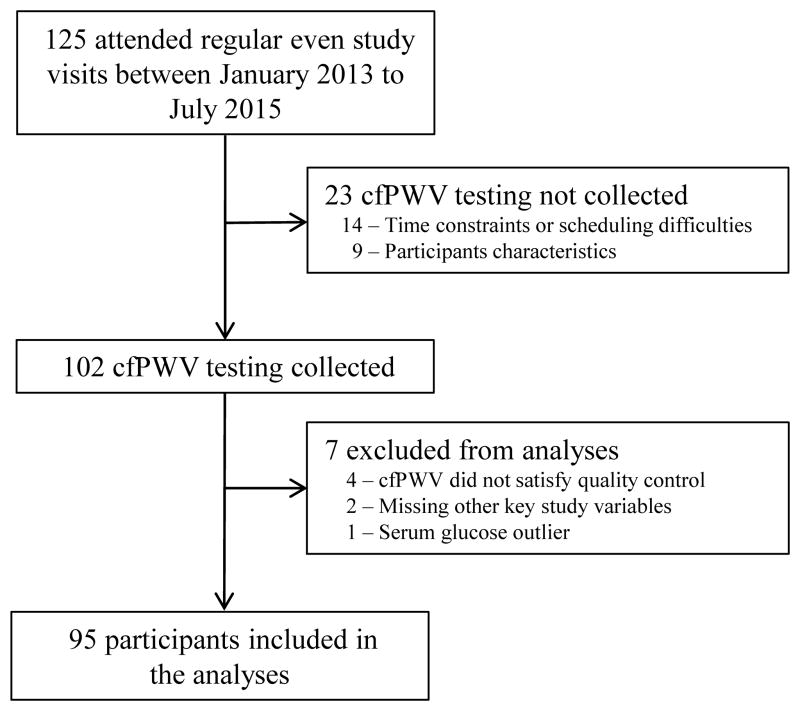
The number of eligible participants and description of excluded participants are summarized in Figure 1. Demographic and clinical characteristics of the 95 included participants and the 30 excluded participants are provided in Table 1. For included participants, the mean age was 15.1 years; 60% were male and 19% were Black. Glomerular diseases accounted for almost half of the underlying diagnoses; median GFR was 63.1 ml/min/1.73m2 (Interquartile range [IQR] 42.9, 78.5). There were 23 participants with CKD Stage 1, 29 with Stage 2, 15 with Stage 3A, 25 with Stage 3B, and 3 with Stage 4. Mean cfPWV was 5.0 m/s (SD 0.8 m/s). Mean cfPWV Z-scores compared with published reference ranges with healthy children based on height/gender and age/gender were both −0.1 (SD 1.1). Mean cfPWV Z-scores were unchanged if we included only those 84 participants within the age range (Z-score −0.10, SD 1.1) and 91 participants within the height range (Z-score −0.06, SD 1.1) of the Reusz et al. formulas.
Figure 1.
“cfPWV, Carotid-femoral pulse wave velocity”.
Table 1.
Baseline characteristics of study participants.
| Patient Characteristics | Included (N=95) | Excluded (N=30) | P |
|---|---|---|---|
| Age (years) | 15.1 ± 3.7 | 13.1 ± 4.4 | 0.01 |
| Male | 60.0 (57) | 70.0 (21) | 0.32 |
| Race/Ethnicity | 0.34 | ||
| White | 66.3 (63) | 50.0 (15) | |
| Black | 19.0 (18) | 26.7 (8) | |
| Hispanic | 8.4 (8) | 10.0 (3) | |
| Other | 6.3 (6) | 13.3 (4) | |
| Height (cm) | 162.6 [147.8, 170.4] | 153.4 [135.6, 163.3] | 0.01 |
| Height Z-score | −0.2 ± 1.2 | −0.5 ± 1.2 | 0.20 |
| BMI (kg/m2) | 20.9 [18.8, 25.1] | 18.5 [16.5, 25.5] | 0.21 |
| Waist circumference (cm) | 74.0 ± 16.0 | 75.0 ± 23.0 | 0.97 |
| Diagnosis (% glomerular) | 46.3 (44) | 26.7 (8) | 0.06 |
| GFR (ml/min/1.73m2) | 63.1 [42.9, 89.0] | 64.7 [46.9, 78.5] | 0.75 |
| GFR Stage | 0.02 | ||
| Stage 1 (≥ 90) | 24.2 (23) | 3.3 (1) | |
| Stage 2 (60 – 89) | 30.5 (29) | 60.0 (18) | |
| Stage 3A (45 – 59) | 15.8 (15) | 13.3 (4) | |
| Stage 3B (30 – 44) | 26.3 (25) | 20.0 (6) | |
| Stage 4 (15 – 29) | 3.2 (3) | 3.3 (1) | |
| Urine protein-creatinine (mg/mg)* | 0.52 | ||
| < 0.5 | 69.2 (63) | 72.4 (21) | |
| 0.5 – 2.0 | 22.0 (20) | 13.8 ( 4) | |
| > 2.0 | 8.8 ( 8) | 13.8 ( 4) | |
| Systolic blood pressure percentile | 44.1 [18.8, 68.6] | 53.6 [26.5, 85.8] | 0.21 |
| Diastolic blood pressure percentile | 57.1 [32.0, 77.7] | 53.3 [33.0, 79.8] | 0.51 |
| Mean arterial pressure (mmHg) | 80.2 ± 9.7 | 83.9 ± 9.5 | 0.07 |
| Heart rate (bpm) | 70.0 [64.0, 78.0] | - | |
| Number of anti-HTN medications* | 0.04 | ||
| 0 | 22.0 (20) | 5.0 ( 1) | |
| 1 | 60.4 (55) | 60.0 (12) | |
| 2 | 15.4 (14) | 20.0 ( 4) | |
