Abstract
Prostate cancer is a prevalent public health problem worldwide. While imaging has played a major role in this disease, there still remain many challenges and opportunities. Positron emission tomography with various physiologically based radiotracers is fundamentally suited to interrogate this biologically and clinically heterogeneous disease along the course of its natural history. In this article, I review briefly the published evidence for the use of positron emission tomography with 18F-fluorodeoxyglucose, 11C-acetate, and 18F- or 11C-choline in the imaging evaluation of prostate cancer. Although the focus of the article will be on these radiotracers given the accumulated experience with them, but I will also comment on the outlook for the use of other emerging PET radiotracers such as those targeted to the prostate-specific membrane antigen and the amino acid metabolism pathway. It is anticipated that PET will play major role in the evaluation of prostate cancer in the current evidence-based medicine environment. There will also be exciting novel prospects for the use of therapeutic-diagnostic (theransotic) pairs in the management of patients with prostate cancer.
Keywords: Prostate, Cancer, PET, Staging, Restaging, Response, Prognosis
Imaging evaluation of prostate cancer remains challenging. This relates to the need for patient-specific and risk-adapted imaging strategy that optimizes diagnostic yield. The conventional imaging modalities include transrectal ultrasonography, multiparametric magnetic resonance (MR) imaging, computed tomography (CT), bone scintigraphy, and In-111 capromab pendetide scintigraphy. However, these imaging modalities do not fully meet the clinical needs of the remarkably heterogeneous biological behavior of prostate cancer. Positron emission tomography (PET) when used with various biologically relevant radiotracers is fundamentally suited to interrogate the underlying pathology in a quantitative manner. Over the past several years, there has been a plethora of research and development activity on the potential utility of PET in the imaging evaluation of prostate cancer. The aim of this article is to review briefly the value of PET along the various clinical phases of the natural history of prostate cancer.
Primary diagnosis and staging
Prostate cancer is typically considered as a suspect diagnosis after an abnormal digital rectal examination and/or high or rising serum prostate-specific antigen (PSA) level. The usual diagnostic approach includes transrectal ultrasonography (TRUS)-guided biopsy using standard 10–12 core biopsy of the gland. With this procedure, less than 1% of gland volume is retrieved for histologic assessment, and therefore, there is significant probability for missing cancer. In fact, up to 40% miss rate has been reported for TRUS-guided biopsy that may then lead to repeat biopsies at still higher miss rates of up to 70% [1, 2].
It is of note that TRUS is only used for guiding the biopsy needle pass to the organ (anatomic localization) and generally lacks the sufficient sensitivity and specificity to detect and localize prostate cancer. Image-guided biopsy optimizes the probability of detection of clinically relevant tumors (e.g., aggressive tumors) and reduces the biopsy rate of clinically insignificant tumors. Image-guided tumor localization and characterization will also pave the way for rational treatment decision-making including active surveillance for low-grade tumors and focal therapy (male lumpectomy) for intraglandular high-grade tumors that is expected to improve overall outcome and quality of life.
Multiparametric MR imaging including diffusion-weighted imaging (DWI) and dynamic contrast enhancement (DCE) at 3-T using pelvic-phased array and endorectal coils has offered an improved diagnostic performance for the imaging evaluation of the prostate gland [3, 4]. In some instances, image-guided biopsy may be performed by either direct MR-guided approach or through dynamic fusion of MR and TRUS images [5]. A recent meta-analysis of the accuracy of multiparametric MR imaging reported relatively high specificity but variable sensitivity for prostate cancer detection [6]. Although a similar finding was also reported by another systematic review and meta-analysis, interestingly, it also found that MRI-targeted biopsy and standard TRUS-guided biopsy did not differ significantly in the overall prostate cancer detection [7]. Nevertheless, MR imaging is particularly helpful for differentiating between organ-confined disease (stage T1 or T2) and early extracapsular extension or seminal vesicle invasion (stage T3). The delineation of the extent of local disease can have important ramification on the treatment selection and patient management.
There is an increasing interest in the potential role of PET in prostate cancer [8]. Given the remarkable biological and clinical heterogeneity of prostate cancer, PET would be an ideal imaging tool for noninvasive interrogation of the underlying tumor biology in different phases of this prevalent disease. The cumulative current experience with PET and the most studied radiotracers, namely 18F-fluorodeoxyglucose (FDG), 11C-acetate, and 18F- or 11C-choline, suggests a generally limited role for these radiotracers in the imaging-based localization and characterization of primary prostate tumor due to the overlap of uptake among normal, benign prostatic hyperplasia, and prostate cancer tissues [9].
