Abstract
Background:
Self-administration is a hallmark of all addictive drugs, including alcohol. Human laboratory models of alcohol self-administration have characterized alcohol-seeking behavior and served as surrogate measures of the effectiveness of pharmacotherapies for alcohol use disorders. Intravenous alcohol self-administration is a novel method that assesses alcohol exposure driven primarily by the pharmacological response to alcohol and may have utility in characterizing unique behavioral and personality correlates of alcohol-seeking and consumption.
Methods:
This study examined exposure-response relationships for i.v. alcohol self-administration, and the influence of impulsivity and alcohol expectancy, in healthy, nondependent drinkers (n=112). Participants underwent a 2.5-hour free-access i.v. alcohol self-administration session using the Computerized Alcohol Infusion System. Serial subjective response measures included the Drug Effects Questionnaire and Alcohol Urge Questionnaire. To characterize the motivational aspects of alcohol consumption prior to potential acute adaptation, the number of self-infusions in the first 30 minutes of the free-access session was used to classify participants as low- and high-responders.
Results:
High-responders showed greater subjective responses during i.v. alcohol self-administration compared with low responders, reflecting robust exposure-driven hedonic responses to alcohol. High-responders also reported heavier drinking patterns and lower scores for negative alcohol expectancies on the Alcohol Effects Questionnaire. High-responders also showed higher measures of impulsivity on a delayed discounting task, supporting previous work associating impulsivity with greater alcohol use and problems.
Conclusions:
These findings indicate that early-phase measures of free-access i.v. alcohol self-administration are particularly sensitive to the rewarding and motivational properties of alcohol and may provide a unique phenotypic marker of alcohol-seeking behavior.
Keywords: alcohol self-administration, i.v., expectancy, impulsivity, reward
Significance Statement
Self-administration is a hallmark of all addictive drugs, including alcohol. Human alcohol self-administration studies have been used to understand alcohol-seeking behavior and evaluate the potential effectiveness of medications for alcohol use disorder. This study aimed to evaluate a novel i.v. alcohol self-administration method and the role of impulsivity and alcohol expectancy on alcohol consumption. A total of 112 nondependent drinkers underwent an i.v. alcohol self-administration session, resulting in a wide range of self-administration patterns. Participants were classified as low- or high responders based on the number of self-administrations in the first 30 minutes of the session. High responders reported greater subjective responses for rewarding and pleasurable effects of alcohol compared with low responders. High responders also reported heavier drinking patterns, lower scores for negative alcohol expectancies, and higher measures of impulsivity. The early-phase measures of alcohol selfadministration are particularly sensitive to the rewarding properties of alcohol and may provide a unique measure of alcoholseeking behavior.
Introduction
The study of addictive drugs commonly involves self-administration paradigms that are designed to measure drug-seeking and consumption behavior. Measures such as the amount and rate of consumption of the drug can be used to characterize the reinforcing and addictive properties of the drug. Alcohol self-administration (ASA) studies typically use an oral route of administration via ingestion of standardized or preferred alcoholic drinks. Such studies impose various parameters to control variability in the subsequent trajectories of breath alcohol concentrations (BrAC) achieved by participants, such as using a fixed alcohol concentration of the drink or adjusting the amount based on the body weight of the participant (de Wit and McCracken, 1990; Davidson et al., 1999). Nonetheless, even with the most careful adjustments, there can be up to a 3-fold difference between participants in the BrAC exposure after ingestion, primarily related to the substantial interindividual variability in the rates of absorption, distribution, and metabolism of alcohol following oral administration (Ramchandani et al., 2006, 2009).
The Computerized Alcohol Infusion System (CAIS) ameliorates these interindividual differences: i.v. administration of alcohol bypasses gastrointestinal absorption and accounts for individual differences in distribution and elimination. All CAIS paradigms employ a physiologically based pharmacokinetic (PBPK) model-based algorithm (Ramchandani et al., 1999) where the parameters of age, sex, height, and weight are used to determine the infusion rate profile required to achieve and maintain a predetermined BrAC overall (investigator-prescribed) or incremental (ASA) trajectory. Past studies have used prescribed i.v. alcohol administration using the alcohol clamp method to examine a variety of factors regarding the pharmacokinetics of alcohol such as sex, body composition and liver volume (Kwo et al., 1998); food and food composition (Ramchandani et al., 2001); as well as ethnic and genetic influences (Neumark et al., 2004; Marshall et al., 2014). Other studies have explored various determinants of the pharmacodynamics of alcohol, including family history of alcoholism and its associations with self-report intoxication (Morzorati et al., 2002), saccadic eye movements (Blekher et al., 2002), recent drinking history (Ramchandani et al., 2002), and genetic influences on alcohol-induced brain dopamine release measured using PET (Ramchandani et al., 2011) and drinking history effects on brain activation measured using fMRI (Gilman et al., 2012).
