Abstract
Background
Most Lyme disease cases in the Midwestern United States are reported in Minnesota and Wisconsin. In recent years, however, a widening geographic extent of Lyme disease has been noted with evidence of expansion eastwards into Michigan and neighboring states with historically low incidence rates.
Methods
We collected confirmed and probable cases of Lyme disease from 2000 through 2014 from the Michigan Department of Health and Human Services, entering them in a geographic information system. We performed spatial focal cluster analyses to characterize Lyme disease expansion. We compared the distribution of human cases with recent Ixodes scapularis tick distribution studies.
Results
Lyme disease cases in both the Upper and Lower Peninsulas of Michigan expanded more than 5-fold over the study period. Although increases were seen throughout the Upper Peninsula, the Lower Peninsula particularly expanded along the Indiana border north along the eastern shore of Lake Michigan. Human cases corresponded to a simultaneous expansion in established I scapularis tick populations.
Conclusions
The geographic distribution of Lyme disease cases significantly expanded in Michigan between 2000 and 2014, particularly northward along the Lake Michigan shore. If such dynamic trends continue, Michigan—and possibly neighboring areas of Indiana, Ohio, and Ontario, Canada—can expect a continued increase in Lyme disease cases.
Keywords: Borrelia burgdorferi, Ixodes scapularis, Lyme disease, Michigan, geographic information systems
Lyme disease is a multisystem bacterial infection caused by Borrelia burgdorferi and transmitted by Ixodes species ticks. With approximately 30000 reported cases and an estimated 300000 cases per year, it is the most common vector-borne infectious disease in the United States [1, 2]. Approximately 90% of all confirmed cases in the United States are acquired along the northeast coast, with most of the remainder in Minnesota and Wisconsin. Between these eastern and Midwestern foci, locally acquired Lyme disease has been comparatively rare. Michigan has a historically low incidence of Lyme disease compared with neighboring Wisconsin, particularly in the Lower Peninsula of the state. This paucity of cases is corroborated by acarological studies between 1985 and 2006, demonstrating the rarity of B burgdorferi-infected Ixodes scapularis ticks in Michigan’s Lower Peninsula [3, 4].
Recent epidemiologic and entomologic surveillance from 2000 to 2014 have revealed an overall increase in Lyme disease cases in Michigan, including the emergence of the disease in the Lower Peninsula. In this study, we report the results of spatial and spatiotemporal analyses of Lyme disease case data from the Michigan Department of Health and Human Services (MDHHS) over this period, indicating that the expanding distribution of human cases is geographically concordant with expansion of I scapularis populations in Michigan.
MATERIALS AND METHODS
This study was exempted from institutional review board (IRB) review by the Duke University Human Subjects Committee, and the protocol was approved by the MDHHS IRB.
Data Sources
Human case data were made available by data use agreement with the MDHHS. The MDHHS provided case records of probable and confirmed Lyme disease cases from 2000 through 2014. These cases were aggregated by zip code according to each patient’s address of residence. In addition, MDHHS also provided annual aggregate data on the county of Lyme disease exposure. These exposure data were collected by interview from patients. Spatial data layers included zip code centroid points, zip code polygons, and county polygons; these layers included United States Census data (eg, 2010 census population and population per square mile). These were obtained through esri (Redlands, CA) online data services.
Case Definitions
Cases of Lyme disease were classified by the MDHHS according to the Centers for Disease Control and Prevention (CDC) surveillance definitions. Three case definitions were active during this period: the 1996 definition (for 2000–2007 cases), the 2008 definition (for 2008–2010 cases), and the 2011 definition (for 2011–2013 cases) [5–7]. Until 2008, the CDC only classified cases as “confirmed”; the subsequent definitions have classified cases as “suspected,” “probable,” or confirmed. For this study, we pooled probable and confirmed cases, but we excluded suspected cases.