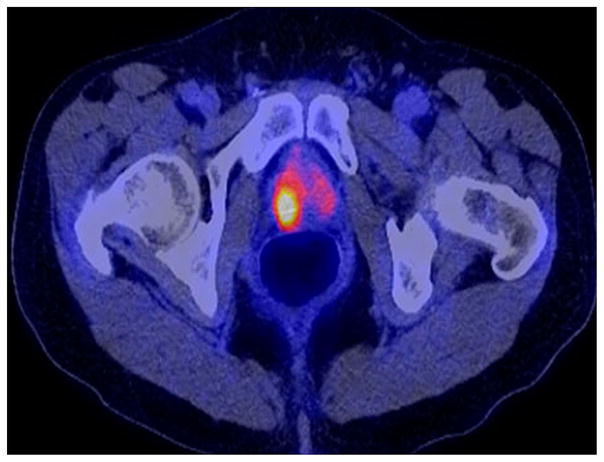
The tumor uptake of FDG is based on the Warburg effect that leads to complex biological mechanisms involved in malignancy-induced enhanced glucose metabolism [10]. Shiiba et al correlated the FDG uptake in primary prostate cancer with the biopsy specimen’s Gleason score [11]. At a cutoff SUVmax of 2.8, the sensitivity and specificity for differentiating between biopsy specimens with a summed Gleason score of 5 or less and specimens with a summed Gleason score of 6 or greater were 62%, and 80.0%, respectively. Minamimoto et al. evaluated FDG PET/CT for detecting prostate cancer in 50 men with elevated serum PSA levels who underwent subsequent prostate biopsy [12]. The sensitivity and specificity were 51.9% and 75.7% for the entire prostate gland; 73% and 64% for the peripheral zone; and 22.7% and 85.9% for the central zone, respectively. The authors concluded that FDG PET/CT may be useful for detection of peripheral zone prostate cancer in men at more than intermediate risk. A recent systematic review and meta-analysis of 47,935 men reported a pooled prevalence of 1.8% for incidental high FDG uptake in the prostate gland [13]. The pooled risk of malignancy with biopsy verification was 62% (95% CI 54–71%). In another similar investigation from South Korea that included 47,109 patients, the prevalence of incidental high FDG uptake in the prostate gland was 2.8% with the rate of observed malignancy that was related to the serum PSA level (3.8% rate of cancer with PSA less than 2.5 ng/mL but 60% rate of cancer with PSA greater than 2.5 ng/mL) [14]. These studies suggest that, in some cases, FDG PET may be able to characterize prostate tumors of sufficient size and malignancy grade (Gleason score of 7 or higher) (Fig. 1). FDG PET/CT is typically not employed for initial staging of disease, although in selected cases with high clinical suspicion for metastatic spread, it may be useful to delineate the extent of the metabolically active disease.
Fig. 1.
Incidental high FDG uptake (maximum SUV 7.7) in right prostate lobe in a 67-year-old man who presented for restaging colon cancer; further follow-up revealed a serum PSA level of 14.6 ng/mL. A subsequent biopsy confirmed prostate cancer with Gleason score of 8. (Reproduced with permission from Ref. 9).
The results for the utility of lipogenesis radiotracers, 11C-acetate, and 11C- or 18F-choline in the imaging evaluation of prostate cancer are generally similar [15]. Acetate is transported across the cellular membrane through the monocarboxylate transporter and participates in production of phospholipids in the cellular membranes facilitated by the fatty acid synthase enzymatic reaction that is up-regulated in cancer [16]. Choline enters the cell through choline transporters and is catalyzed by choline kinase (which is up-regulated in cancer) to form phosphorylcholine, followed by generation of phosphatidylcholine in the tumor cell membrane [17].
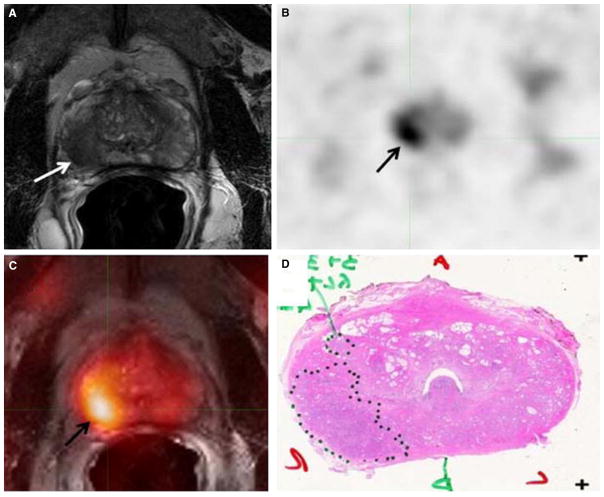
A systematic review and meta-analysis of 11C-acetate PET/CT reported a pooled sensitivity of 75.1% (95% CI 69.8–79.8%) and pooled specificity of 75.8% (95% CI 72.4–78.9%) for detection of primary prostate cancer [18] (Fig. 2). Similar to the case with FDG, the uptake level of lipogenesis tracers in benign and neoplastic prostate tissues can overlap, which is fundamentally related to the nonspecificity of these tracers for cancer [19].