| 3+ | 2.2 (2) | 15.0 (3) | |
| Triglycerides (mg/dL) | 90.0 [68.0, 131.0] | 97.0 [65.0, 145.0] | 0.82 |
| LDL (mg/dL) | 86.5 [72.0, 113.0] | 85.5 [70.0, 106.0] | 0.79 |
| HDL (mg/dL) | 54.5 [45.0, 66.0] | 54.0 [44.0, 62.0] | 0.40 |
| Calcium x Phosphate (mg2/dL2) | 38.3 [33.3, 42.7] | 40.6 [32.2, 45.9] | 0.23 |
| Uric acid (mg/dL) | 6.5 ± 1.6 | 6.3 ± 1.5 | 0.64 |
| Serum glucose (mg/dL) | 87.0 [81.0, 92.0] | 90.5 [87.0, 95.0] | 0.01 |
| cfPWV (m/s) | 5.0 ± 0.8 | - | |
| cfPWV Z-score by age/gender | −0.1 ± 1.1 | - | |
| cfPWV Z-score by height/gender | −0.1 ± 1.1 | - | |
| Length of time with CKD (years) | 10.6 [3.8, 15.2] | 12.2 [7.8, 15.5] | 0.22 |
Data are means ± SD, medians [25th, 75th percentiles] or n (%). BMI, body mass index; LDL, low density lipoproteins; HDL, high density lipoproteins; cfPWV, carotid-femoral pulse wave velocity.
Included N=91
The univariable analysis with cfPWV as the dependent variable is shown in Table 2. cfPWV was significantly associated with age, race, height, BMI, waist circumference, glomerular diagnosis, urine protein-creatinine ratio, SBP percentile, mean arterial pressure (MAP), and uric acid. Results from the multivariable analysis (Table 3) revealed cfPWV had significant, independent associations with age, MAP, and race.
Table 2.
Outcome cfPWV: Univariable Analysis.
| Patient Characteristics | Estimate ± SE | P-value |
|---|---|---|
| Age (years) | 0.10 ± 0.02 | < 0.0001 |
| Male | −0.16 ± 0.17 | 0.35 |
| Black | 0.47 ± 0.21 | 0.03 |
| Height (cm) | 0.02 ± 0.00 | < 0.001 |
| BMI (kg/m2) | 0.05 ± 0.01 | 0.001 |
| Waist circumference (cm) | 0.01 ± 0.01 | 0.03 |
| Glomerular diagnosis | 0.38 ± 0.17 | 0.03 |
| GFR (per 10 ml/min/1.73m2) | −0.00 ± 0.03 | 0.96 |
| Urine protein-creatinine ratio (mg/mg) | ||
| < 0.5 | Reference | Reference |
| ≥ 0.5 | 0.38 ± 0.19 | 0.05 |
| Systolic blood pressure percentile | 0.01 ± 0.00 | 0.02 |
| Diastolic blood pressure percentile | 0.00 ± 0.00 | 0.14 |
| Mean arterial pressure (mmHg) | 0.03 ± 0.01 | 0.001 |
| Heart rate (bpm) | −0.01 ± 0.01 | 0.12 |
| Number of anti-HTN medications | ||
| 0 | Reference | Reference |
| 1 | 0.34 ± 0.21 | 0.11 |
| ≥ 2 | 0.46 ± 0.28 | 0.10 |
| Triglycerides (per 10 mg/dL) | 0.01 ± 0.02 | 0.40 |
| LDL cholesterol (per 10 mg/dL) | 0.03 ± 0.02 | 0.14 |
| HDL cholesterol (per 10 mg/dL) | 0.05 ± 0.05 | 0.26 |
| Calcium x phosphate (mg2/dL2) | −0.01 ± 0.01 | 0.60 |
| Uric acid (mg/dL) | 0.12 ± 0.05 | 0.03 |
| Serum glucose (per 10 mg/dL) | 0.05 ± 0.09 | 0.57 |
| Length of time with CKD | 0.01 ± 0.01 | 0.71 |
SE, standard error; BMI, body mass index; LDL, low density lipoproteins; HDL, high density lipoproteins; cfPWV, carotid-femoral pulse wave velocity.