Intravenous ASA (IV-ASA) paradigms provide the investigator control over the ascending and descending rates, amount, and duration of the incremental exposure to alcohol (Zimmermann et al., 2008, 2013), while the subject determines the timing and number of increments delivered. The participant can self-administer with the press of a button to receive another incremental exposure to alcohol at their chosen pace and level of exposure, with the assurance that the increments will be identical across subjects. The CAIS method therefore enables the assessment of alcohol seeking and use behavior that is driven primarily by the pharmacological effects of alcohol (Zimmermann et al., 2013).
The first IV-ASA studies using a free-access paradigm included an examination of test-retest reliability in 9 participants across 3 sessions where the incremental BrAC exposure for each infusion was an increase of 7.5 mg%; however, the duration of the infusion varied from 1.5 to 3.5 minutes across the 3 sessions (Zimmermann et al., 2008). Results indicated that the number of infusions during a session was highest with the 1.5-minute infusion rate, suggesting higher rewarding effects of a faster infusion rate. By maintaining ASA more readily than oral alcohol and at a faster rate of onset, the i.v. alcohol method is a key component to maintaining self-administration behavior. The number of infusions as well as the average and peak BrAC were highly correlated between day 2 and day 3, but not with day 1, suggesting the need for a practice session to familiarize the participant with i.v. alcohol exposure procedures. A second study examined the effects of family history of alcoholism (Zimmermann et al., 2009) on IV-ASA measures and found that the peak and average BrAC and number of infusions were significantly higher in the family history-positive (n=12) vs the family history-negative participants (n=10). More recently, IV-ASA has been used to evaluate the genetic influences on self-administration and subjective response. The GABRG1 polymorphism was evaluated in nondependent drinkers pretreated with either lorazepam or placebo, and their motivation for reward was assessed with a progressive ratio IV-ASA paradigm (Plawecki et al., 2013). Another study found that the OPRM1 A118G minor (G) allele variant that is associated with greater dopamine release following alcohol (Ramchandani et al., 2011) was associated with greater free-access IV-ASA, including a greater likelihood of surpassing a binge threshold (BrAC = 80 mg%) during the session (Hendershot et al., 2014). Most recently, variation in the glucagon-like peptide gene that was associated with a higher risk of alcohol use disorder was found to be associated with increased IV-ASA (Suchankova et al., 2015). The IV-ASA method has also been employed in characterizing sex differences in alcohol preference and self-administration in an adolescent sample (Jünger et al., 2016). These studies expand on the utility of IV-ASA for characterizing sources of inter-individual variation in phenotypes of alcohol-seeking and consumption behavior and point to the need for a comprehensive evaluation of predictors of IV-ASA.
The purpose of this study was to characterize IV-ASA behavior using a free-access (open-bar) CAIS paradigm in nondependent drinkers and evaluate the role of several predictors that may be associated with increased risk for alcohol use disorder. These predictors included measures of drinking history, priming effects of alcohol following initial exposure, expectancy, and personality. In addition, this study evaluated the early phase of ASA behavior as a more sensitive measure of motivation for alcohol in this sample. The significance of this work is the identification of the influence of these predictors on ASA behavior that will help improve the understanding of inter-individual differences in this alcohol consumption phenotype and how it may relate to risk for alcohol use disorder.
Methods
Participants
Healthy 21- to 45-year-old male and female nondependent drinkers (n=112) were recruited via local newspaper advertisements and through the NIH Normal Volunteer Office. The study was approved by the NIH Addictions Institutional Review Board and conducted at the NIH Clinical Center in Bethesda, MD. All participants provided written informed consent prior to participating in the study.
Respondents were prescreened in a telephone interview and then brought in for further screening in the outpatient clinic. The screening procedures included medical history and physical examination, as well as ECG, blood tests for routine blood chemistry and liver function, and urine screen for illicit drugs. Recent drinking history was assessed using the 90-day timeline followback (TLFB) (Sobell and Sobell, 1992) and the Alcohol Use Disorder Identification Test (AUDIT) (Babor et al., 1989). The Structured Clinical Interview for DSM-IV-TR psychiatric diagnoses was conducted to assess to evaluate presence of major Axis-I disorders, including alcohol or substance abuse and dependence. Participants also completed the delay discounting task as a measure of choice impulsivity (Bjork et al., 2009). This task presents an option to participant where the participant can choose between a smaller, immediate monetary reward or a fixed greater reward ($100) that is delayed in time (0–30 days). The rate of discounting of the delayed outcome is represented by the discounting factor k. Because k is not a normally distributed value, a natural log-transformation was applied called the ln(k), which was used in our analyses. A higher ln(k) value represents a greater preference for immediate rewards. The Alcohol Effects Questionnaire was a measure of alcohol expectancies (Rohsenow, 1983). This measure included 40 true/false statements about how alcohol typically makes respondents feel. True statements are summed across various subscales, including cognitive and physical impairment and power and aggression.
