Das and Knust preview work from Perez-Mockus et al. describing how Neuralized controls Crumbs and epithelial cell polarity via distinct Stardust isoforms.
Abstract
The Drosophila melanogaster scaffolding protein Stardust (Sdt) stabilizes the transmembrane protein Crumbs, a conserved regulator of apical–basal epithelial polarity. In this issue, Perez-Mockus et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201611196) report that a subset of Sdt isoforms are targeted by the ubiquitin ligase Neuralized, thus fine tuning the endocytosis and activity of this apical determinant.
Epithelia are a hallmark of metazoa and are the tissues that are the most affected in human diseases, including cancer. Therefore, unraveling the mechanisms that regulate their development and homeostasis is also instrumental to understanding the origin of the diseased state. Because of its superb genetic tools and the ease of imaging, the Drosophila melanogaster serves as an excellent model to study epithelial tissues during morphogenesis and homeostasis. Drosophila crumbs (crb) was the first gene identified as a guardian of cell polarity and tissue integrity. crb encodes an evolutionarily conserved transmembrane protein, which not only controls apical–basal polarity but performs a variety of other tissue-specific functions, including cell proliferation, tissue growth, and cell survival under stress. It is well established that the proper amount of Crb is essential in maintaining epithelial integrity: although loss of Crb can lead to a loss of apical–basal polarity, too much Crb can expand the apical portion of epithelial cells or can even result in the formation of a second apical pole. Therefore, several mechanisms are set in place to tightly control the amount of Crb. One of these depends on Crb stabilization by the scaffolding protein Stardust (Sdt), one of the core members of the Crb complex. Sdt binds to the short, highly conserved cytoplasmic tail of Crb, and loss of sdt results in the loss of apical Crb and impaired epithelial integrity in the embryo (Bachmann et al., 2001; Hong et al., 2001). This function of Sdt is antagonized by the AP-2 complex, binding of which to Crb promotes its endocytosis (Lin et al., 2015). These examples reveal the complexity of Crb regulation, which is crucial to fine tune the amount of Crb on the apical surface, and hence its activity. In this issue, Perez-Mockus et al. shed light on a novel mechanism by which Sdt proteins ensure the correct amount of apical Crb. Perez-Mockus et al. (2017) show that in some epithelia, the E3 ubiquitin ligase Neuralized (Neur) regulates Crb by controlling the stability of a subset of Sdt isoforms that are able to bind to Neur.
The Schweisguth group previously showed that loss of the Bearded (Brd) complex results in the disintegration of several embryonic epithelia because of down-regulation of apical Crb, Sdt, DPatj (Drosophila protein associated with tight junctions), Par6, and atypical PKC (aPKC). This function of Brd is mediated by increased activity of the neur E3 ubiquitin ligase (Chanet and Schweisguth, 2012), which raises the question of what proteins Neur targets in this context. Perez-Mockus et al. (2017) focused on Sdt, a member of the membrane-associated guanylate kinase family, as the primary target of Neur. A total of 12 Sdt isoforms are predicted in Drosophila, which fall into two classes: those containing exon 3 (represented by Sdt-PB in this study) and those lacking exon 3 (represented by Sdt-PF in this study). Perez-Mockus et al. (2017) revealed that the 433 amino acids encoded by exon 3 (which is present in Sdt-PB but not in Sdt-PF) contain two putative Neur binding motifs (NBMs) similar to those present in other Neur target proteins, e.g., Delta and Serrate. This suggests that Sdt-PB may be a target of Neur.
Overexpression of either Sdt isoform in wing imaginal discs resulted in increased Sdt protein and, as a consequence, increased endogenous Crb protein. Strikingly, upon overexpression of Neur with Sdt-PB, the enrichment of Sdt and Crb was abolished. In contrast, overexpressed Neur did not affect the enrichment of overexpressed Sdt-PF, suggesting that the down-regulation of Sdt depends on exon 3. In fact, Neur, when coexpressed with either of the two Sdt isoforms in S2R+ cells, could coimmunoprecipitate Sdt-PB but not Sdt-PF, suggesting that exon 3, which contains two NBMs, is required for this association.