Fig. 2.
58-Year-old male with prostate cancer. A Axial T2-weighted MRI show low-signal intensity in the right mid-gland peripheral zone (white arrow), B concurrent high 11C-acetate uptake is seen in the axial PET image (black arrow), C fused 11C-acetate PET/MRI localizes the tumor (black arrow), D corresponding pathology revealed a Gleason 7 tumor. (Reproduced with permission from Ref. 19).
With regards to initial staging, both acetate and choline may be useful in patients with intermediate to high risk for lymph node involvement. Haseebuddin and colleagues performed 11C-acetate PET/CT in 107 men with prostate cancer with intermediate to high risk (either Gleason score of 7 and serum PSA greater or equal to 10 ng/mL, or Gleason score greater than or equal 8, or serum PSA greater than or equal to 20 ng/mL) for lymphadenopathy who were scheduled to undergo radical prostatectomy [20]. The sensitivity and specificity for detection of pelvic lymphadenopathy were 68% and 78%, respectively. Patients with positive PET scans had 3.3 times higher risk for therapy failure after surgery. Therefore, in selected intermediate to high-risk cases, 11C-acetate may provide useful information that then can lead to management change at the time of initial staging [21].
The detection of prostate tumor with 11C-choline may depend on tumor configuration with unifocal cancers detected more often than those that are multifocal or rindlike. Moreover, the extent of actual tumor may not completely overlap the area with abnormal uptake [22, 23]. Scher et al. reported a sensitivity of 87% and specificity of 62% for the detection primary prostate cancer with histopathological examination of the resection specimen or biopsy as reference standard [24]. However, another group of investigators from Italy reported a sensitivity of 66% and specificity of 81% for localization of primary prostate cancer on a sextant histopathologic analysis [25]. Martorana et al. reported a sensitivity of 83% in detection of primary tumor nodules of 5 mm larger although the sensitivity for assessment of extraprostatic extension was inferior to MR imaging (22% for choline PET vs. 63% for MR imaging, P < 0.001) [26]. Overall, while choline PET may be helpful in detecting primary prostate cancer, but the diagnostic performance may depend on several important factors such as tumor grade, size, and location. Eschmann et al. compared 11C-choline PET/CT with whole-body MRI for staging of prostate cancer with histologic analysis and follow-up as validation criteria [27]. The sensitivity and specificity were 97% and 77%, respectively, for 11C choline PET and 79% and 94% for whole-body MRI. These results suggested that PET and MRI might provide complementary diagnostic information in the initial staging of prostate cancer.
The potential use of other PET radiotracers in the setting of primary tumor detection and initial staging is uncertain in view of little published reports. There is one case report of the potential use of 68Ga-labeled ligand of prostate-specific membrane antigen (PSMA) in the initial diagnosis setting [28]. However, another recent case report, using this tracer, has indicated limitations with false-negative results in the poorly differentiated tumors with neuroendocrine transdifferentiation [29]. We recently reported a clinical case example of a patient with elevated serum PSA level of 10.5 ng/mL and a prior negative standard TRUS biopsy who underwent a clinical 3-T multiparametric MRI and a research protocol PET/CT with the cellular proliferation radiotracer 18F-FMAU (2′-deoxy-2′-[18F]fluoro-5-methyl-1-β-D-arabi-nofuranosyluracil). The PET/CT and multiparametric MRI were fused with TRUS for real-time hybrid image-based targeting of the biopsy needle to an area with abnormal tracer localization, which on histopathology revealed early malignancy [30].
