A new study explains Pseudomonas aeruginosa’s strong resistance to osmotic down-shock.
Abstract
A new study explains Pseudomonas aeruginosa’s strong resistance to osmotic down-shock.
Water is the stuff of life, but cells must carefully manage their water content when environmental changes alter the osmotic pressure driving water into or out of the cell. In their paper published this month in JGP, Çetiner et al. compare the resilience of two bacteria, Escherichia coli and Pseudomonas aeruginosa, to osmotic shifts (1).
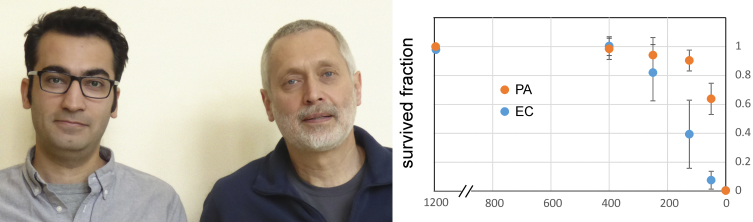
First author Uğur Çetiner (left) and colleagues working in the laboratory of Sergei Sukharev (right) explain the comparative robustness of P. aeruginosa (PA) and E. coli (EC) to osmotic down-shocks. Photo courtesy of the authors.
The sudden addition of water to the environment, such as during a rainstorm, causes an osmotic down-shock that drives water into a cell and threatens to burst it. To guard against water loss in high-osmolarity environments and to cope with down-shock threats, bacteria stockpile small osmolytes that can be released to reduce the tendency of water to enter the cell. The gut bacterium E. coli releases osmolytes through mechanosensitive channels (MSCs), which open after water flows into the cell and stretches the cell membrane. MSCs fall into two groups: MscS, which open at low to intermediate levels of membrane tension to pass modest amounts of osmolytes (2); and MscL, large-conductance channels that open at higher tension levels (3). Mutated E. coli lacking both channel types are extremely fragile to osmotic down-shocks (2).
“The peptidoglycan layer around the bacterial cells, the cell wall, is totally insufficient to provide osmotic protection. The channels are much more important; they extend the bacterium’s range of osmotic tolerance approximately three times,” observes Sergei Sukharev, Professor of Biology at the University of Maryland.
Sukharev wondered whether other types of bacteria handle osmotic shocks similarly to E. coli. Led by graduate student Uğur Çetiner, Sukharev’s group investigated the osmotic tolerance of P. aeruginosa, a soil bacterium that acts as an opportunistic pathogen in people. The researchers found that P. aeruginosa can survive larger osmotic shocks than E. coli.
What explains P. aeruginosa’s relative ruggedness? Çetiner et al. theorized that the bacteria differ in their rate of osmolyte release, so they perfected an optical technique to measure this process.
“When osmolytes are released from the cell, the refractive index inside drops,” causing a decline in light scattering by bacteria in solution, notes Sukharev. These experiments showed that P. aeruginosa releases osmolytes more rapidly than E.coli and that this response only saturates at larger down-shocks.
“The cell wall is totally insufficient to provide osmotic protection.”
These differences could be explained by differential composition of cell membrane MSCs in the two bacteria. Patch-clamp recordings showed that P. aeruginosa has both MscS-type and MscL-type channels, but has a greater membrane density of MscL-type channels than does E. coli. Furthermore, comparison of genetic databases predicted that P. aeruginosa possesses two MscS-type and one MscL-type channel. To investigate the behavior of these channels, the researchers cloned them and expressed them in an E. coli strain lacking its own MSCs. The P. aeruginosa variants behaved similarly to their E. coli counterparts, although the MscL channel had a lower conductance than the E. coli version, and the P. aeruginosa MscS channels were more likely than their E. coli counterparts to inactivate after prolonged moderate membrane tension.
“This is an important termination step,” explains Sukharev, because although MscL channels can effect strong osmolyte release, they do not undergo inactivation. Therefore, MscS channels limit osmolyte loss by keeping membrane tension below MscL’s activation threshold, then inactivating to seal the membrane. Amino acid sequence alignments of P. aeruginosa MSCs, based on earlier studies of channel structures (4–6), supported this conclusion.
Compared with E. coli, the different characteristics of P. aeruginosa MSCs, combined with its smaller size, rounder shape, and a cell membrane that’s relatively impermeable to water, may explain its heightened resiliency to osmotic shock. This comparative study therefore opens a window to understanding bacterial strategies for managing osmotic down-shocks. Now, Sukharev’s group is working to characterize the osmotic adaptations of other pathogenic bacteria and to determine what osmolytes bacteria use to counter osmotic down-shock.
References
- 1.Çetiner U., et al. J. Gen. Physiol. 2017;149 doi: 10.1085/jgp.201611699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levina N., et al. 1999. EMBO J. 18:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukharev S.I., et al. 1994. Nature. 368:265–268. [DOI] [PubMed] [Google Scholar]
- 4.Bass R.B., et al. 2002. Science. 298:1582–1587. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z., et al. 2009. Nature. 461:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anishkin A., and Sukharev S.. 2009. J. Biol. Chem. 284:19153–19157. [DOI] [PMC free article] [PubMed] [Google Scholar]