This assumption was further corroborated by elegant transgenomic approaches resulting in the establishment of fly lines in which either all Sdt isoforms or only exon 3–containing isoforms were endogenously tagged (sdtGFP and sdtGFP3, respectively). In a third variant, exon 3 was replaced by GFP (sdtΔ3GFP). Animals of all three genotypes were viable and fertile, even those in which none of the Sdt variants contained exon 3. This is particularly striking because previous data showed that sdt alleles carrying nonsense mutations in exon 3 (sdtEH681 and sdtM120), which result in premature stop codons, lead to embryonic lethality (Hong et al., 2001; Berger et al., 2007). One may speculate that, in these cases, nonsense-mediated decay of the mutant mRNA may have an effect on sdt mRNAs lacking exon 3, thus resulting in a complete loss of sdt function. sdtGFP, sdtGFP3, and sdtΔ3GFP expressed Sdt proteins in the embryo and in wing imaginal discs. Upon Neur overexpression in wing discs, SdtGFP3 and SdtGFP isoforms were completely or almost completely down-regulated, respectively. Some residual apical Crb was still detectable in both cases because Neur-resistant Sdt variants were not affected by Neur overexpression. In line with this assumption, SdtΔ3GFP isoforms were not affected by Neur overexpression.
The next set of experiments addressed the mechanism by which overexpressed Neur regulates apical Crb levels. To support their initial findings that apical Crb is affected by Neur activity through Sdt-PB, Perez-Mockus et al. (2017) performed cleverly designed endocytotic uptake assays in S2R+ cells. When Crb was expressed in S2R+ cells alone, low levels of endocytosis were observed. However, Crb was increasingly endocytosed upon increasing levels of both Neur and Sdt-PB but not upon Neur or Sdt-PB expression alone. The specific involvement of the binding between Neur and the NBM of Sdt-PB isoforms in Crb endocytosis was conclusively proven by verifying that endocytosis of Crb upon coexpression of Neur and SdtΔNBM, which carried a deletion in exon 3 that removed the NBM was minimal.
Finally, to understand the physiological relevance of this proposed regulation of Crb via Neur, Perez-Mockus et al. (2017) studied embryonic epithelia. They previously showed that the epidermis of Brd mutant embryos lose epithelial polarity and integrity as a result of increased neur activity (Chanet and Schweisguth, 2012). In this study, they now show that this goes along with strongly reduced expression of SdtGFP3. Strikingly, Brd mutant embryos expressing only Sdt variants lacking exon 3 (SdtΔ3GFP) develop a properly polarized epidermis. This suggests that the epithelial defects in Brd mutants are, at least partially, caused by reduced Sdt-PB levels, and hence reduced Crb levels.
Furthermore, the posterior midgut anlage of wild-type embryos down-regulates Crb, and this down-regulation is a prerequisite for epithelial remodeling to allow trans-epithelial migration of the primordial germ cells (PGCs). neur mutant embryos fail to down-regulate Crb, and as a consequence, trans-epithelial migration of the PGCs is delayed (Chanet and Schweisguth, 2012). Embryos expressing all Sdt variants (sdtGFP) behave like the wild type, i.e., they down-regulate Crb in the anlagen of the posterior midgut. However, embryos expressing only Sdt variants that lack exon 3 and hence the NBM (sdtΔ3GFP and sdtΔ3, a variant in which the whole exon 3 was deleted) do not down-regulate apical Crb and exhibit delayed PGC migration similar to neur embryos. From this, Perez-Mockus et al. (2017) concluded that in the posterior midgut, Sdt isoforms that contain exon 3, and hence an NBM, are targeted by Neur for degradation, which in turn results in Crb destabilization and hence allows epithelial remodeling.