Inclusion criteria were: 21 to 45 years of age and good health as determined by screening tests. Females were required to have normal menstrual cycles and were tested during the follicular phase of their cycle (within 10 days of offset of menses). In addition, all females had a negative urine pregnancy (hCG) test at the start of each study session. Participants were excluded if they exhibited current or prior history of any central nervous system, cardiovascular, respiratory, gastrointestinal, hepatic, renal, endocrine, or reproductive disorders; positive hepatitis or HIV test at screening; current history of Axis-I psychiatric illness; current or lifetime diagnosis of alcohol or substance dependence; currently seeking treatment for alcohol use disorders; presence of clinically significant withdrawal symptoms (Clinical Institute Withdrawal Assessment score >8) at screening; nondrinkers (alcohol-naïve individuals or current abstainers) or no experience drinking 5 or more drinks on at least one occasion in their lifetime; current or prior history of alcohol-induced flushing reactions; regular tobacco users (occasional use of tobacco products of up to 20 cigarettes/week was acceptable); positive urine drug screen or positive breathalyzer during screening or at the start of any study visit; use of prescription or OTC medications known to interact with alcohol within 2 weeks of the study; or use of drugs known to inhibit or induce enzymes that metabolize alcohol within 4 weeks of the study.
Design
Eligible participants were enrolled in 2 groups: Group 1 comprised 52 participants who participated in 2 identical sessions to assess the test-retest reliability of IV-ASA behavior. Group 2 comprised 60 participants who participated in a single session. Participants were enrolled in Group 1 first, and once that had been filled, enrolled into Group 2. All sessions were identical free-access (open-bar) IV-ASA sessions, each lasting 150 minutes. Each session included a 25-minute priming phase followed by a 125-minute voluntary free-access phase. Before starting the session, CAIS computed the PBPK model parameters for the individual participant using age, height, weight, and sex and derived the incremental infusion rate profile for each button press by the participant. An Alcotest 7410 handheld breathalyzer (Drager Safety Diagnostics) was administered approximately every 15 minutes during the session and entered into the CAIS software program to enable real-time adjustments to the model-based algorithm.
During the directed priming phase (25 minutes), participants were prompted when to push the button to receive 4 increments in BrAC over the first 10 minutes. Each increment comprised an increase of 7.5 mg% over a period of 2.5 minutes. Prompting allowed no pause between priming increments, resulting in a BrAC level of ~30 mg% at 10 minutes, followed by a 15-minute rest period while the BrAC declined at 1.0 mg%/min. This phase allowed the participant to practice pushing the button for an infusion and to give the participant an opportunity to experience i.v. alcohol. This was immediately followed by a 125-minute voluntary free-access phase where participants could press a button whenever they chose to experience the same increment in BrAC increase until the participant pressed the button for another infusion (Zimmermann et al., 2008). The button was inactivated during the ascending phase of the infusion. The button was also inactivated whenever the next push would yield a BrAC increase that exceeded a preset safety limit of 100 mg%. Both limits were imposed with the participant’s knowledge and communicated on the computer screen.
Procedure
Study sessions were scheduled between 3 and 30 days apart. Participants typically arrived at the day hospital unit at 11:00 am on the day of testing after abstaining from alcohol for 48 hours. A breathalyzer test was performed to ensure a zero BrAC. A urine sample was collected for a urine drug screen for all participants and a urine beta-hCG test for females; both were required to be negative to continue participation in the study. Participants received a light snack and completed brief medical and drinking history questionnaires. An indwelling i.v. catheter was then inserted into a vein in the antecubital fossa of (preferably) the nondominant arm using sterile technique. This catheter was used for the alcohol infusion.
The participant was seated in a comfortable chair in a study room in the day hospital unit, out of sight of the infusion pumps and technician’s screen, then instructed in the procedures and limits for selecting alcohol self-infusions in the paradigm. Participants were told to administer alcohol as if they were in a social situation in which they usually drink alcohol. To control the ambient environment during the self-administration session, participants were allowed to watch television or listen to music. The experimenter was available to monitor the infusion and obtain breathalyzer readings as well as to answer any questions raised by the participants and to occasionally inquire about the well-being of the participant.
Subjective measures were obtained serially to assess alcohol effects. These measures were collected at baseline as well as during the directed priming phase (at the 10- and 20-minute time points) and 8 times during the IV-ASA session every 15 minutes, with a final postinfusion measure 15 minutes after the IV-ASA had ended. These measures took roughly 5 minutes to complete. Subjects were allowed to press for more alcohol during data collection so as to not interfere with the subject’s opportunity to self-administer alcohol. These included the Alcohol Urge Questionnaire (AUQ) (Bohn et al., 1995) and Drug Effects Questionnaire (DEQ) (Fischman and Foltin, 1991).
