Abstract
Background
Effective symptom palliation can be achieved with low-dose palliative thoracic radiotherapy. In several studies, median survival was not improved with higher doses of radiation. More controversy exists regarding the impact of higher doses on 1- and 2-year survival rates. Therefore, a comparison of survival outcomes after radiotherapy with different biologically equivalent doses (equivalent dose in 2-Gy fractions, EQD2) was performed.
Methods
This was a retrospective single-institution study of 232 patients with small or non-small cell lung cancer. Most commonly 2 fractions of 8.5 Gy were prescribed (34%), followed by 10 fractions of 3 Gy or equivalent regimens (30%, EQD2 circa 33 Gy). The highest EQD2 consisted of 45 Gy. Intention-to-treat analyses were performed.
Results
Survival was significantly shorter with regimens of intended EQD2 < 33 Gy, e.g., 2 fractions of 8.5 Gy (median 2.5 months compared to 5.0 and 7.5 months with EQD2 of circa 33 and 45 Gy, respectively). The 2-year survival rates were 0%, 7% and 11%, respectively. In 128 prognostically favorable patients, median survival was comparable for the three different dose levels (6 - 8.3 months). The 2-year survival rates were 0%, 10%, and 13%, respectively (not statistically significant).
Conclusion
Although most of the observed survival differences diminished after exclusion of poor prognosis patients with reduced performance status and/or progressive extrathoracic disease, a slight increase in 2-year survival rates with higher EQD2 cannot be excluded. Because of relatively small improvements, a confirmatory randomized trial in this subgroup would have to include a large number of patients.
Keywords: Biologically equivalent dose, Non-small cell lung cancer, Palliative radiotherapy, Prognostic factors, Small cell lung cancer
Introduction
Palliative external beam thoracic radiotherapy is an integral component of treatment algorithms for patients with incurable lung cancer [1]. Its ability to improve local symptoms even after low doses is well known [2]. Several generations of clinical studies have tried to identify suitable fractionation regimens. A wide range of strategies have been explored, including conventional once daily fractionation, accelerated twice daily fractionation, moderate hypofractionation and extreme hypofractionation [3-6]. These strategies differ with regard to biological effectiveness (equivalent dose in 2-Gy fractions, EQD2), side effects, patient convenience and resource utilization. Illustrative examples include 2 fractions of 8.5 Gy, 10 - 13 fractions of 3 Gy, 15 fractions of 2.8 - 3 Gy and 20 fractions of 2.5 Gy. Patients with adverse prognostic features such as large disease burden, which result in short survival, should not spend most of their remaining life time on active therapy [7, 8]. However, a subgroup of patients referred for palliative radiotherapy consists of those unsuitable for state-of-the-art curative regimens, but nevertheless with limited disease extent and a certain probability of survival beyond 2 years [9, 10]. Undertreatment might compromise survival in such cases, because low radiation doses likely translate into insufficient local control [11]. In patients unsuitable for curative first-line approaches, effective salvage strategies for local failures cannot be offered either. The purpose of our study was to evaluate the probability of survival beyond 2 years in patients treated with different fractionation regimens, hypothesizing that regimens with low EQD2 are unlikely to result in extended survival.
Patients and Methods
We performed a retrospective analysis of palliative thoracic radiotherapy covering the years 2007 - 2016. Only patients with histologically confirmed lung cancer (small cell (SCLC) or non-small cell (NSCLC)) irradiated at Nordland Hospital Bodo were included. Patients who received thoracic reirradiation after previous curative or palliative radiotherapy were excluded. The same holds true for patients with extensive disease SCLC who received consolidation radiotherapy together with prophylactic cranial irradiation after response to systemic platinum-based chemotherapy. Patients who failed to complete the prescribed number of fractions were included (intention to treat, i.e. a patient who received 5 of 10 planned fractions of 3 Gy was assigned to the 30 Gy in 10 fractions of 3 Gy group). All systemic and local cancer treatment was based on national guidelines developed by the Norwegian Lung Cancer Group (www.nlcg.no) and discussed by a multidisciplinary tumor board which meets on a weekly basis. Choice of fractionation regimen was at the discretion of the treating clinical oncologist.