Ixodes scapularis Distribution
To document the expansion of I scapularis in Michigan during the period of interest, field reports on these ticks were collated and used to map counties where this species was detected in 2001, 2007, and 2014. The 2001 and 2007 datasets were based on the county status reported in Dennis et al [8], supplemented by the results of additional field reports undertaken since that publication. The 2014 dataset was based on field reports collated by Eisen et al [9]. The Dennis et al [8] county records were obtained from published reports, unpublished reports by CDC and other experts, the US National Tick Collection database [10], and questionnaire surveys sent to public health officials, acarologists, and Lyme disease researchers. Ixodes scapularis was categorized as “reported” in a Michigan county if at least 1 tick of any life stage had been found there; and I scapularis populations were categorized as “established” if at least 6 individuals or 2 life stages had been found within 1 year.
From the late 1990s until the present, Michigan State University (MSU) and MDHHS field teams undertook 5 field surveys for I scapularis populations in various counties. The most common survey method was to collect questing adult ticks in the spring or fall, by “dragging” a 1-m2 corduroy cloth through vegetation transects [11]. Each tick was identified to species, life stage, and sex using dichotomous keys [12, 13]. Surveys undertaken before 2008 were used to identify Michigan counties that had changed status since 1998. Surveys undertaken from 2008 onwards were used to make equivalent updates to the maps in Eisen et al [9] (described below).
In 2016, Eisen et al [9] updated the Dennis et al [8] database by collating studies published since the original, by visiting individual state health department web sites to identify county-level tick surveillance data, and by recontacting public health officials, acarologists, and Lyme disease investigators who had information on ticks in Michigan. The surveys by MSU and MDHHS, described above, provided much of the new Michigan data reported by Eisen et al [9]. If a county had been classified as established at a prior time point, it retained this designation in the 2007 and 2014 classifications, unless more recent collection records changed that county’s classification from reported to established.
Geoprocessing
We used ArcGIS 10.3.1 (ESRI, Redlands, CA) for all geoprocessing operations. We appended case data to a zip code polygon and county polygon layers in ArcGIS, using 5-digit zip code and county name as common fields, respectively. We used cartographic and layout features in ArcGIS to produce all map figures in this paper.
Spatial Cluster Analysis
We performed a focal cluster analysis using the spatial scanning statistic in SaTScan (www.satscan.org) [14]. SaTScan uses circular scanning windows, comparing case counts to population values, to identify areas where observed numbers of cases exceed those expected under a Poisson distribution. The advantage of this technique is it inherently accounts for geographic heterogeneity in the underlying population at risk. Our analysis parameters specified a maximum spatial cluster size up to 5% of the population at risk, and to limit overlapping clusters we did not allow cluster centers to fall within other clusters. We performed our analysis for 3 intervals of 5 years: 2000–2004, 2005–2009, and 2010–2014. We analyzed the Upper and Lower Peninsulas of Michigan separately because of their spatial discontinuity. Population figures were obtained from 2010 United States Census data. We retained only clusters with a P value below 0.01. The circular clusters were then geometrically clipped to the boundaries of underlying zip code areas in order that the cluster shape conform to the underlying geography. We removed from the clusters any zip code in which there had been no Lyme disease cases during that 5-year interval.
RESULTS
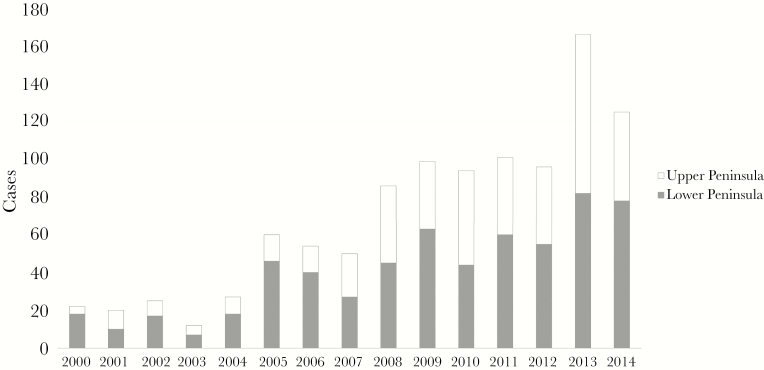
Between 2000 and 2014, there were 1057 confirmed and probable cases of Lyme disease reported by the MDHHS; 1045 of these could be mapped to zip codes. Of these, 612 cases were from residents of the Lower Peninsula and 433 from the Upper Peninsula. There were fewer than 30 Lyme disease cases reported in Michigan in any year between 2000 and 2004 (Figure 1). Notwithstanding some year-to-year variation, the total case count began expanding in 2005; by 2009, the case count surpassed 90, and in 2013 there were 166 cases. This increase in case volume occurred simultaneously in both the Upper and Lower Peninsulas.