At the end of the free-access phase, the infusion pump was disconnected, and the i.v. catheter was removed from the participant’s arm. Lunch was provided and serial breathalyzer tests tracked the BrAC. Participants were asked to stay in the hospital for at least 2 hours after the end of the self-administration or until their BrAC level fell <20 mg%, whichever was later. At this time, participants were debriefed and sent home in a taxi paid for by NIH. The total duration of the session was approximately 7 hours. Study participants were instructed to refrain from medications and operating any machinery requiring concentration for at least 2 hours following their release from the unit.
Data Analysis
Primary self-administration measures included peak BrAC (mg%), which was the highest BrAC achieved during the free-access phase, the average BrAC (mg%) achieved during the free-access phase, total number of infusions received (not including the 4 priming infusions), and total EtOH delivered (g) during the free-access phase. Test-retest reliability of IV-ASA measures was assessed using Pearson’s correlation coefficients of measures between session 1 and session 2 in Group 1. For the remaining data analysis, data from session 1 across groups 1 and 2 were combined. To characterize the early-phase IV-ASA behavior, participants were grouped based on the number of infusions they administered during the first 30 minutes of the voluntary free-access ASA phase. Not only is this early interval associated with the greatest variability in response, but it should be the least influenced by acute tolerance or sensitization to alcohol and therefore a potentially more precise marker of ASA behavior (Supplemental Figure 1). This early phase also showed significantly higher rewards pressed in comparison with those at the 60-, 90-, and 120-minute phases, suggesting an initial strong priming response to reach a high BrAC (P<.01). Based on the 2.5-minute ascending phase of each incremental alcohol exposure, the maximum number of infusions that could be selected within the first 30 minutes of the session was 12; thus, participants were categorized into low (1–6 infusions) and high (7–12 infusions) responders. A third group of nonresponders included those who chose not to self-administer during the session to determine if these subjects were distinctly different from those that did elect to self-administer alcohol and to assess their subjective response following the directed priming phase. Associations between IV-ASA measures and sex and recent drinking history as well as priming subjective responses were initially examined using bivariate correlations. Multiple regression analyses were used to determine the amount of variance accounted for by predictor variables on IV-ASA outcome measures. Comparison of early-phase responder groups on measures of drinking history, subjective response, expectancy, and personality measures were evaluated using ANOVA. Measures that were not normally distributed or did not show homogeneity of variances were analyzed using nonparametric Kruskal-Wallis tests. Analyses were conducted using SPSS for Windows, Version 22.0 (IBM Corporation), and the level of significance for all analyses was set at .05.
Results
Participant Demographics and Test-Retest Reliability
Males (n=29) and females (n=23) in Group 1 were not significantly different on measures of recent drinking history as reported by the TLFB 90 days and the AUDIT (Supplemental Table 1). Similar lack of differences was found between males (n=34) and females (n=26) in Group 2 (Supplemental Table 1). Overall, sessions were well tolerated with minimal transient adverse events that resolved within minutes of their occurrence, including discomfort at the i.v. site (n=1), mild chest pain (n=1), and light-headedness associated with vasovagal responses during i.v. insertion (n=2). There were very few smokers in the sample (n=7) to allow any meaningful analysis of smoking influences on IV-ASA.
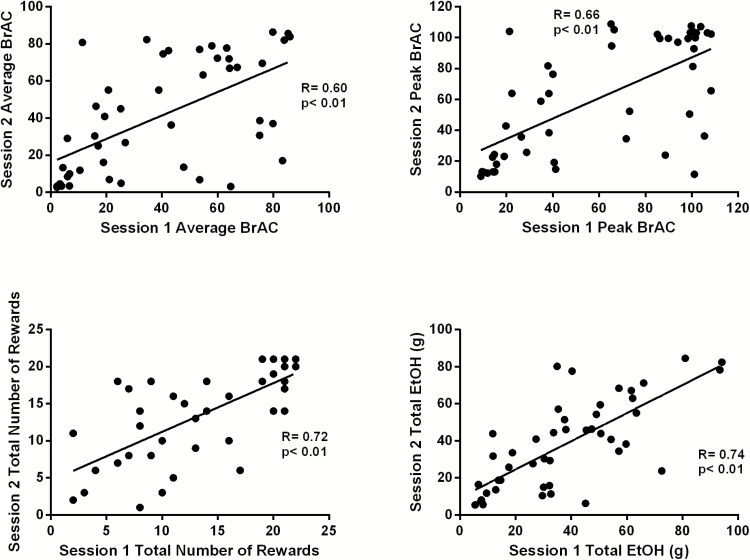
Figure 1 shows the correlations between IV-ASA measures obtained in the same Group 1 participants in sessions 1 and 2. Correlation coefficients indicated moderate to high values for average BrAC (r=0.60), peak BrAC (r=0.66), total number of rewards (r=0.72), and total amount of EtOH consumed (r=0.74) (all P <.01). Correlation coefficients among ASA exposure measures for session 1 ranged from 0.88 to 0.97 (Supplemental Figure 2) and from 0.86 to 0.97 for session 2 (Supplemental Figure 3), indicating extremely high internal consistency among IV-ASA measures within session.