Biochemical recurrence and restaging
Localized disease is treated with curative intent (radical prostatectomy, radiation therapy). However, up to 35% of patients (or higher in select high-risk groups) may experience biochemical recurrence (PSA relapse) within a decade of primary “curative” therapy [31]. Localization of disease in this group of patients is pivotal as it directs appropriate management, which may include salvage therapy (surgery, radiation) for local recurrence and systemic therapy for metastatic disease, or both. Biochemical failure is defined as an increase in serum PSA level with negative standard imaging studies after definitive therapy for primary prostate cancer. The American Urologic Association (AUA) defines biochemical recurrence in post-prostatectomy patients as an initial serum PSA level of 0.2 ng/mL or higher, with a second confirmatory level higher than 0.2 ng/mL [32]. The American Society for Therapeutic Radiology and Oncology consensus definition for biochemical failure after primary external beam radiotherapy is an increase of 2 ng/mL or more above the nadir PSA level, regardless of hormonal therapy [33].
In general, FDG PET appears to have a limited role in this clinical setting, although higher PSA levels may be associated with higher probability of detection of metabolically active disease. In one study, FDG PET demonstrated sensitivity and specificity of 75% and 100%, respectively, for the detection of pelvic lymph node metastases, with validation based on histopathologic examination of the surgically harvested nodes [34]. We have reported our findings of a prospective investigation on the potential utility of FDG PET/CT and 18F-NaF PET/CT in detection of occult metastases in 37 men with PSA relapse (range, 0.5–40.2 ng/mL) and strictly negative standard imaging studies (as required by definition) [35]. FDG PET/CT only was positive in one patient, 18F-NaF PET/CT only was positive in eight patients and both were positive in another two patients. Overall we found a detection rate of 8.1% for FDG PET/CT in the setting of biochemical recurrence.
Most studies with 11C- and 18F-choline in prostate cancer have been in the biochemical recurrence phase of the disease. Umbehr et al provided a systematic review and meta-analysis of 11C- and 18F-choline in restaging patients with biochemical recurrence. They reported, on a per-patient basis (12 studies, 1055 patients), a pooled sensitivity and specificity of 85% (95% CI 79–89%) and 88% (95% CI 73–95%), respectively [36]. A similar report by von Eyben et al examined 47 articles and data from 3167 patients with regards to the diagnostic utility of choline PET/CT in staging and restaging of prostate cancer [37]. They found that there were statistically significant more positive results in the prostate bed of biochemically relapsed patients who had previously undergone external beam radiation therapy than those patients who had radical prostatectomy as the initial treatment strategy. Moreover, choline PET/CT led to a change in treatment in 381 (41%) of 938 patients, leading to complete PSA response in 101 of 404 (25%) patients. Another systematic review and meta-analysis by Evangelista and colleagues (19 studies, including 12 studies for all sites of disease, 3 for lymph node metastases, and 4 for local recurrence, 1555 patients), on the use of choline PET and PET/CT in biochemical relapse of prostate cancer reported a pooled sensitivity of 85.6% (95% CI 82.9–88.1%) and pooled specificity of 92.6% (95% CI 90.1–94.6%) for all sites of disease (prostatic fossa, lymph nodes, and bone), a pooled sensitivity of 75.4% (95% CI 66.9–82.6%) and pooled specificity of 82% (95% CI 68.6–91.4%) for prostatic fossa recurrence, and a pooled sensitivity of 100% (95% CI 90.5–100%) and pooled specificity of 81.8% (95% CI 48.2–97.7%) for lymph node metastases [38]. The reported 100% pooled sensitivity for detection of lymph node metastases may have been overestimated given the small number of publications that were included in the meta-analysis.
It has been noted that the diagnostic performance of choline PET/CT may depend on serum prostate-specific antigen (PSA) level and kinetics. Treglia et al. performed a systematic review of 14 articles with the specific focus on the relationship between PSA level and kinetics (e.g., PSA doubling time or PSAdt, and PSA velocity or PSAvel) on the lesion detection rate in restaging prostate cancer [39]. The overall pooled detection rate of choline PET/CT in restaging prostate cancer was 58% [95% CI 55–60]. The pooled detection rate increased to 65% (95% CI 58–71) when PSAdt was ≤6 months and to 71% (95% CI 66–76) and 77% (95% CI 71–82) when PSAvel was >1 or >2 ng/(mL year), respectively. More recently, a retrospective multicenter study of 374 patients with biochemical relapse showed that a Gleason score of <5 or greater than or equal 8 could differentiate positive from negative PET. In this regard, the optimal threshold values for trigger PSA and PSAdt were 3 ng/mL and 6 months, respectively. Interestingly, in patients with PSA less than 1.5 ng/mL, about 31% had evidence disease on choline PET, with 7% demonstrating metastases [40].