Figure 1.
Cases of probable and confirmed Lyme disease in Michigan’s Upper and Lower Peninsulas, 2000–2014. Case counts for both Peninsulas increased more than 5-fold over the course of the 15-year study period.
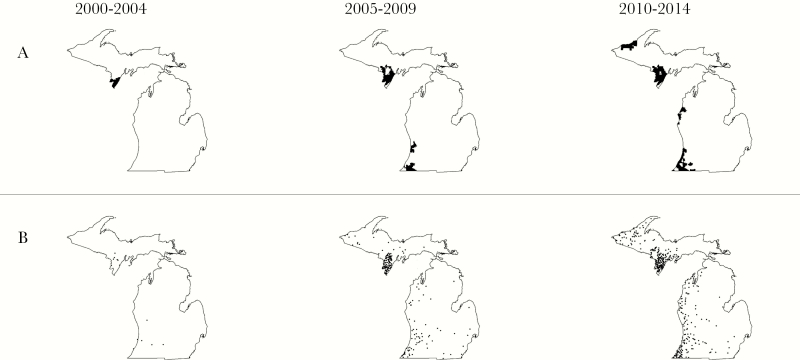
From 2000 to 2004, there were 108 total Lyme disease cases reported statewide. The heaviest single concentration occurred in the Upper Peninsula, with 27 total cases in the southern part of Menominee County (Figure 2A). During these 5 years, no significant concentration of cases was seen in the Lower Peninsula. Only a single zip code in Ann Arbor recorded more than 2 total cases between 2000 and 2004. However, this pattern changed in the subsequent 5 years. The primary concentration of Lyme disease cases in the Upper Peninsula expanded into neighboring Dickinson and Delta Counties, and a second concentration appeared in Gogebic County along the western border with Wisconsin. At the same time, cases began to accumulate in the southwestern Lower Peninsula near the border with Indiana and along the Lake Michigan shore. This was true for population-weighted maps of cases mapped by residential zip code (Figure 2A) and for cases mapped by county of exposure (Figure 2B). Cases were more scattered elsewhere in the Lower Peninsula, particularly in urban centers such as Ann Arbor and Grand Rapids. The next 5-year interval, between 2010 and 2014, saw further extension of transmission in the Upper Peninsula, particularly along the Lake Superior coast. Cases in the Lower Peninsula accumulated farther north along the Lake Michigan coast during these years. The county of acquisition (Figure 2B) bore a close geographic resemblance to our cluster analysis in Figure 2A.
Figure 2.
(A) Sequential cluster analysis of Lyme disease cases, grouped by 5-year interval. Clusters of Lyme disease both expanded and appeared in the Upper and Lower Peninsulas throughout the study period. The underlying case data were initially aggregated annually at the zip code level based on the patient’s residential address. The cluster detection method incorporated zip code level population data and identified clusters where more cases were observed than would be expected under a Poisson distribution. (B) Cases of Lyme disease by county of acquisition. In this map, each case is represented by a dot placed randomly in the county where the infection was likely acquired.