Figure 1.
Correlations between session 1 and session 2 self-administration measures. Positive associations are displayed between alcohol self-administration (ASA) measures of average breath alcohol concentration (BrAC) (A), peak BrAC (B), total number of rewards (C), and Total EtOH (D). All P<.01.
IV-ASA exposure measures showed considerable inter-individual variability (Supplemental Figure 1) and were not significantly different by sex, except for total EtOH, which was lower in females than males (Supplemental Table 1). This difference is not unexpected, given that females have smaller total body water volumes of alcohol distribution than males and the CAIS program adjusts for these pharmacokinetic differences.
Recent Drinking History
TLFB measures of total drinks and number of drinks per drinking day and AUDIT scores were highly correlated with all ASA exposure measures (P <.05) (Supplemental Table 3). Regression analyses of the influence of recent drinking history measures revealed the number of drinks per drinking day to be the strongest predictor of IV-ASA measures (all P <.01).
Subjective Response Measures
Subjective response measures on the DEQ following the priming phase were predictive of IV-ASA during the free-access phase. Participants who reported less “intoxication” (P <.02), less “feeling of alcohol effects” (P <.02), and greater “wanting alcohol” (P <.01) following priming also consumed more alcohol during the free-access phase. Peak DEQ scores for “feel,” “like,” “want,” “high,” and “intoxicated” across the session were all positively associated with all IV-ASA exposure measures (all P <.01). Similar associations were found for alcohol urges measured on the AUQ. Participants who craved more alcohol at the end of the priming phase and who reported greater peak scores for craving during the free-access phase also self-administered more alcohol (all P <.01).
Personality Measures
Participants with lower expectancy scores at baseline for cognitive and physical impairment self-administered more alcohol as measured by the average BrAC (r= -0.32, P <.01), peak BrAC (r=-0.28, P <.02), and number of button presses (r=-0.28, P <.02). Participants who had higher values for the delayed discounting steepness constant (log K) also consumed more alcohol as measured by average BrAC (r=0.27), peak BrAC (r=0.24), and number of button presses (r=0.27) (all P <.05).
Early-Phase IV-ASA Response: Correlates
Table 1 shows the IV-ASA outcomes by category of responder during the early phase of IV-ASA and reflects the tautological significant differences expected between non-, low, and high responders. Comparison of recent drinking history measures among categories, however, is not tautological, and indicated heavier drinking in high responders compared with non- and low responders. Specifically, high responders had higher total AUDIT scores (P <.01) and higher TLFB total drinks, drinks per drinking day, and heavy drinking days than either of the other categories (all P <.05). There was no effect of sex on response rate category.
Table 1.
Comparison of Responder Groups during the Early Phase of IV-ASA
| Criteria for each group |
Nonresponders
(n=12) |
Low
Responders (n=50) |
High
Responders (n=43) |
|---|---|---|---|
| Rewards earned in the first 30 minutes | 0 | 1–6 | 7–12 |
| Self-administration measures within the first 30 minutes of open-bar phase | |||
| Rewards earned | 0.0 ± 0.0 | 3.2 ± 1.3 | 8.7 ± 1.9 |
| Peak BrAC (mg%) | 11.6 ± 2.4 | 23.2 ± 8.4 | 65.5 ± 19.4 |
| Average BrAC (mg%) | 6.1 ± 1.3 | 15.8 ± 6.6 | 40.8 ± 9.6 |
| Demographic and drinking history measures | |||
| AUDIT** | 4.2 ± 2.8 | 5.3 ± 2.3 | 6.7 ± 2.8 |
| AUDIT-C* | 3.9 ± 2.2 | 4.8 ± 1.7 | 5.4 ± 1.7 |
| Total drinks in past 90 days*,a | 62.4 ± 67.4 | 69.0 ± 46.9 | 98.2 ± 95.2 |
| Drinking days in past 90 days | 22.4 ± 20.5 | 25.0 ± 10.9 | 26.2 ± 16.8 |
| Drinks per drinking day in past 90 days* | 2.6 ± 1.6 | 2.8 ± 1.4 | 3.6 ± 1.6 |
| Heavy drinking days in past 90 days**,a | 4.0 ± 6.3 | 5.3 ± 7.3 | 8.3 ± 13.1 |
Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; AUDIT-C, AUDIT Consumption sub-score; BrAC, breath alcohol concentration.
All data are reported as mean and SD. *P<.05 for difference between high responders and non- and low responders. **P<.01 for difference between high responders and non- and low responders.
a Analyzed using nonparametric Kruskal-Wallis test.