Table 3.
Adjusted associations between cfPWV and selected factors.
| Patient Characteristics | Estimate ± SE | P-value |
|---|---|---|
| Age (years) | 0.08 ± 0.03 | 0.004 |
| Male | 0.07 ± 0.18 | 0.68 |
| Black | 0.45 ± 0.21 | 0.03 |
| Glomerular diagnosis | 0.26 ± 0.17 | 0.14 |
| Urine protein-creatinine ratio (mg/mg) | ||
| < 0.5 | Reference | Reference |
| ≥ 0.5 | −0.06 ± 0.19 | 0.74 |
| BMI (kg/m2) | −0.00 ± 0.02 | 0.87 |
| Mean arterial pressure (mmHg) | 0.02 ± 0.01 | 0.01 |
| Heart rate (bpm) | −0.00 ± 0.01 | 0.91 |
| Number of anti-HTN medications | ||
| 0 | Reference | Reference |
| 1 | 0.10 ± 0.20 | 0.63 |
| ≥ 2 | 0.29 ± 0.27 | 0.27 |
| LDL cholesterol (per 10 mg/dL) | 0.00 ± 0.03 | 0.88 |
| Uric acid (mg/dL) | 0.05 ± 0.05 | 0.38 |
SE, standard error; BMI, body mass index; LDL, low density lipoproteins; cfPWV, carotid-femoral pulse wave velocity.
One participant had a measured cfPWV value above the third quartile plus 1.5 the interquartile range. In a subsequent analysis this point of high influence was excluded from the regression models. In this analysis, the independent associations with age and MAP persisted. The point estimate for the association with AA race was still large, but the significance of the association with race was lessened. The estimate of the association between AA race and cfPWV was modified from 0.45 ± 0.21(p=0.03) to 0.23 ± 0.19 (p=0.24) when this one point was removed from the analysis. Given that the cfPWV data collected for this one participant satisfied all quality control parameters and was a physiologically plausible value (8.2 m/s), we did not exclude it from our analyses.
Discussion
In this study we assessed central arterial stiffness via carotid-femoral pulse wave velocity (cfPWV) using applanation tonometry in a well-characterized cohort of children with mild to moderate CKD. The assessment of arterial stiffness in children with early CKD is important as arterial stiffness may lead to left ventricular hypertrophy and left ventricular dysfunction, both of which are widely reported in pediatric CKD.24,25 Our analysis demonstrated that cfPWV was independently and significantly associated with increasing age and MAP, both of which are well-known primary determinants of PWV7,26, as well as Black race, a novel finding in children with early CKD. Our study serves to confirm the findings of Sinha et al.15, namely that that the cfPWV of children with early CKD was not significantly different from healthy children nor was related to GFR, and also extends these findings to a North American cohort using applanation tonometry and measured GFR.