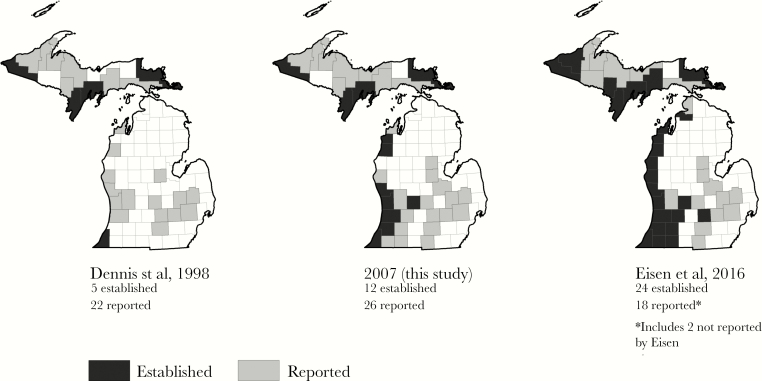
In 1998, Dennis et al [8] indicated that I scapularis had been reported from 22 Michigan counties and was established in 5 (only 1 of which was in the Lower Peninsula; Figure 3). By 2007, 7 additional counties (all in the Lower Peninsula) were known to have established populations, and 4 additional counties had reported ticks. By 2016, the totals had increased to 24 counties with established populations and an additional 18 with reports of ticks. This expansion of tick populations is geographically concordant with the expanding distribution of human cases.
Figure 3.
Expanding distribution of established and reported Ixodes scapularis populations in Michigan, 1998–2016. The expansion of I scapularis populations is geographically concordant with the expansion in human cases observed over this same time period.
DISCUSSION
From 2000 through 2014, Michigan saw both a numerical and geographic expansion of its total Lyme disease case volume. Early during this period, Lyme disease cases were largely confined to 1 county in the Upper Peninsula bordering Wisconsin. In the subsequent years, the endemic range expanded within the Upper Peninsula. At the same time, cases began to emerge in the Lower Peninsula along the Lake Michigan shore and the Indiana border and subsequently extended farther northward and inland.
Standard case reporting for Lyme disease records location of patient residence, and this may differ from location of exposure. As a consequence, Lyme disease cases were identified in populous areas, such as Ann Arbor and Grand Rapids. However, our cluster analyses were adjusted for the underlying population distribution, allowing us to highlight regions where the observed cases exceeded expectation. This analysis closely corroborated the subset of cases in which the location of exposure was known.
In the Upper Midwestern United States, I scapularis populations have been reported throughout Wisconsin and Minnesota since the late 1960s and have since expanded [8, 9]. In Michigan, initial passive and active surveillance suggested that the only established population of blacklegged ticks was in Menominee County in the western Upper Peninsula [8], although there had been sporadic reports of I scapularis ticks on humans and dogs in many other counties since the late 1980s [4].
By applying a blacklegged tick habitat suitability model developed in Wisconsin to Michigan’s landscape, Foster [15] made the first detections of established populations of I scapularis in southwestern Lower Peninsula Michigan. A multiyear surveillance (2004–2009) documented progressive northward spread of I scapularis populations along the coast of Lake Michigan, but less so at inland sites [16]. Blacklegged ticks had become established at 3 of 4 inland sites by 2010 [17] and at all 4 inland sites by 2014 [9]. Borrelia burgdorferi has been detected in ticks at sites in the Upper Peninsula [4, 16, 17], western coastal Lower Peninsula sites [15–17], and inland Lower Peninsula sites (J. T., unpublished data, 2014). The spread of blacklegged ticks across the Lower Peninsula of Michigan supports predictions of a recent spatial study mapping the environmental risk of Lyme disease [3, 18].
Our study is primarily limited by challenges and biases in current Lyme disease surveillance. Physicians report only a small minority of Lyme disease cases, perhaps as little as 10% in some states, and underreporting is most likely not spatially uniform. On the other hand, surveillance definitions leave room for false-positive misclassification of cases due to a variety of factors. Finally, the ability of a state public health program to address these biases can be strongly influenced by resource allocation and labor.
CONCLUSIONS
Although we acknowledge these limitations, our approach uses data from more than 1000 Lyme disease cases over a 15-year period. These data illustrate (1) a trend of northeastward expansion around the shore of Lake Michigan into the Lower Peninsula of Michigan as well as (2) expansion within the Upper Peninsula. These trends are consistent with both national and local trends demonstrating expansion of the endemic range for Lyme disease in other geographic regions. These trends are also supported by recent expansion in the national distribution of seropositive canines [19]. We do not fully understand the environmental and biological factors that have facilitated spread of this tick and pathogen nor which factors may ultimately constrain it. In the meantime, further study is needed to optimize both ecological and case surveillance methods to best understand these changes in geographic range of this common disease.