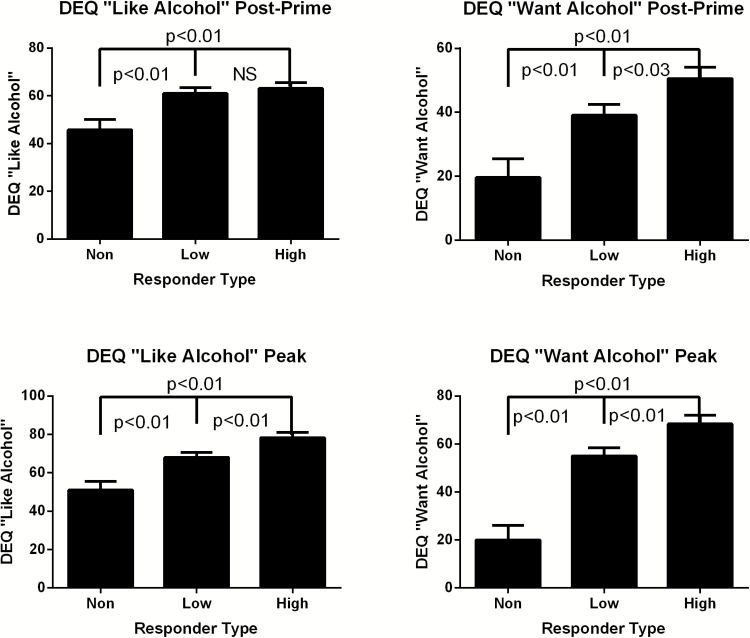
With regard to subjective response measures, high and low responders reported greater changes in postpriming “liking” (P <.01) and “wanting” (P <.01) alcohol compared with nonresponders (Figure 2). High responders also reported greater postpriming “wanting” compared with low responders (P <.03). Examination of peak subjective responses indicated that high responders showed greater peak scores on DEQ measures during the open-bar phase compared with non- and low responders (all P <.01 except “high,” which was P <.05) (Figure 2). Low responders reported greater peak “liking” and “wanting” than nonresponders (all P <.01). AUQ total score following the priming phase and AUQ peak total score during the open-bar phase was also greater for high responders compared with other categories (all P <.01), while low responders also reported significantly higher AUQ scores compared with nonresponders (all P <.01) (Supplemental Figure 4).
Figure 2.
Drug Effects Questionnaire (DEQ) priming effect and peak subjective response across early phase response groups. Significant differences are displayed for group comparisons between non-, low, and high responders across DEQ postpriming “liking” (A), “wanting” (B), and DEQ peak subjective response for “liking” (C) and “wanting” (D). High responders showed significantly higher priming effects and peak subjective responses indicating robust exposure-response relationships during free-access i.v. alcohol self-administration (IV-ASA). All P<.05.
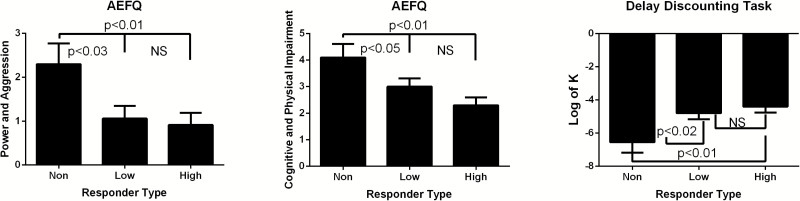
With regard to expectancy measures, high responders had lower expectancy scores for self-reported power and aggression (P <.01) as well as cognitive and physical impairment (P <.01) compared with nonresponders (Figure 3). High and low responders also had higher values (P <.01) for the delayed discounting steepness constant (log K), indicating higher impulsivity, compared with nonresponders.
Figure 3.
Expectancy and impulsivity measures across early phase response groups. Significant differences are displayed for group comparisons between non-, low, and high responders across the Alcohol Effects Questionnaire power and aggression (A), cognitive and physical impairment (B), and delayed discounting task (C). Nonresponders showed significantly greater expectancy of power and aggression as well as cognitive and physical impairment. High and low responders also showed significantly higher steepness constants (log K), suggesting higher impulsivity compared with nonresponders. All P<.05.
Discussion
The goal of this study was to characterize IV-ASA in nondependent drinkers, and the results indicated a robust relationship between exposure and psychopharmacological responses during IV-ASA. IV-ASA measures reflected recent drinking history and were associated with perceptions of rewarding effects following priming and during the session. The early phase of IV-ASA showed substantial interindividual variability in IV-ASA measures, allowing for comparisons between low and high responders, and was particularly sensitive to the motivational properties of alcohol in nondependent drinkers. Self-administration during the session was driven by perceptions of intoxication and urges for more alcohol. Specifically, high responders had greater subjective responses to the priming effects of alcohol and greater peak subjective response measures during the entire open bar session, indicative of a hedonic response to alcohol.