There are several different methods to measure arterial stiffness, the most commonly recommended is carotid-femoral PWV (cfPWV) via applanation tonometry.8 cfPWV is a simple, non-invasive assessment of functional stiffness of the central arterial system, and is the “gold-standard” due to its ability to predict future CV events and agreement with invasive measures in adults (e.g., cardiac catheterization), although comparison to direct invasive measures in children have not been performed.8,27 cfPWV can also be measured using an oscillometric device (cuff-based) instead of using applanation tonometry (transducer-based). Proponents of the oscillometric method cite that it is quicker and easier to perform and may be better tolerated by children. One study comparing different methods of collecting cfPWV in children found that tonometric assessment failed in 22% of the 156 participants, although the timing of the procedure (conducted after oscillometric assessment) may have contributed to this high failure rate.28 While our study did have missing cfPWV in 18% of eligible participants, only 7% were due to failure of tonometric acquisition (Figure 1). However, multiple reports have concluded that PWV values obtained from oscillometric and tonometric devices are not interchangeable28,29, and our group decided to utilize tonometric cfPWV due to its extensive prior use in clinical research and its endorsement as method of choice by consensus bodies.7,27
We observed that the cfPWV in our cohort was comparable to published reference ranges for healthy children standardized by height and gender and separately by age and gender. Our study population had relatively preserved kidney function, evidenced by a median GFR of 63 ml/min/1.73m2. In addition, only 9% had a urine protein-creatinine ratio greater than 2.0 mg/mg, and blood pressure was seemingly well-controlled, with 78% of participants being prescribed at least one anti-HTN medication and median SBP and DBP percentiles of 44% and 57%, respectively. Similarly, our study population had a relatively large proportion of children with glomerular cause of CKD with shorter disease duration, hence less exposure to reduced GFR, elevated BP, and other factors that may contribute to increased PWV. Given the evidence supporting elevated PWV in pediatric ESRD populations,10–14 our results are consistent with the concept that increased arterial stiffness becomes more prominent as CKD progresses toward ESRD.
Several studies conducted in adults with CKD have shown an association between increased PWV and decreased GFR.4,7 Fewer data exist on arterial stiffness in children with CKD. A very recent study by Sinha et al. compared oscillometric cfPWV in 188 children ages 2 – 18 years with CKD stages 1, 2, 3, 4, and 5 (26%, 25%, 30%, 16%, and 3%, respectively) with 38 age- and BP-matched healthy controls. Similar to our results, the authors found that cfPWV values did not differ between those with CKD and healthy controls (5.3±0.9 m/s vs. 5.3±0.8 m/s, respectively) and were not significantly related to eGFR.15 While Sinha et al. did observe differences in other measures of arterial stiffness, such as carotid augmentation index and circumferential wall stress, between CKD patients with suboptimal BP (defined as ≥75th percentile) and normotensive controls, this relationship did not persist for cfPWV.15 A study by Patange et al. found that radial augmentation index was inversely proportional to eGFR and was worse in patients with any CKD as compared to healthy controls.30 Although Patange et al. used a surrogate measure of arterial stiffness and had relatively few patients with mild to moderate CKD, these results suggest that arterial stiffness worsens with kidney disease progression in children.30 Similarly, in a study by Dursun et al., children on dialysis had higher Doppler-ultrasound aortic PWV as compared to non-dialysis CKD patients, while non-dialysis CKD patients were similar to healthy controls.31
We also found that Black race, which was significantly associated with elevated cfPWV in univariable analysis, retained significance after controlling for other factors in multivariable analysis. Several reports have suggested that arterial stiffness may be worse in Blacks compared to Whites in healthy adults and adults with CKD8,32, but there is few data regarding cfPWV in healthy Black children and, to our knowledge, whether this relationship exists in children with CKD has not been described. As the number of Black participants in our study was small, these findings will need to be explored further in other studies. Given the increased burden of CVD among Black Americans as compared to Whites33, the interaction between race and the progression of arterial stiffness and kidney disease will be important to further delineate in future research.
We did not find that the number of prescribed anti-HTN medications was significantly related to cfPWV. This may suggest that blood pressure control was generally similar across our study population regardless of the number of prescribed anti-hypertensive medications. Lastly, to further explore the impact of GFR on cfPWV, we performed an additional limited regression analysis looking at cfPWV across different CKD stages with adjustment for age, race, BMI, and MAP. Similar to our primary model, the major covariates significantly associated with cfPWV were age, Black race, and MAP, with no significant relation to CKD staging.
