Molday and Goldberg discuss work by Salinas et al. describing formation of the light-sensing outer segment organelle of photoreceptor cells.
Abstract
Formation of membrane discs in photoreceptor cells requires evagination of its ciliary plasma membrane by an unknown molecular mechanism. Salinas et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201608081) show that peripherin (also known as peripherin-2 or peripherin-2/rds) diverts membrane traffic to photoreceptor disc formation by inhibiting ectosome release from the cilium.
Rod and cone photoreceptors use light-sensitive ciliary organelles called outer segments (or photoreceptor sensory cilia) to detect photons via a well-characterized G protein–coupled signal transduction cascade (Molday and Moritz, 2015; Goldberg et al., 2016). Photon capture by outer segments relies on a long stack of membranous “discs” packed with the photopigment protein rhodopsin in rods and cone opsin in cones. Many hundreds of discs are stacked in precise register and oriented for maximum photon capture. Like other cilia, outer segments are anchored in the photoreceptor cell by a microtubule-based axoneme. A thin ciliary bridge known as the connecting cilium, comparable to the transition zone of primary cilia, projects 1–2 µm out from the inner segment of the photoreceptor cell body and expands to a larger diameter outer segment housing the stacked discs (Fig. 1). The mechanisms by which these light-sensitive discs are initially formed from the photoreceptor cilium and continually renewed in the process known as disc morphogenesis are not well understood and are of longstanding interest. In this issue, Salinas et al. show that these discs are formed by suppressing the release of ciliary ectosomes.
Figure 1.
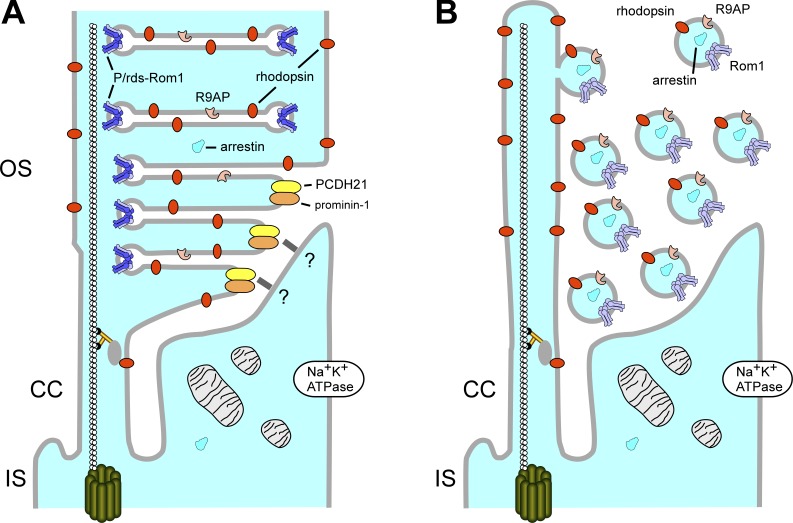
Peripherin initiates outer segment disc formation by suppressing ciliary ecotosome release. (A) Outer segment discs generated by peripherin-containing photoreceptors of wild-type mice are formed by expansion of disc surface area, followed by circumferential growth of a high-curvature rim that promotes disc internalization (Steinberg et al., 1980). CC, connecting cilium; IS, inner segment; OS, outer segment. Adapted from Goldberg et al. (2016). (B) No outer segment discs are produced by photoreceptors of rds−/− mice lacking peripherin; instead, ciliary ecotosomes containing outer segment proteins are present, including rhodopsin, Rom-1, R9AP, and arrestin.