Statistical analysis
Patients were selected from the hospital’s electronic patient record system and the IBM SPSS 23 software package (IBM SPSS Statistics, Somers, NY, USA) was employed for the database and statistical analyses. The Kaplan-Meier method was used to analyze overall survival and different groups were compared with the log-rank test. Multivariate analysis was performed using the forward conditional Cox proportional hazards model. Twelve patients were alive at the time of analysis and their median follow-up was 11 months (range 7 - 88). As a retrospective quality of care analysis, no approval from the Regional Committee for Medical and Health Research Ethics (REK Nord) was necessary. Similarly no approval from the Norwegian Social Science Database (NSD) had to be obtained.
Results
Overall 232 patients with a median age of 71 years (range 41 - 89) fulfilled the inclusion and exclusion criteria. Eleven percent had a diagnosis of SCLC. Most commonly 2 fractions of 8.5 Gy were prescribed (34%), followed by 10 fractions of 3 Gy (30%), as shown in Table 1. Failure to complete radiotherapy occurred in 11% of the patients. Actuarial median survival was 4.3 months. Univariate analyses of the parameters shown in Table 1 demonstrated that performance status, N and M stage, serum hemoglobin, lactate dehydrogenase, progressive extrathoracic disease, pleural effusion, and fractionation were significantly associated with overall survival (all P < 0.05). Median survival was 2.5 months after 2 fractions of 8.5 Gy, 5.0 months after 10 fractions of 3 Gy (or other regimens with comparable EQD2 of approximately 33 Gy, calculated with α/β value 10 Gy) and 7.5 months after 15 fractions of 2.8 Gy (or other regimens with EQD2 ≥ 34 Gy) (Fig. 1). The multivariate Cox regression analysis included all eight parameters with significant P-value < 0.05 in univariate log-rank tests. Four of these remained independently associated with survival: performance status, lactate dehydrogenase, progressive extrathoracic disease, and fractionation (all P < 0.001).
Table 1. Baseline Parameters in 232 Patients.
| Parameter | Number of patients | Percentage |
|---|---|---|
| Sex | ||
| Male | 140 | 60 |
| Female | 92 | 40 |
| Age | ||
| ≤ 69 years | 105 | 45 |
| ≥ 70 years | 127 | 55 |
| ECOG performance score | ||
| 0 - 1 | 76 | 33 |
| 2 | 89 | 38 |
| 3 - 4 | 67 | 29 |
| Histology | ||
| Small cell lung cancer | 26 | 11 |
| Non-small cell lung cancer | 206 | 89 |
| Smoking history | ||
| No | 15 | 6 |
| Yes | 199 | 86 |
| Unknown | 18 | 8 |
| Timing of radiotherapy | ||
| Upfront (within 3 months) | 110 | 47 |
| Delayed (> 3 months from diagnosis) | 122 | 53 |
| Fractionation | ||
| 8.5 Gy × 2 | 79 (eight incomplete) | 34 |
| 3 Gy × 10 (or equivalent) | 69 (nine incomplete) | 30 |
| 2.8 Gy × 15 (or equivalent) | 68 (four incomplete) | 29 |
| 4 Gy × 5 - 6 | 16 (four incomplete) | 7 |
| Serum hemoglobin | ||
| Low | 126 | 54 |
| Normal | 98 (eight unknown) | 42 |
| Serum lacate dehydrogenase | ||
| High | 53 | 23 |
| Normal | 108 (71 unknown) | 47 |
| N stagea | ||
| N0-N1 | 45 | 19 |
| N2-N3 | 187 | 81 |
| M stagea | ||
| M0 | 91 | 39 |
| M1 | 141 | 61 |
| Pleural effusion | ||
| Absent | 177 | 76 |
| Present | 53 (two unknown) | 23 |
| Previous systemic therapy | ||
| No | 131 | 56 |
| Yes | 99 (two unknown) | 43 |
| Extrathoracic disease status | ||
| Absent/stable | 163 | 70 |
| Progression | 67 (two unknown) | 29 |
aSeventh edition of the TNM classification (based on imaging).