The described mechanism of Crb regulation by Neur adds yet another way to fine tune the levels of the apical determinant Crb at the surface (Fig. 1). At the same time, these results trigger several follow-up questions. For example, what is the phenotypic consequence of Crb down-regulation induced by neur overexpression in wing imaginal discs? Previous work has shown that the loss of crb in wing imaginal discs does not affect epithelial tissue integrity but affects the Hippo pathway and hence induces cell proliferation and tissue growth (Flores-Benitez and Knust, 2016). Moreover, studies conducted in our laboratory have shown that loss of Crb results in Notch endocytosis in pupal wings, followed by the activation of the ligand-independent Notch pathway and wing-vein phenotypes. Are the Neur-resistant Sdt variants still present after neur overexpression able to keep sufficient Crb at the surface to allow normal Hippo and Notch signaling? Because wing epithelia normally do not express neur, this mechanism is unlikely to play a role in Crb regulation in developing wings under normal conditions. Also, what is the function of Sdt isoforms containing exon 3 since flies lacking these isoforms are viable and fertile?
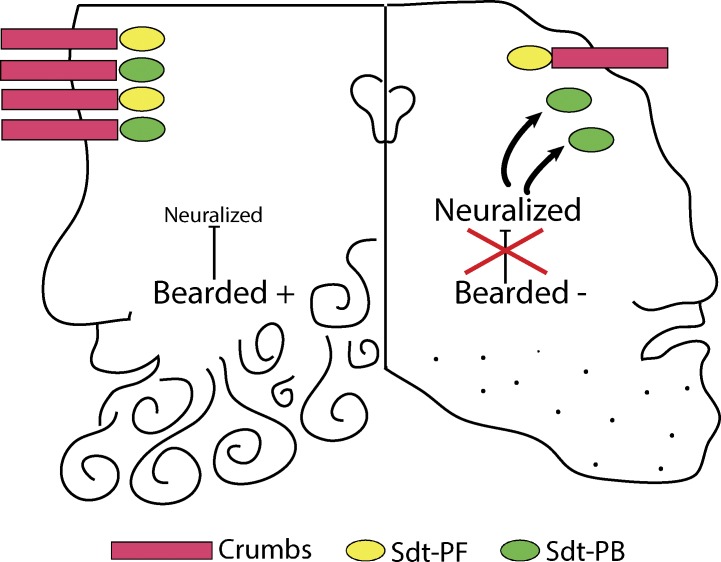
Figure 1.
The figure shows two faces (inspired by the two-faced Janus god) that represent two cells. On the left is a wild-type cell with normal Bearded function, and on the right is a Bearded mutant cell. In the wild-type cell, the presence of Bearded leads to a polarized cell with normal morphology with Crb in the subapical region. Crb binds to Sdt (both the Sdt-PF and Sdt-PB isoforms), and this leads to stabilization of the Sdt protein and thus apical enrichment of Crb. In contrast, in the Bearded mutant cell (represented by the lack of a beard), neuralized is hyperactive, which in turn results in reduction of Sdt-PB and Crb from the apical membrane and thus compromises the morphology of this cell.
It was previously shown that exon 3 contains a signal required for apical targeting of sdt mRNA (Horne-Badovinac and Bilder, 2008). These authors showed that Gal4-mediated overexpression of a Sdt variant containing exon 3 (called Sdt-A in their paper) was more efficient in reducing the strong mutant phenotype of sdtEH651 embryos than overexpression of a variant lacking exon 3 (called Sdt-B). However, these results were based on overexpression. Perez-Mockus et al. (2017) used knock-in modifications of the sdt locus to demonstrate that exon 3–containing sdt mRNAs are dispensable for viability (as in sdtΔ3GFP animals). These animals are now ideally suited to unveil whether exon 3 is indeed required for mRNA localization in early embryogenesis and in the follicle epithelium and, if so, the extent to which loss of exon 3 affects protein localization and function.