The rate of IV-ASA during the early phase of free access was also associated with expectancy and impulsivity measures. In particular, high responders had lower expectancy scores for power and aggression as well as cognitive and physical impairment. This association may be a contributing factor to drinking patterns during the session and is in agreement with previous findings demonstrating that such expectancies are strong correlates of alcohol use, abuse, and alcoholism (Brown, 1985; Lundahl et al., 1997; Mann et al., 1987). High responders also had greater impulsive personality scores compared with non- and low responders. This finding supports previous studies associating higher impulsivity with drug-seeking and consumption behavior (Belin et al., 2008) and higher stimulant response to i.v. alcohol challenge (Leeman et al., 2014). This study may represent one of the first reports of the relationship between impulsivity and ASA in a human laboratory setting and underscores the significance of impulsivity as a risk factor for alcohol abuse and dependence (Poulos et al., 1995; Petry, 2001; Bjork et al., 2004; Dick 2010).
The free-access IV-ASA paradigm demonstrated a high degree of test-retest reliability in self-administration measures across Group 1 participants. Although a practice session had been advised for preparing the participant for the test session (Zimmermann et al., 2008, 2009), this study found strong correlations between the first and second sessions on all self-administration measures. This level of reliability strongly supports the utility of IV-ASA paradigms for studies evaluating the effectiveness of medications to reduce alcohol drinking and problems, where pre- and posttreatment comparisons are standard. Within sessions, ASA measures were highly inter-correlated, signifying a high level of consistency among measures. On the other hand, there was also substantial inter-individual variability in i.v. self-administration patterns. The variability between participants and the within-participant consistency of behavior offer exciting prospects for examining predictors of self-administration behavior. One of these predictors is recent drinking history. The results of this study showed that free-access IV-ASA was highly sensitive to drinking history and pattern and was driven by the rewarding effects of alcohol. Heavier drinkers showed higher IV-ASA, demonstrating that the free-access paradigm can accurately reproduce recent drinking behavior inside the laboratory, allowing for examination of the pharmacologically rewarding properties of alcohol and its determinants.
There were no significant sex differences in IV-ASA measures in the free-access paradigm, and this was reflected in the lack of sex differences in recent drinking history of participants as measured by TLFB. The only exception was the total EtOH consumed, which is attributable to the natural sex difference in total body water and was managed by the CAIS PBPK model such that the increment in BrAC in response to a button press was identical for men and women. These findings are in contrast to a recent study that demonstrated sex differences in IV-ASA between adolescent males and females, with adolescent females demonstrating lower IV-ASA compared with adolescent males; this difference was mirrored in their recent drinking history as measured by TLFB (Jünger et al., 2016). The current study included males and females that were older than the adolescent sample in the other study, and the sample did not demonstrate sex differences in recent drinking which may have translated into a lack of difference in IV-ASA measures.
This study had several strengths, including a large sample size with a substantial group evaluated for test-retest reliability and exquisite precision in predicting and providing consistent alcohol exposure across participants. One limitation of this study, and of i.v. alcohol studies in general, is that the alcohol is administered i.v., representing a nonnaturalistic route. Moreover, the i.v. route does not provide typical olfactory and gustatory cues associated with oral ingestion. However, by removing these cues, the method allows the consumption behavior to be driven by the pharmacological effects of alcohol, which may be better defined and elucidated by the IV-ASA measures. In addition, the CAIS algorithm provides precision in the BrAC levels following consumption, thus minimizing overexposure (i.e., improving safety) that cannot be achieved with oral self-administration. Another limitation is the lack of a placebo condition. Although we did not provide an alternative reinforcer to the i.v. ethanol, Plawecki et al. (2013) found alcohol to be more rewarding than the alternative reinforcer (saline infusion) in pretreatment conditions using a progressive ratio CAIS paradigm.
IV-ASA using a free-access paradigm is a promising method to examine alcohol consumption behavior while maintaining a high degree of control over the pharmacokinetics of alcohol metabolism. IV-ASA reflects recent drinking history and is consistent between sessions, thus providing a translational tool for examining promising drugs that have modified ASA in animal models of alcoholism (Zimmermann et al., 2013). The initial rate of IV-ASA is particularly sensitive to the rewarding and motivational properties of alcohol and provides a unique and early marker of potential sources of inter-individual variation, including genetic and personality traits, in alcohol craving that may drive risky and more abusive drinking behavior in nondependent drinkers.
Statement of Interest
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Division of Clinical and Biological Research (Z1A AA 000466). The CAIS software was developed with funding from the Indiana Alcohol Research Center (P60 AA 07611). There are no financial interests to disclose.
Supplementary Material
Acknowledgments
The authors express their thanks to Victor Vitvitskiy at the Indiana Alcohol Research Center for assistance with the CAIS software development, support, and technical assistance. The authors gratefully acknowledge the NIH Clinical Center Alcohol Clinic and 5SWS Day Hospital staff for clinical support; Mary Lee, MD, and the late Dan Hommer, MD for medical support; additional laboratory research staff for data collection support; and the study participants for their participation in the study.