Our study has several potential limitations. First, our study is cross-sectional, and thus represents a snapshot of arterial stiffness. The prospective design of the CKiD study will allow us to perform longitudinal assessments of cfPWV in the cohort, which could help elucidate changes in arterial stiffness as kidney function declines or as exposure to CKD increases. Second, participants enrolled at cfPWV participating centers of the CKiD study may differ from the general population of children with CKD. However, the demographic characteristics and distribution of underlying diagnoses is similar to the typical North American pediatric ESRD population as reported by the United States Renal Data System.34 Third, despite our efforts to standardize cfPWV collection procedures and the intensive qualification process, the presence of multiple operators at different clinical sites may bias the results. Similarly, given the different certification process for the collection of BP and cfPWV possibly necessitating two different operators at a given clinical site, these separate measurements may not have been performed in immediate succession. Lastly, we measured cfPWV using a SphygmoCor device and compared these results to normative data collected using a PulsePen device.18 Although some authorities suggest that results are comparable between these two applanation tonometric devices29, we cannot exclude that there were additional problems related to comparability. Despite these limitations, our analysis is strengthened by our relatively large sample size, a diverse cohort with a mixture of glomerular and non-glomerular CKD diagnoses, an measurement of kidney function using plasma disappearance of iohexol (as opposed to GFR estimating equations), and systematically collected CV and laboratory parameters.
Perspectives
This study represents an examination of children enrolled in a national cohort with mild to moderate CKD using cfPWV, an understudied topic with implications for the treatment of childhood CKD. Our initial cross-sectional findings provide some assurance that early CKD is not characterized by substantial arterial stiffness in children. We also found in our analysis that Black race was associated with increased arterial stiffness. This should be examined in future studies. The longitudinal nature of CKiD should help us determine the effects of aging, ongoing CKD, and worsening of kidney function on arterial stiffness in children with CKD.
Novelty and Significance.
What is New?
We examined arterial stiffness using carotid-femoral pulse wave velocity by applanation tonometry in a nationally representative North American sample of children with mild to moderate chronic kidney disease (CKD) and a measured glomerular filtration rate.
What is Relevant?
We found that arterial stiffness was not significantly related to measures of kidney function in children with mild to moderate CKD, and was not increased when compared to data collected in healthy children.
Summary
Carotid-femoral pulse wave velocity was significantly associated with higher age and mean arterial pressure as well as Black race in multivariable analysis.
The impaired arterial stiffness seen in children with end stage renal disease was not evident in our sample of children with mild to moderate CKD, suggesting that these vascular changes occur later in the disease process.
Acknowledgments
Funding provided by the National Institute of Diabetes and Digestive and Kidney Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116). Partial findings presented at the ASN Kidney Week 2015 in San Diego, CA.
Sources of Funding
Funding provided by the National Institute of Diabetes and Digestive and Kidney Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01DK-082194, U01-DK-66116).
Footnotes
Conflicts of Interest/Disclosures
None.
References
- 1.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen C-H, Cruickshank JK. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 3.Khoshdel AR, Carney SL, Nair BR, Gillies A. Better management of cardiovascular diseases by pulse wave velocity: combining clinical practice with clinical research using evidence-based medicine. Clin Med Res. 2007;5:45–52. doi: 10.3121/cmr.2007.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerin AP, Pannier B, Metivier F, Marchais SJ, London GM. Assessment and significance of arterial stiffness in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:635–641. doi: 10.1097/mnh.0b013e32830dcd5c. [DOI] [PubMed] [Google Scholar]
- 5.Karras A, Haymann J-P, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–1457. doi: 10.1161/HYPERTENSIONAHA.112.197210. [DOI] [PubMed] [Google Scholar]
- 6.Briet M, Boutouyrie P, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:388–400. doi: 10.1038/ki.2012.131. [DOI] [PubMed] [Google Scholar]