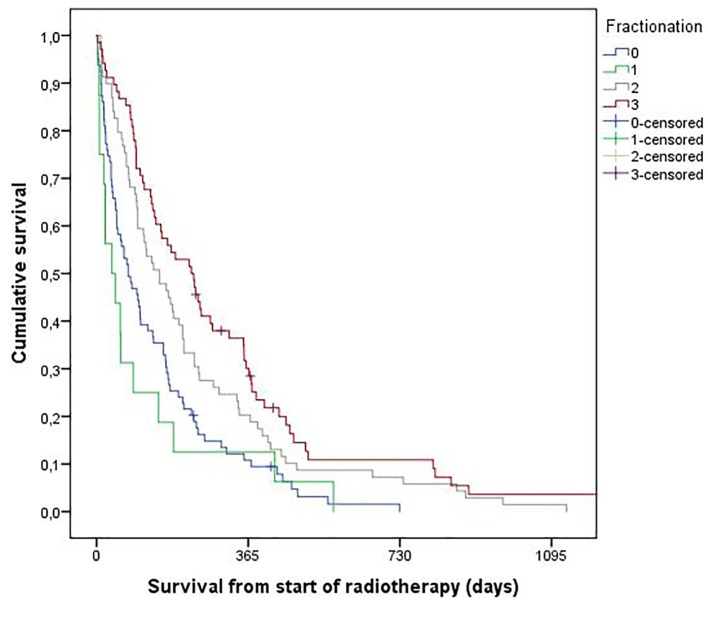
Figure 1.
Overall survival (Kaplan-Meier method, intention to treat analysis). Median survival was 2.5 months in patients treated with 2 fractions of 8.5 Gy (group 0). The corresponding figures were 2.2 months (20 - 24 Gy, group 1), 5.0 months (30 Gy, group 2) and 7.5 months (> 30 Gy, group 3), respectively (P = 0.0001, pooled over strata). The 2-year survival rates were 0%, 0%, 7% and 11%, respectively. For individual comparisons, significance level was 0.34 (0 vs. 1), 0.085 (2 vs. 3), 0.009 (0 vs. 2), 0.009 (1 vs. 2), 0.001 (1 vs. 3), and 0.0001 (0 vs. 3).
Since radiotherapy with high EQD2 might achieve the best results in prognostically favorable patients, we performed a separate analysis of patients with performance status 0 - 2 and absent or stable extrathoracic disease (n = 128). Only three of these patients were treated with 4-Gy fractions (20 - 24 Gy), therefore we pooled this small group with the 33 patients who received 2 fractions of 8.5 Gy. Their median survival was 6.0 months, exactly the same as in the group treated with 10 fractions of 3 Gy or comparable EQD2. The 50 patients treated with higher EQD2 had a median survival of 8.3 months. The 2-year survival rates were 0%, 10%, and 13%. These differences were not statistically significant (P = 0.17 pooled over all three strata; P > 0.05 for all individual comparisons; Kaplan-Meier curves not shown).
Discussion
The purpose of this study was to analyze the impact of fractionation regimens with different EQD2 on survival in patients with lung cancer who received palliative thoracic radiotherapy. Previous analyses suggested that symptom palliation could be achieved with low doses of radiation and that efficacy was not improved with higher doses [2, 3, 12]. Most studies reported no impact of EQD2 or fractionation regimen on median survival. However, there was uncertainty about 1- and 2-year survival rates, which appeared better when higher radiation doses were prescribed, at least in patients with better baseline prognostic features [1, 13]. Recently, Janssen et al reported that regimens with EQD2 of 47 - 52 Gy resulted in 2-year survival rates of 20%, whereas lower doses (31 - 46 Gy) resulted in approximately 15% (estimated from the published Kaplan-Meier graphs) [9]. In multivariate analysis, EQD2 of 47 - 52 Gy, completion of planned radiotherapy, and lower stage were positively associated with survival. In our study, the maximum radiation dose was 42 Gy in 15 fractions of 2.8 Gy without concomitant chemotherapy (EQD2 45 Gy). In the subgroup with the highest biologically equivalent radiation doses, 2-year survival was 11%. We found that low-dose radiation (2 fractions of 8.5 Gy or 5 - 6 fractions of 4 Gy) was associated with significantly inferior overall survival, also in multivariate analysis where performance status, stage and a surrogate marker of overall cancer burden (lactate dehydrogenase) were included. None of the patients treated with these regimens survived beyond 2 years. After exclusion of poor prognosis patients (performance status > 2, progressive extrathoracic disease), differences in median survival diminished. However, 2-year survival was still numerically different (P = 0.17). With 128 eligible patients, these analyses had limited statistical power. A larger prospective randomized trial, also from Norway, reported comparable 2-year survival rates of 8% (2 fractions of 8.5 Gy), 13% (42 Gy in 15 fractions of 2.8 Gy, EQD2 45 Gy) and 10% (50 Gy in 25 fractions of 2 Gy) [6]. In this study, no significant difference was seen with regard to median survival either.