Acknowledgement
Financial support. P. M. L. was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2 TR001115. L. E. N. was funded by a Boston Children’s Hospital Pilot research grant, Harvard Catalyst, and the Bay Area Lyme Foundation. P. G. A. was supported by the Ken and Sherrilyn Fisher Center for Environmental Infectious Diseases and has performed expert medical-legal reviews concerning Lyme disease. V. F. and F. R. were funded by the National Institute for Allergy and Infectious Diseases of the National Institutes of Health under award number K24 AI093969. G. H. and J. T. were funded by the Michigan Lyme Disease Association. In addition, G. H. was funded by cooperative agreement cI00171-01 from the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Reported cases of Lyme disease by state or locality, 2004–2013. Available at: http://www.cdc.gov/lyme/stats/chartstables/reportedcases_statelocality.html Accessed 12 January 2015
- 2. Hinckley AF, Connally NP, Meek JI, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014; 59:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diuk-Wasser MA, Hoen AG, Cislo P, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg 2012; 86:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker ED, Stobierski MG, Poplar ML, et al. Geographic distribution of ticks (Acari: Ixodidae) in Michigan, with emphasis on Ixodes scapularis and Borrelia burgdorferi. J Med Entomol 1998; 35:872–82. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Lyme disease (Borrelia burgdorferi): 1996 case definition. Available at: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=750&DatePub=1/1/1996%2012:00:00%20AM Accessed 21 January 2015
- 6. Centers for Disease Control and Prevention. Lyme disease (Borrelia burgdorferi): 2008 case definition. Available at: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=751&DatePub=1/1/2008%2012:00:00%20AM Accessed 21 January 2015
- 7. Centers for Disease Control and Prevention. Lyme disease (Borrelia burgdorferi): 2011 case definition. Available at: http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=752&DatePub=1/1/2011%2012:00:00%20AM Accessed 18 December 2014
- 8. Dennis DT, Nekomoto TS, Victor JC, et al. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol 1998; 35:629–38. [DOI] [PubMed] [Google Scholar]
- 9. Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol 2016; 53:349–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. National Tick Collection. Available at: http://cosm.georgiasouthern.edu/icps/collections/the-u-s-national-tick-collection-usntc/ Accessed 4 October 2016
- 11. Ginsberg HS, Ewing CP. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari:Ixodidae). Exp Appl Acarol 1989; 7:313–22. [DOI] [PubMed] [Google Scholar]
- 12. Keirans JE, Hutcheson HJ, Durden LA, Klompen JS. Ixodes (Ixodes) scapularis (Acari:Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol 1996; 33:297–318. [DOI] [PubMed] [Google Scholar]
- 13. Sonenshine DE. Ticks of Virginia (Acari, Metastigmata). Blacksburg, VA: Virginia Polytechnic Institute and State University; 1979. [Google Scholar]
- 14. Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med 1995; 14:799–810. [DOI] [PubMed] [Google Scholar]
- 15. Foster ES. Ixodes scapularis (Acari: Ixodidae) and Borrelia burgdorferi in Southwest Michigan: Population Ecology and Verification of a Geographic Risk Model [master’s thesis]. East Lansing, MI: Michigan State University; 2004. [Google Scholar]
- 16. Hamer SA, Tsao JI, Walker ED, Hickling GJ. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. Ecohealth 2010; 7:47–63. [DOI] [PubMed] [Google Scholar]
- 17. Hamer SA, Hickling GJ, Walker ED, Tsao JI. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the Midwestern United States. Infect Genet Evol 2014; 27:531–42. [DOI] [PubMed] [Google Scholar]
- 18. Diuk-Wasser MA, Vourc’h G, Cislo P, et al. Field and climate based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glob Ecol Biogeogr 2010; 19:504–14. [Google Scholar]
- 19. Little SE, Beall MJ, Bowman DD, et al. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010-2012. Parasit Vectors 2014; 7:257. [DOI] [PMC free article] [PubMed] [Google Scholar]