Once viewed as evolutionary vestiges, primary cilia are now recognized as important signal-transducing organelles that can both receive and transmit essential information. One form of signal transmission involves extracellular vesicle release via ectosome shedding (Wood and Rosenbaum, 2015). Ectosomes (also called microvesicles and microparticles) are a class of large extracellular vesicles that is derived from the plasma membrane. Mammalian primary cilia and flagella of unicellular organisms have each been documented to release these extracellular vesicles. Ectosomes can serve a variety of functions, including intercellular communication, extracellular matrix proteolysis, cellular disposal, and signal-dependent removal of G protein–coupled receptors from the cilium. Ectosomes are distinct from exosomes, which are smaller and of endosomal or multivesicular body origin. The widespread roles played by extracellular vesicles for biology and human health are currently being hotly debated, so the discovery of ectosome shedding from photoreceptor cilia is an exciting new development in a rapidly developing area.
Salinas et al. (2017) show that ectosomes can be released by developing photoreceptor sensory cilia. They further demonstrate a role for peripherin (also known as peripherin-2 or peripherin-2/rds) in suppressing ectosome release and redirecting membrane traffic to outer segment disc morphogenesis. In these studies, they used a classic animal model for progressive retinal degeneration, the retinal degeneration slow (rds) mouse, which carries a null mutation in its Prph2 gene (Travis et al., 1992). Early electron microscopic studies characterized a striking retinal phenotype in this animal—a highly specific and dose-dependent disruption of photoreceptor outer segment biogenesis. Although early stages of photoreceptor development proceed normally, outer segment morphogenesis, which occurs postnatally (at approximately postnatal day [P]10–21), does not. The connecting cilia of rds+/− heterozygotes project large whirls of overgrown/misshapen discs into the subretinal space. In contrast, the connecting cilia of rds−/− homozygotes are largely devoid of membrane projections and, instead, the subretinal space around them is packed with membranous material. These structures have been generally thought to be abortive attempts at outer segment formation, consistent with the presence of several outer segment proteins within them, including rhodopsin and S-antigen (arrestin).
The disruption of outer segment morphogenesis in rds mice results from a decrease (rds+/−) or absence (rds−/−) of the Prph2 gene product, peripherin (Connell et al., 1991). This integral membrane tetraspanin protein resides only at the highly curved rims of outer segment disc membranes, where it likely plays a structural role (Goldberg et al., 2016; Milstein et al., 2017). Outer segment discs are formed by an initial expansion of disc surface area, followed by the circumferential growth of a high-curvature rim that promotes disc internalization (Steinberg et al., 1980). It is therefore conceivable that a failure in disc rim formation caused by insufficient peripherin prevents rds photoreceptors from generating normal outer segments.
Using improved fixation procedures, Salinas et al. (2017) report that the extracellular membranous structures filling the subretinal space in the rds−/− mouse are ectosomes released from the photoreceptor cilium. The ectosomes contain photoreceptor outer segment proteins including rhodopsin, R9AP, and Rom-1 (a homologue of peripherin), but lack Na+/K+ ATPase, a protein normally confined to the photoreceptor inner segments (illustrated in Fig. 1). As part of this study, the time course for ciliogenesis in photoreceptors of wild-type (WT) and rds−/− mice was examined. Early stages of photoreceptor ciliogenesis including the attachment of ciliary vesicles to the basal body and primary cilium elongation were similar in rod photoreceptors of WT and rds−/− mice. However, at P10, nascent discs extended from the distal end of the rod cilium in the WT mouse, whereas only ectosomes were seen surrounding the rod cilium in the rds−/− mouse. In the WT mouse rod outer segment morphogenesis and maturation continued through P21. In contrast, ectocytosis persisted in photoreceptors of the rds−/− mouse and the length of the primary cilium remained constant. To determine if ectosomes are also produced during normal outer segment morphogenesis, Salinas et al. (2017) examined wild-type photoreceptors for the presence of ectosomes at various stages of development. Rhodopsin-containing ectosomes were observed at the distal tips in a small number of primary cilia at P10, but not at later stages of photoreceptor development. These experiments and the finding that the rds+/− mouse with reduced peripherin expression shows an increase in the number of ectosomes relative to WT mice support a role for peripherin in inhibiting ectosome release and diverting membrane component trafficking through the cilium to disc morphogenesis.