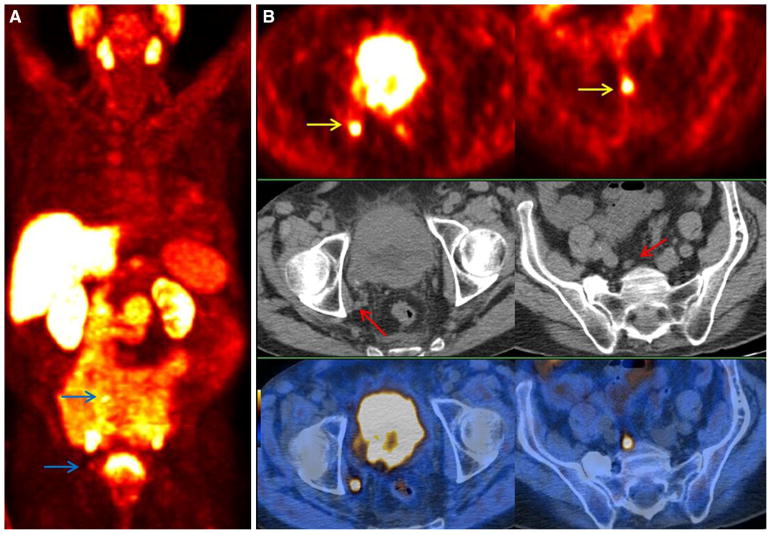
Overall, there is relatively convincing evidence for the first-line use of choline PET/CT in restating of patients with biochemical relapse of prostate cancer with a detection rate that is positively associated with increasing serum PSA level, increasing PSA velocity and decreasing PSA doubling time [41] (Fig. 3). It is of note that on September 12, 2012, the U.S. Food and Drug administration approved the production and use of 11C-choline at the Mayo Clinic PET Radiochemistry Facility (Rochester, MN) for “PET imaging of patients with suspected prostate cancer recurrence and noninformative bone scintigraphy, CT, or MR imaging.” While the Mayo Clinic was the first approved facility, the list of approved facilities is anticipated to grow since the current National Comprehensive Cancer Network guidelines (version 1.2015) includes 11C-choline PET as a possible imaging test to consider in the evaluation of men with biochemical recurrence. 11C-choline is also reimbursable by the Centers for Medicare and Medicaid Services through local Medicare Administrative Contractors who determine coverage within their respective jurisdictions for PET using radiopharmaceuticals for their labeled indications for oncologic imaging that are approved by the U.S. Food and Drug Administration.
Fig. 3.
A 63-year-old patient who had underwent radical prostatectomy and radiotherapy of primary prostate followed by androgen deprivation therapy for primary prostate cancer (pT3a, N0, Gleason score of 9, and primary PSA level of 9.0 ng/mL). At the time of imaging he presented with increasing PSA level of 22.56 ng/mL. A 18F-fluorocholine PET maximum intensity projection image shows multiple sites of pathologic increased tracer in pelvis and retroperitoneum (blue arrow). B Transaxial PET/CT images (top: 18F-fluorocholine PET; middle: CT; bottom: PET/CT) show LN metastases with high tracer uptake in right external iliac (left, yellow arrow) and common iliac (right, yellow arrow) nodal basins; CT (middle) demonstrates only small lymph nodes (red arrows). (Reproduced with permission from Ref. 66).
There is limited experience with other PET radiotracers in the clinical setting of biochemical recurrence. Recently, our group at the University of Southern California reported on a comprehensive extraction and reanalysis of the PET detection data for 5 tracers ((18)F-fluorodeoxyglucose (FDG), 11C-acetate, 11C- or 18F-choline, anti-1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid (FACBC), and radiolabeled ligand targeted to prostate-specific membrane antigen, PSMA) that have been explored in prostate cancer [42]. We found that FDG exhibited the lowest detection rate for any suspected disease. 11C-acetate tended to be advantageous over radiolabeled choline in detecting local recurrence and lymph node lesions, although the difference was not statistically significant. FACBC had greater likelihood of detecting local recurrence, when compared to radiolabeled choline, although again this difference was not statistically significant. PSMA-based tracers tended to show higher proportion of patients with suspected disease compared to the other four tracers [43].