In contrast, a Dutch randomized study found significantly improved survival after 10 fractions of 3 Gy compared to 2 fractions of 8 Gy (EQD2 33 vs. 24 Gy) (P = 0.03) [13]. The researchers randomized 303 patients with stage III/IV NSCLC. One-year survival was 20% vs. 11%. The 2-year rates were estimated from the published Kaplan-Meier graphs and are shown in Table 2 [2, 6, 9, 10, 13-17], together with other results. The survival difference was driven by patients with performance status 0 - 1, all of whom had stage IV disease. Patients with stage III NSCLC were only eligible for this study if they had reduced performance status. Thus, heterogeneous impact of EQD2 on survival was observed in two different randomized studies. The next paragraphs summarize results from recent retrospective analyses.
Table 2. Comparison of Different Results From the Literature of the Last 15 Years [2, 6, 9, 10, 13-17].
| Authors | Equivalent dose EQD2 | Percentage of 2-year survival |
|---|---|---|
| Kramer et al* [13] | 24 | 3 |
| Present study | 26 | 0 |
| Sundstrom et al* [6] | 26 | 8 |
| Schroder et al [14] | 31 | 2 |
| Kramer et al* [13] | 33 | 7 |
| Present study | 33 | 7 |
| Nawrocki et al* [15] | 33 | 6 |
| Erridge et al* [2] | 33 | 8 |
| Jeremic et al* [16] | 42 | 9 |
| Present study | 45 | 11 |
| Sundstrom et al* [6] | 45 | 13 |
| Sundstrom et al* [6] | 50 | 10 |
| Schroder et al [14] | 52 | 5 |
| Janssen et al [9] | 31 - 46 | 15 |
| Janssen et al [9] | 47 - 52 | 20 |
| Van Oorschot et al [10] | 42 - 49 | 10 |
| Jeremic et al* [16] | 17 or 24 (+platinum-based chemo) | 12 (no stage IV in this study) |
| Nawrocki et al* [15] | 33 (+cisplatin/vinorelbine) | 24 (no stage IV in this study) |
| Strom et al* [17] | 45 (+carboplatin/vinorelbine) | 28 (no stage IV in this study) |
*Randomized study.
Van Oorschot et al reported on 120 NSCLC patients treated with 13 - 15 fractions of 3 Gy [10]. The median survival of all patients was 5.8 months and the 2-year rate was 10%. Those with non-metastatic disease survived significantly longer than patients with metastatic disease (median 11.7 months vs. 4.7 months) and 18.6% of non-metastatic patients survived longer than 2 years. In the multivariate analysis, good general condition, non-metastatic disease, and a stable or improved general condition at the end of radiotherapy were significant. Patients with unfavorable stage III disease, e.g. tumors larger than 8 cm or performance status 2, treated with 15 fractions of 2.8 Gy and up to 4 cycles of carboplatin/vinorelbine in a randomized Norwegian trial had a median survival of 12.6 months and 2-year survival rate of 28% (Table 2) [17].