In a series of elegant experiments, Salinas et al. (2017) dissected the contributions of peripherin domains for the suppression of ectosome release from the photoreceptor cilium and the generation of membrane curvature. They created two fusion protein constructs for these experiments. The first fused the rhodopsin C terminus to peripherin lacking only its C terminus (Per-TS). This construct (amino acids 1–290) includes the peripherin N terminus, the four membrane spanning segments, and the large extracellular/intradiscal EC2 domain; the latter mediates several stages of oligomerization and interaction with Rom-1 (Goldberg et al., 2016). The second construct fused the C terminus of peripherin (amino acids 310–349) downstream of full-length rhodopsin (Per-CT). This construct includes only the protein’s intrinsically disordered cytoplasmic C terminus, which mediates protein targeting and contains a membrane-inducible amphipathic helix of uncertain significance (Goldberg et al., 2016; Milstein et al., 2017). These constructs and full-length peripherin and rhodopsin constructs were individually electroporated into neonatal (P1) rds−/− retina and their localization and effect on disc morphogenesis was examined by immunofluorescence and electron microscopy at P21, when outer segment formation is complete. Full-length peripherin resulted in normal outer segments as expected, whereas rhodopsin produced punctate structures resembling ectosomes. Per-CT expression produced membrane structures protruding from the primary cilium. Similar ciliary membrane projections were observed when the serotonin-6 or somatostatin-3 receptors containing the C terminus of peripherin were expressed in developing photoreceptors. In contrast, Per-TS expression produced clusters of Per-TS–containing membrane vesicles and tubular structures not attached to the photoreceptor cilium. Collectively, these experiments strongly support a role of the C terminus of peripherin in the suppression of ectosome release from photoreceptor cilia. These peripherin constructs were also expressed in cultured cells to further dissect out the role of particular peripherin domains in generating membrane curvature. Full-length peripherin and Per-TS produced intracellular tubular membranes, not observed when Per-CT was expressed in culture cells. These experiments support the crucial role of peripherin domains other than the C terminus in generating membrane curvature required for disc morphogenesis and stabilization of the mature disc rim structure.
Importantly, disc morphogenesis is not only essential for outer segment biogenesis, but is also required for continued photoreceptor function and viability over the lifetime of the organism. Outer segments undergo a continuous renewal process: aged discs at the tip of the outer segment are shed and then phagocytosized by adjacent retinal pigment epithelial cells, while new discs are formed at the proximal end of the outer segment. Because up to 10% of the outer segment must be replaced each day, even small perturbations in disc morphogenesis may result in damaged photoreceptors and lead to a loss in vision. Disc morphogenesis during outer segment biogenesis and renewal likely proceed by essentially similar mechanisms, and have been extensively studied at an ultrastructural level, but the underlying molecular components and mechanisms are not yet understood. Salinas et al. (2017) have significantly advanced our understanding of the initial steps in disc formation at a molecular level by showing that peripherin, and more specifically its C terminus, prevents ectosome release from the primary cilium, enabling membrane components transported through the cilium to be diverted to disc formation. Their studies further highlight the importance of the remainder of peripherin for generating membrane curvature required for the formation of nascent disc structures.