Treatment response evaluation and prognostication
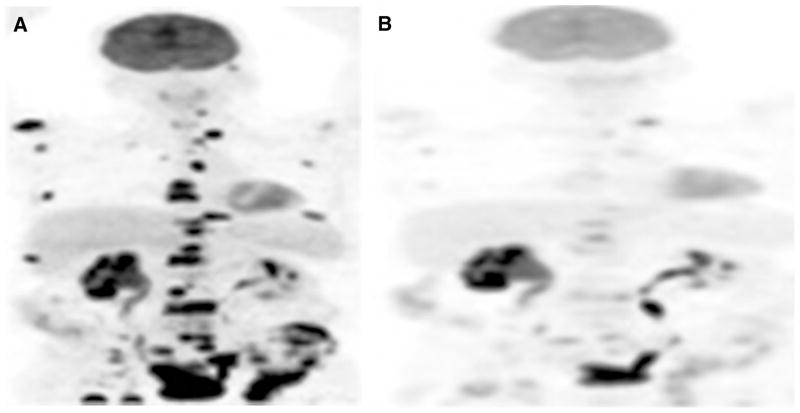
Literature on the potential utility of PET with various tracers in the imaging evaluation of treatment response in prostate cancer is relatively limited. Our preliminary results show that tumor FDG uptake decreases with successful treatment (using androgen deprivation or various chemotherapy regimens), although imaging findings may be discordant with those of other manifestations of disease including changes in the levels of serum PSA or circulating tumor cells (Fig. 4). Also, there may be differences in imaging-based assessment based on the response criteria that are employed in the data analysis such as Response Evaluation Criteria In Solid Tumors (RECIST 1.0 and RECIST 1.1) or PET Response Criteria in Solid Tumors (PERCIST 1.0) [44, 45]. Another preliminary study from our group using 18F-NaF PET/CT showed that semiquantitative 18F-NaF PET-based analysis performs better than PSA-based response assessment criteria and is associated better than qualitative criteria with changes in therapy [46]. Clearly additional studies are needed to decipher the optimal combination of relevant data that can most accurately reflect the effect of various current and novel therapies.
Fig. 4.
67-Year-old male with castrate-resistant metastatic prostate cancer. FDG PET maximum intensity projection images before (A, PSA of 223.3 ng/mL) and after (B, PSA of 52.5 ng/mL) chemotherapy show marked decline in metabolic activity of multiple metastatic lesions in response to therapy.
Yu et al. reported in separate studies that 11C-acetate and 18F-NaF PET may be helpful in response assessment of bone metastases to therapy [47, 48]. Single case reports or small case series have been reported that suggest 18F-NaF and 11C-choline may be useful in the assessment response to Ra-223 dichloride therapy [49, 50]. Similar preliminary results have been reported for effects of neodjuvant androgen deprivation and radical prostate radiotherapy with concurrent androgen deprivation therapy on uptake level of 11C-choline in tumors [51, 52]. There is currently no experience with other PET radiotracers in the clinical setting of treatment response assessment in patients with prostate cancer.
Prognostication
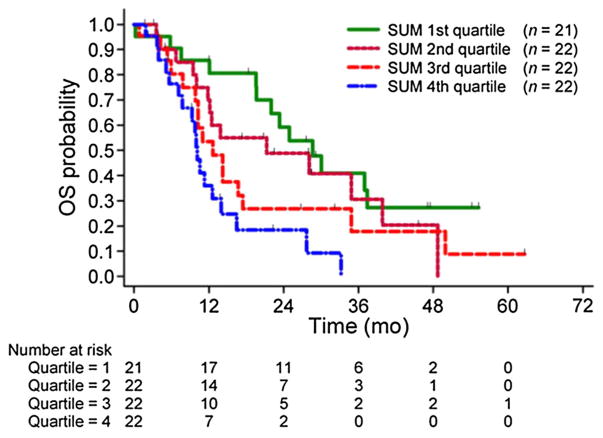
Until recently most published articles focused on the diagnostic rather than the prognostic utility of PET in prostate cancer. Oyama et al. investigated the prognostic value of glucose metabolism of the primary tumors in 42 men with prostate cancer [53]. These authors showed that the FDG uptake level in the primary was positively correlated to relapse-free survival after radical prostatectomy. Patients with higher tumor uptake had a significantly poorer prognosis compared with those patients with tumors that showed lower FDG uptake. In another investigation of 43 patients with metastatic castrate-resistant prostate, the FDG uptake in most active lesion was positively correlated with overall survival [54]. Jadvar and colleagues reported on a cohort of 87 patients with metastatic castrate-resistant prostate cancer who underwent FDG PET/CT and were followed-up with prospectively for overall survival. In the multivariate analysis that adjusted for the potentially prognostic clinical parameters (age, serum PSA level, serum alkaline phosphatase level, use of pain medication, prior chemotherapy, and Gleason score at initial diagnosis), the sum of the maximum standardized uptake value (sum of up to 25 active lesions including lymph nodes, bone, and soft tissue metastases) was significant with a hazard ratio of 1.01 (95% CI 1.001–1.020; P = 0.053) [55] (Fig. 5).