- 7.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282–289. doi: 10.1038/ajh.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savant JD, Furth SL, Meyers KE. Arterial stiffness in children: Pediatric measurement and considerations. Pulse. 2014;2:69–80. doi: 10.1159/000374095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoun B, Lorton F, Wannous H, Levy B, Ulinski T. Aortic stiffness in ESRD children before and after renal transplantation. Pediatr Nephrol. 2010;25:1331–1336. doi: 10.1007/s00467-010-1509-y. [DOI] [PubMed] [Google Scholar]
- 11.Covic A, Mardare N, Gusbeth-Tatomir P, Brumaru O, Gavrilovici C, Munteanu M, Prisada O, Goldsmith DJ. Increased arterial stiffness in children on haemodialysis. Nephrol Dial Transplant. 2006;21:729–735. doi: 10.1093/ndt/gfi196. [DOI] [PubMed] [Google Scholar]
- 12.Cseprekal O, Kis E, Schaffer P, Othmane Tel H, Fekete BC, Vannay A, Szabo AJ, Remport A, Szabo A, Tulassay T, Reusz GS. Pulse wave velocity in children following renal transplantation. Nephrol Dial Transplant. 2009;24:309–315. doi: 10.1093/ndt/gfn494. [DOI] [PubMed] [Google Scholar]
- 13.Kis E, Cseprekal O, Horvath Z, Katona G, Fekete BC, Hrapka E, Szabo A, Szabo AJ, Fekete A, Reusz GS. Pulse wave velocity in end-stage renal disease: influence of age and body dimensions. Pediatr Res. 2008;63:95–98. doi: 10.1203/PDR.0b013e31815b47ff. [DOI] [PubMed] [Google Scholar]
- 14.Tawadrous H, Kamran H, Salciccioli L, Schoeneman MJ, Lazar J. Evaluation of arterial structure and function in pediatric patients with end-stage renal disease on dialysis and after renal transplantation. Pediatr Transplant. 2012;16:480–485. doi: 10.1111/j.1399-3046.2012.01721.x. [DOI] [PubMed] [Google Scholar]
- 15.Sinha MD, Keehn L, Milne L, Sofocleous P, Chowienczyk PJ. Decreased arterial elasticity in children with nondialysis chronic kidney disease is related to blood pressure and not to glomerular filtration rate. Hypertension. 2015;66:809–815. doi: 10.1161/HYPERTENSIONAHA.115.05516. [DOI] [PubMed] [Google Scholar]
- 16.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber T, Ammer M, Rammer M, Adji A, O’Rourke MF, Wassertheurer S, Rosenkranz S, Eber B. Noninvasive determination of carotid–femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27:1624–1630. doi: 10.1097/HJH.0b013e32832cb04e. [DOI] [PubMed] [Google Scholar]
- 18.Reusz GS, Cseprekal O, Temmar M, Kis É, Cherif AB, Thaleb A, Fekete A, Szabó AJ, Benetos A, Salvi P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension. 2010;56:217–224. doi: 10.1161/HYPERTENSIONAHA.110.152686. [DOI] [PubMed] [Google Scholar]
- 19.Flynn J, Mitsnefes M, Pierce C, Cole S, Parekh R, Furth S, Warady B. Chronic Kidney Disease in Children Study Group: Blood pressure in children with chronic kidney disease: A report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: Methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 22.Schwartz GJ, Abraham AG, Furth SL, Warady BA, Munoz A. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77:65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matteucci MC, Wühl E, Picca S, Mastrostefano A, Rinelli G, Romano C, Rizzoni G, Mehls O, de Simone G, Schaefer F. Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol. 2006;17:218–226. doi: 10.1681/ASN.2005030276. [DOI] [PubMed] [Google Scholar]
- 25.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137–144. doi: 10.1681/ASN.2009060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Rourke MF, Mancia G. Arterial stiffness. J Hypertens. 1999;17:1–4. doi: 10.1097/00004872-199917010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 28.Keehn L, Milne L, McNeill K, Chowienczyk P, Sinha MD. Measurement of pulse wave velocity in children: comparison of volumetric and tonometric sensors, brachial-femoral and carotid-femoral pathways. J Hypertens. 2014;32:1464–1469. doi: 10.1097/HJH.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kis E, Cseprekál O, Kerti A, Salvi P, Benetos A, Tisler A, Szabó A, Tulassay T, Reusz GS. Measurement of pulse wave velocity in children and young adults: a comparative study using three different devices. Hypertens Res. 2011;34:1197–1202. doi: 10.1038/hr.2011.103. [DOI] [PubMed] [Google Scholar]
- 30.Patange AR, Valentini RP, Du W, Pettersen MD. Vitamin D deficiency and arterial wall stiffness in children with chronic kidney disease. Pediatr Cardiol. 2012;33:122–128. doi: 10.1007/s00246-011-0101-y. [DOI] [PubMed] [Google Scholar]
- 31.Dursun I, Poyrazoglu HM, Gunduz Z, Ulger H, Yykylmaz A, Dusunsel R, Patyroglu T, Gurgoze M. The relationship between circulating endothelial microparticles and arterial stiffness and atherosclerosis in children with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2511–2518. doi: 10.1093/ndt/gfp066. [DOI] [PubMed] [Google Scholar]
- 32.Morris AA, Patel RS, Binongo JNG, Poole J, al Mheid I, Ahmed Y, Stoyanova N, Vaccarino V, Din-Dzietham R, Gibbons GH. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2:e002154. doi: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ. Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 34.United States Renal Data System. 2015 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]