Many issues related to the molecular basis of outer segment disc morphogenesis require further study. First, the mechanism by which peripherin inhibits ectocytosis remains to be discovered. Current knowledge suggests that ciliary ectosome formation includes actin-dependent membrane remodeling by ESCRT complexes (Wood and Rosenbaum, 2015). It is possible that peripherin inhibits this process. Alternatively, loss of peripherin could cause ectocytosis by disrupting ciliary exit. Many types of primary cilia organize G protein–coupled receptor–mediated signaling cascades, and signal-dependent removal of G protein–coupled receptors from cilia (“ciliary exit”) via actin-dependent ectosome shedding is a recently documented mechanism for modulation of signaling (Nager et al., 2017). Although not extensively documented in the literature, extracellular vesicle shedding by photoreceptors has been observed in several (nonperipherin) animal models and supports the possibility that disruption of ciliary exit can activate ectosome release. Proteomic studies have identified a broad variety of molecules in isolated cilia (Wood and Rosenbaum, 2015). Similar approaches can be used to identify low abundance proteins that may play crucial roles in photoreceptor disc formation and maturation. Although actin, prominin-1, and protocadherin-21, are well-documented to play important roles in disc formation, it is likely that additional proteins are involved as well (Goldberg et al., 2016). Such studies may also shed light on the molecular mechanism by which membrane proteins transported through the cilium are selectively sorted to either the disc or plasma membrane of rod photoreceptors. Finally, the studies by Salinas et al. (2017) focused on rods, which represent ∼97% of murine photoreceptors. Interestingly, a complete loss of peripherin (in the rds−/− mouse) leaves cones with atypical outer segment membranes (Farjo et al., 2006), an outcome different than that documented here for rods. Therefore, it remains to be determined if cone photoreceptor cilia release ectosomes and whether peripherin plays a role in suppressing their release.
In summary, the studies of Salinas et al. (2017) significantly contribute to our understanding of initial steps in disc morphogenesis. This information and techniques used in this study should prove valuable for further advancing our knowledge of disc morphogenesis.
Acknowledgments
Research in the laboratory of R.S. Molday is funded by National Institutes of Health grant EY002422 and Canadian Institutes of Health Research grant PJT 148649. Research in the laboratory of A.F.X. Goldberg is funded by National Institutes of Health grant EY025291.
The authors declare no competing financial interests.
References
- Connell G., Bascom R., Molday L., Reid D., McInnes R.R., and Molday R.S.. 1991. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc. Natl. Acad. Sci. USA. 88:723–726. 10.1073/pnas.88.3.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R., Skaggs J.S., Nagel B.A., Quiambao A.B., Nash Z.A., Fliesler S.J., and Naash M.I.. 2006. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J. Cell Biol. 173:59–68. 10.1083/jcb.200509036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A.F., Moritz O.L., and Williams D.S.. 2016. Molecular basis for photoreceptor outer segment architecture. Prog. Retin. Eye Res. 55:52–81. 10.1016/j.preteyeres.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein M.L., Kimler V.A., Ghatak C., Ladokhin A.S., and Goldberg A.F.. 2017. An inducible amphipathic helix within the intrinsically disordered C-terminus is not required for protein biosynthesis, trafficking, or GARP2 interaction, but can participate in membrane curvature generation by peripherin-2/rds. J. Biol. Chem. 10.1074/jbc.M116.768143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday R.S., and Moritz O.L.. 2015. Photoreceptors at a glance. J. Cell Sci. 128:4039–4045. 10.1242/jcs.175687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nager A.R., Goldstein J.S., Herranz-Pérez V., Portran D., Ye F., Garcia-Verdugo J.M., and Nachury M.V.. 2017. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell. 168:252–263.e14. 10.1016/j.cell.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas R.Y., Pearring J.N., Ding J.-D., Spencer W.J., Hao Y., and Arshavsky V.Y.. 2017. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J. Cell Biol. 10.1083/jcb.201608081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R.H., Fisher S.K., and Anderson D.H.. 1980. Disc morphogenesis in vertebrate photoreceptors. J. Comp. Neurol. 190:501–518. 10.1002/cne.901900307 [DOI] [PubMed] [Google Scholar]
- Travis G.H., Groshan K.R., Lloyd M., and Bok D.. 1992. Complete rescue of photoreceptor dysplasia and degeneration in transgenic retinal degeneration slow (rds) mice. Neuron. 9:113–119. 10.1016/0896-6273(92)90226-4 [DOI] [PubMed] [Google Scholar]
- Wood C.R., and Rosenbaum J.L.. 2015. Ciliary ectosomes: Transmissions from the cell’s antenna. Trends Cell Biol. 25:276–285. 10.1016/j.tcb.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]