Fig. 5.
Kaplan–Meier plot of overall survival probability in men with castrate-resistant metastatic prostate cancer against sum of SUVmax of FDG-avid metastatic lesions grouped into quartiles. Patients in fourth-quartile group (blue line) have significantly poorer survival probability than reference first-quartile group (green line). (Reproduced with permission from Ref. 55).
In a study that reported on the comparative utility of 11C-choline PET/CT over clinical staging nomograms for preoperative staging of lymph nodes in intermediate-risk and high-risk prostate cancer, 11C-choline PET/CT performed better than clinical nomograms with equal sensitivity and better specificity [56]. Gacci and colleagues in a longitudinal study of 103 patents with biochemical recurrence showed that an increase in serum PSA from baseline by greater than 5 ng/mL, decrease in PSA doubling time by less than 6 months, and increase in PSA velocity by greater than 6 ng/mL/month were highly associated with the outcome of progression on the follow-up PET/CT (6 months after baseline PET/CT) [57].
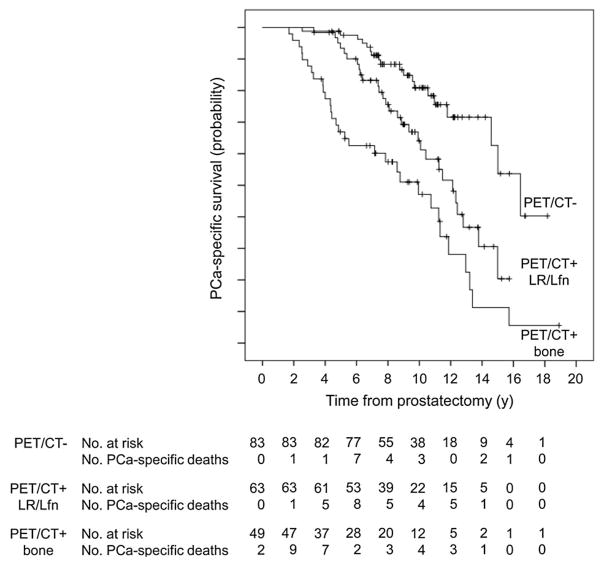
Breeuwsma and colleagues associated the findings on 11C-choline PET/CT with disease-specific survival in 64 men with biochemical recurrence after radical prostatectomy [58]. The investigators found that disease-specific survival was significantly higher in the negative PET/CT group than the group with positive PET/CT. In another similar study from Italy, the investigators evaluated retrospectively the potential utility of 11C-choline PET/CT in prediction of prostate cancer-specific survival in 195 patients who presented with biochemical failure (PSA > 0.2 mg/mL during androgen deprivation therapy) after radical prostatectomy. The median prostate cancer-specific survival in patients with positive and negative 11C-choline PET/CT was 11.2 and 16.4 years, respectively [59] (Fig. 6). Kwee et investigated the prognostic significance of metabolically active tumor volume (MATV) and activity distribution within the lesion volume, termed total lesion activity (TLA) on 18F-fluorocholine PET/CT in 30 men with castrate-resistant prostate cancer. The authors found that both net MATV and net TLA were significantly associated with overall survival [60].
Fig. 6.
Prostate cancer-specific survival probability curves in patients with negative 11C-choline PET/CT (PET/CT−), in patients with 11C-choline PET/CT suggestive of local recurrence or lymphadenopathy (PET/CT+ LR/Lfn), and in patients with 11C-choline PET/CT suggestive of bone metastases (PET/CT+ bone). Patients with abnormal tracer uptake in the prostatic bed or in lymph nodes but no pathologic bone uptake had shorter prostate cancer-specific survival in comparison to patients with negative PET/CT but longer prostate cancer-specific survival in comparison to patients with skeletal metastatic disease. (Reproduced with permission from Ref. 59).
Other emerging PET radiotracers
This article focused on PET radiotracers for which more experience has been accumulated. However, there are a number of other emerging PET radiotracers that are being actively pursued in this clinical setting, especially not only in Europe and Japan, but also under investigational new drug application at some centers in the U.S. These radiotracers include but are not limited to those that target the PSMA, the gastrin-releasing peptide receptor, and the amino acid metabolic pathway [8]. The recently developed PET radiotracers that target the extracellular moiety of the PSMA employ labeled agents that involve antibodies, antibody fragments, aptamers, and PSMA inhibitors [61]. Particularly, the 68Ga-labeled PSMA inhibitor, Glu-NH-CO-NHLys-(Ahx)-[68Ga-HBED-CC], abbreviated as 68Ga-PSMA, has received much attention demonstrating high-diagnostic performance in early human studies. The construct may also be amenable for use in targeted radionuclide therapy with an appropriate particle emitter [62].