As previously shown (Chanet and Schweisguth, 2012), loss of neur or Brd has opposite effects on trans-epithelial migration of PGCs through the midgut epithelium, in that migration was delayed in neur mutant embryos but accelerated in Brd mutant embryos. This raises the question of whether the latter phenotype can be abolished when Brd mutant embryos express only exon 3–lacking Sdt variants, which are not susceptible to Neur-mediated degradation. Chanet and Schweisguth (2012) had also shown that apical markers, including Crb, aPKC, and Par6, are strongly down-regulated in the ectoderm of Brd mutant embryos as early as stage 6. Because Par6 can bind both Crb (Kempkens et al., 2006) and Sdt (Wang et al., 2004), the question remains of whether Crb or Par6 is the primary target of Sdt-mediated Neur action at this stage and whether Neur acts via ubiquitinating Sdt or other Sdt-associated proteins.
Finally, we are left with the question of the importance of Crb regulation via exon 3–containing Sdt isoforms because animals expressing only Sdt variants lacking exon 3 are fully viable, suggesting that the embryo has put in place backup systems. Hence, this paper by Perez-Mockus et al. (2017) supports previous observations suggesting there is not one Crb complex, but that the composition and hence the stability and function of the complex are highly dynamic (Flores-Benitez and Knust, 2016) in order to fine tune epithelial morphogenesis by regulating Crb levels.
Acknowledgments
Research in the group of E. Knust is funded by the Max Planck Society.
The authors declare no competing financial interests.
References
- Bachmann A., Schneider M., Theilenberg E., Grawe F., and Knust E.. 2001. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 414:638–643. 10.1038/414638a [DOI] [PubMed] [Google Scholar]
- Berger S., Bulgakova N.A., Grawe F., Johnson K., and Knust E.. 2007. Unraveling the genetic complexity of Drosophila stardust during photoreceptor morphogenesis and prevention of light-induced degeneration. Genetics. 176:2189–2200. 10.1534/genetics.107.071449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanet S., and Schweisguth F.. 2012. Regulation of epithelial polarity by the E3 ubiquitin ligase Neuralized and the Bearded inhibitors in Drosophila. Nat. Cell Biol. 14:467–476. 10.1038/ncb2481 [DOI] [PubMed] [Google Scholar]
- Flores-Benitez D., and Knust E.. 2016. Dynamics of epithelial cell polarity in Drosophila: how to regulate the regulators? Curr. Opin. Cell Biol. 42:13–21. 10.1016/j.ceb.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Hong Y., Stronach B., Perrimon N., Jan L.Y., and Jan Y.N.. 2001. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 414:634–638. 10.1038/414634a [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S., and Bilder D.. 2008. Dynein regulates epithelial polarity and the apical localization of stardust A mRNA. PLoS Genet. 4:e8 10.1371/journal.pgen.0040008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempkens O., Médina E., Fernandez-Ballester G., Ozüyaman S., Le Bivic A., Serrano L., and Knust E.. 2006. Computer modelling in combination with in vitro studies reveals similar binding affinities of Drosophila Crumbs for the PDZ domains of Stardust and DmPar-6. Eur. J. Cell Biol. 85:753–767. 10.1016/j.ejcb.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Currinn H., Pocha S.M., Rothnie A., Wassmer T., and Knust E.. 2015. AP-2-complex-mediated endocytosis of Drosophila Crumbs regulates polarity by antagonizing Stardust. J. Cell Sci. 128:4538–4549. 10.1242/jcs.174573 [DOI] [PubMed] [Google Scholar]
- Perez-Mockus G., Roca V., Mazouni K., and Schweisguth F.. 2017. Neuralized regulates Crumbs endocytosis and epithelium morphogenesis via specific Stardust isoforms. J. Cell Biol. 10.1083/jcb.201611196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Hurd T.W., and Margolis B.. 2004. Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/Stardust. J. Biol. Chem. 279:30715–30721. [DOI] [PubMed] [Google Scholar]