Schroder et al compared two standard regimens used at their institution for palliation of SCLC and NSCLC, 5 fractions of 5 Gy (EQD2 31 Gy, 126 patients) and 20 fractions of 2.5 Gy (EQD2 52 Gy, 81 patients) [14]. No significant survival difference was observed (median 4.8 vs. 5.3 months, 2-year rates of 2% and 5%, respectively). A subgroup analysis of patients with performance status 0 - 2 revealed no significant improvement of survival with higher radiation dose either. Possibly, the relatively small survival differences beyond 2 years observed in some of the retrospective studies and our inter-study comparison can be explained by confounding factors that persisted despite multivariate analyses. Differences in size of the gross tumor volume, number of involved nodal levels and difficulty in adhering to normal tissue dose constraints might have been reasons to prescribe lower doses of radiation. In addition, development of distant metastases is a competing cause of death, especially in patients for whom curative treatment was impossible because of disease characteristics rather than comorbidity. Mutation status (EGFR, ALK, etc.) might also contribute to confounding results, because increased survival can be caused by administration of targeted systemic therapy [18-20]. A drawback of our study is the lack of data on utilization of systemic therapy after thoracic irradiation. Without systemic therapy, uncontrolled extrathoracic distant metastases limit survival. Comparable to previous analyses, comorbidity assessment was not included. If patients with short predicted survival because of severe comorbidity receive short-course radiotherapy without systemic treatment, survival will inevitably be short, either as a result of comorbidity or uncontrolled lung cancer. Another self fulfilling prophecy worth mentioning is the inverse relationship between target volume and radiation dose. Very large target volumes cannot be safely irradiated to higher total doses. Therefore, limited doses will result in poor local control and earlier death. Target volume size was not evaluated in our study, comparable to other publications reviewed here.
Overall, our own results and the literature displayed in Table 2 suggest that regimens with higher EQD2 might be associated with relatively small, if any, improvements of 2-year survival probability. It is interesting to note that 2-year survival was < 10% in all studies where radiotherapy with EQD2 ≤ 33 Gy was administered. In contrast, survival rates ≥ 10% were reported in many studies where EQD2 was higher. The majority of unselected patients have limited survival, regardless of EQD2. A confirmatory randomized superiority trial in the subgroup likely to experience better survival would have to include a large number of patients. Obligatory positron emission tomography staging as part of the study requirements would be advantageous. For the majority of the published studies discussed here, utilization of this diagnostic modality has not been specified. According to a recent randomized study in NSCLC patients with oligometastatic disease without progression after first-line systemic treatment, more aggressive local treatment might prolong survival [21]. However, local consolidative therapy consisted of (chemo)radiotherapy or resection of all lesions with or without subsequent maintenance treatment. It is therefore tempting to argue that established curative approaches are needed to ensure optimal local control. Radiotherapy with EQD2 of less than 54 Gy, as evaluated in the present study and those included in Table 2, provides long-term local control in a minority of patients only.
Conclusion
Palliative thoracic radiotherapy should be an integral part of multimodal, interdisciplinary management of patients with incurable lung cancer. It is important to choose wisely from the large number of available fractionation regimens in order to prolong survival in the few patients where this is achievable, and avoid overtreatment when pure symptom palliation is the only realistic goal of treatment.
Disclosures
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Conflicts of interest do not exist.