References
- Babor TF, Kranzler HR, Lauerman RJ. (1989) Early detection of harmful alcohol consumption: comparison of clinical, laboratory, and self-report screening procedures. Addict Behav 14:139–157. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blekher T, Ramchandani VA, Flury L, Foroud T, Kareken D, Yee RD, Li TK, O’Connor S. (2002) Saccadic eye movements are associated with a family history of alcoholism at baseline and after exposure to alcohol. Alcohol Clin Exp Res 26:1568–1573. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. (2004) Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol 34:133–150. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. (2009) Delayed discounting correlates with proportional lateral frontal cortex volumes. Biological Psychiatry 65:710–713. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606. [DOI] [PubMed] [Google Scholar]
- Brown SA. (1985) Reinforcement expectancies and alcoholism treatment outcome after a oneyear follow-up. J Stud Alcohol 46:304–308. [DOI] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. (1999) Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res 23:195–203. [PubMed] [Google Scholar]
- de Wit H, McCracken SG. (1990) Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res 14:63–70. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. (2010) Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol 15:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. (1991) Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict 86:1563–1570. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Crouss T, Hommer DW. (2012) Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology 37:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA. (2014) Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol 21:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jünger E, Gan G, Mick I, Seipt C, Markovic A, Sommer C, Plawecki MH, O’Connor S, Smolka MN, Zimmermann US. (2016) Adolescent women induce lower blood alcohol levels than men in a laboratory alcohol self-administration experiment. Alcohol Clin Exp Res 40:1769–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwo PY, Ramchandani VA, O’Connor S, Amann D, Carr LG, Sandrasegaran K, Kopecky KK, Li T-K. (1998) Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology 115:1552–1557. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Ralevski E, Limoncelli D, Pittman B, O’Malley SS, Petrakis IL. (2014) Relationships between impulsivity and subjective response in an IV ethanol paradigm. Psychopharmacology (Berl) 231:2867–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl LH, Davis TM, Adesso VJ, Lukas SE. (1997) Alcohol expectancies: effects of gender, age, and family history of alcoholism. Addict. Behav 22:115–125. [DOI] [PubMed] [Google Scholar]
- Mann LM, Chassin L, Sher KJ. (1987) Alcohol expectancies and the risk for alcoholism. J Consult Clin Psychol 55:411–417. [DOI] [PubMed] [Google Scholar]
- Marshall VJ, Ramchandani VA, Kalu N, Kwagyan J, Scott DM, Ferguson CL, Taylor RE. (2014) Evaluation of the influence of alcohol dehydrogenase polymorphisms on alcohol elimination rates in African Americans. Alcohol Clin Exp Res 38:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li TK, O’Connor S. (2002) Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcohol Clin Exp Res 26:1299–1306. [DOI] [PubMed] [Google Scholar]
- Neumark YD, Friedlander Y, Durst R, Leitersdorf E, Jaffe D, Ramchandani VA, O’Connor S, Carr LG, Li T-K. (2004) Alcohol dehydrogenase polymorphisms influence alcohol elimination rates in a male Jewish population. Alcohol Clin Exp Res 28:10–14. [DOI] [PubMed] [Google Scholar]
- Petry NM. (2001) Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 154:243–250. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Wetherill L, Vivitskiy V, Kosobud A, Zimmermann US, Edenberg HJ, O’Connor S. (2013) Voluntary intravenous self-administration of alcohol detects an interaction between GABAergic manipulation and GABRG1 polymorphism genotype: a pilot study. Alcohol Clin Exp Res 37Suppl 1:E152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. (1995) Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol 6:810–814. [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. (1999) A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res 23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Kwo PY, Li T- K. (2001) Effect of food and food composition on alcohol elimination rates in healthy men and women. J Clin Pharmacol 41:1345–1350. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Flury L, Morzorati SL, Kareken D, Blekher T, Foroud T, Li T- K, Connor S. (2002) Recent drinking history: association with family history of alcoholism and the acute response to alcohol during a 60 mg/% clamp. J Stud Alcohol 63:734–744. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S. (2006) Studying alcohol elimination using the alcohol clamp method. Alcohol Res Health 29:286–290. [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li T-K, O’Connor S. (2009) Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res 33:938–944. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. (2011) A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry 16:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ. (1983) Drinking habits and expectancies about alcohol’s effects for self versus others. J Consult Clin Psychol 51:752–756. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. (1992) Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Measuring Alcohol Consumption: Psychosocial and Biochemical Methods (Litten RZ, Allen JP, eds), pp41–72. Totowa, NJ: Humana Press. [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, Jerlhag E, Engel JA, Hodgkinson CA, Egli M, Lopez MF, Becker HC, Goldman D, Heilig M, Ramchandani VA, Leggio L. (2015) The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry 5:e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S. (2008) Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans. Alcohol Clin Exp Res 32:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, O’Connor S. (2009) Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE) Psychopharmacology 202:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA. (2013) Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 13:315–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.