References
- 1.Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, Cheung P. et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26(24):4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 2.Erridge SC, Gaze MN, Price A, Kelly CG, Kerr GR, Cull A, MacDougall RH. et al. Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 2005;17(1):61–67. doi: 10.1016/j.clon.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Stevens R, Macbeth F, Toy E, Coles B, Lester JF. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev. 2015;1:CD002143. doi: 10.1002/14651858.cd002143.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma JT, Han CB, Zheng JH, Guo QY. Response to "A meta-analysis comparing higher and lower dose radiotherapy for palliation in locally advanced lung cancer". Cancer Sci. 2015;106(6):783. doi: 10.1111/cas.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestle U, Nieder C, Walter K, Abel U, Ukena D, Sybrecht GW, Schnabel K. A palliative accelerated irradiation regimen for advanced non-small-cell lung cancer vs. conventionally fractionated 60 GY: results of a randomized equivalence study. Int J Radiat Oncol Biol Phys. 2000;48(1):95–103. doi: 10.1016/S0360-3016(00)00607-6. [DOI] [PubMed] [Google Scholar]
- 6.Sundstrom S, Bremnes R, Aasebo U, Aamdal S, Hatlevoll R, Brunsvig P, Johannessen DC. et al. Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol. 2004;22(5):801–810. doi: 10.1200/JCO.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 7.Nieder C, Norum J. Palliative radiotherapy in patients with metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(1):51–53. doi: 10.3978/j.issn.2224-5820.2013.01.10. [DOI] [PubMed] [Google Scholar]
- 8.Koshy M, Malik R, Mahmood U, Husain Z, Weichselbaum RR, Sher DJ. Prevalence and Predictors of Inappropriate Delivery of Palliative Thoracic Radiotherapy for Metastatic Lung Cancer. J Natl Cancer Inst. 2015;107(12):djv278. doi: 10.1093/jnci/djv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen S, Kaesmann L, Schild SE, Rades D. Impact of the Radiation Dose and Completion of Palliative Radiotherapy on Survival in Patients Treated for Locally Advanced Lung Cancer. Anticancer Res. 2016;36(4):1825–1828. [PubMed] [Google Scholar]
- 10.van Oorschot B, Assenbrunner B, Schuler M, Beckmann G, Flentje M. Survival and prognostic factors after moderately hypofractionated palliative thoracic radiotherapy for non-small cell lung cancer. Strahlenther Onkol. 2014;190(3):270–275. doi: 10.1007/s00066-013-0507-y. [DOI] [PubMed] [Google Scholar]
- 11.Willner J, Baier K, Caragiani E, Tschammler A, Flentje M. Dose, volume, and tumor control prediction in primary radiotherapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52(2):382–389. doi: 10.1016/S0360-3016(01)01823-5. [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues G, Macbeth F, Burmeister B, Kelly KL, Bezjak A, Langer C, Hahn C. et al. International practice survey on palliative lung radiotherapy: third international consensus workshop on palliative radiotherapy and symptom control. Clin Lung Cancer. 2012;13(3):225–235. doi: 10.1016/j.cllc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Kramer GW, Wanders SL, Noordijk EM, Vonk EJ, van Houwelingen HC, van den Hout WB, Geskus RB. et al. Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol. 2005;23(13):2962–2970. doi: 10.1200/JCO.2005.01.685. [DOI] [PubMed] [Google Scholar]
- 14.Schroder C, Ivo M, Buchali A. Does high-dose radiotherapy benefit palliative lung cancer patients?: An intradepartmental comparison of two dose regimens. Strahlenther Onkol. 2013;189(9):771–776. doi: 10.1007/s00066-013-0360-z. [DOI] [PubMed] [Google Scholar]
- 15.Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, Kowalski D, Rucinska M, Dziadziuszko R, Sowa A. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol. 2010;5(8):1255–1262. doi: 10.1097/JTO.0b013e3181e15d33. [DOI] [PubMed] [Google Scholar]
- 16.Jeremic B, Fidarova E, Sharma V, Faheem M, Ameira AA, Nasr Ben Ammar C, Frobe A. et al. The International Atomic Energy Agency (IAEA) randomized trial of palliative treatment of incurable locally advanced non small cell lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT) in limited resource setting. Radiother Oncol. 2015;116(1):21–26. doi: 10.1016/j.radonc.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Strom HH, Bremnes RM, Sundstrom SH, Helbekkmo N, Flotten O, Aasebo U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467–1475. doi: 10.1038/bjc.2013.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnio S, Novello S, Mele T, Levra MG, Scagliotti GV. Extending survival of stage IV non-small cell lung cancer. Semin Oncol. 2014;41(1):69–92. doi: 10.1053/j.seminoncol.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch FR, Suda K, Wiens J, Bunn PA Jr. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet. 2016;388(10048):1012–1024. doi: 10.1016/S0140-6736(16)31473-8. [DOI] [PubMed] [Google Scholar]
- 20.Giuliani J, Martelli S, Remo A, Bonetti A. Primary TKI resistance in advanced non-small cell lung cancer with EGFR mutation: an open question. Tumori. 2015;101(4):e115–117. doi: 10.5301/tj.5000317. [DOI] [PubMed] [Google Scholar]
- 21.Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, Doebele RC. et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]