Anti-1-Amino-3-(18F)-Fluorocyclobuate-1-Carboxylic (anti[18F]-FACBC or 18F-fluciclovine) is another PET radiotracer that shows promise in the imaging evaluation of prostate cancer. This agent is a radiolabeled synthetic l-leucine with relatively little renal excretion and cellular accumulation that is based on the expression of the alanine, serine-, and cysteine preferring system-mediated amino acid transport system [63]. A recently published systematic review and meta-analysis reported a pooled sensitivity of 87% and a pooled specificity of 66% on a per-patient-based analysis in detecting prostate carcinoma recurrence [64]. Other investigations suggest that 18F-flucoclovine may be advantageous over 11-choline in the setting of biochemical recurrence of prostate cancer [65].
Conclusion
PET-CT and PET-MRI will have a major role in the imaging evaluation of patients with prostate cancer. The current published evidence is mostly on the diagnostic utility of PET-CT with radiolabeled choline in men with biochemical recurrence. In this clinical setting, PET with radiolabeled choline can be useful to detect and localize local recurrence and distant disease sites with an accuracy that is positively related to the serum PSA level and its derived parameters. There is, however, much room for additional studies with well-defined patient cohorts and select outcomes to decipher the exact role of PET with different radiotracers along the various phases of the natural history of this prevalent disease.
Acknowledgments
Supported by the National Institutes of Health, National Cancer Institute, grants number R01-CA111613, R21-CA142426, R21-EB017568 and P30-CA014089.
Footnotes
Conflict of Interest. The author declares no conflicts of interest.
References
- 1.Presti J., Jr Does the yield of prostate cancer biopsy and repeat biopsy justify the frequency of their use? Nat Clin Pract Urol. 2008;5:246–247. doi: 10.1038/ncpuro1056. [DOI] [PubMed] [Google Scholar]
- 2.Keetch DW, Catalona WJ, Smith DS, et al. Serial prostatic biopsies in men with persistently elevated serum prostate antigen values. J Urol. 1994;151:1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 3.Bonekamp D, Jacobs MA, El-khouli R, et al. Advancements in MR imaging of the prostate: from diagnosis to interventions. Radiographics. 2011;31:677–703. doi: 10.1148/rg.313105139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeks CMA, Barentsz JO, Hambrock T, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 5.Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with negative finding on multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–172. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 6.de Rooj M, Crienen S, Witjes JA, et al. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: a modeling study from a health care perspective. Eur Radiol. 2014;66:430–436. doi: 10.1016/j.eururo.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Radiol. 2015;68:438–450. doi: 10.1016/j.eururo.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Jadvar H. Molecular imaging of prostate cancer: PET radiotracers. AJR Am J Roentgenol. 2012;199:278–291. doi: 10.2214/AJR.12.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C choline. J Nucl Med. 2010;52:81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Shiiba M, Ishihara K, Kimura G, et al. Evaluation of primary cancer using (11C)-methionine PET/CT and (18)F-FDG-PET/CT. Ann Nucl Med. 2011;26:138–145. doi: 10.1007/s12149-011-0551-6. [DOI] [PubMed] [Google Scholar]
- 12.Minamimoto R, Uemura H, Sano F, et al. The potential of FDG PET/CT for detecting prostate cancer in patients with an elevated serum PSA level. Ann Nucl Med. 2011;25:21–27. doi: 10.1007/s12149-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 13.Bertagna F, Sadeghi R, Giovanella L, et al. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland. Systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin. 2014;53(6):249–258. doi: 10.3413/Nukmed-0668-14-05. [DOI] [PubMed] [Google Scholar]
- 14.Kwon T, Jeong IG, You D, et al. Prevalence and clinical significance of incidental (18)F-fluorodeoxyglucose uptake in prostate. Korean J Urol. 2015;56:288–294. doi: 10.4111/kju.2015.56.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchegger F, Garibotto V, Zilli T, et al. First imaging results of an intraindividual comparison of (11)C-acetate and (18)F-fluorocholine PET/CT in patients with prostate cancer at early biochemical first or second relapse after prostatectomy or radiotherapy. Eur J Nucl Med Mol Imaging. 2014;41:68–78. doi: 10.1007/s00259-013-2540-6. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto M, Waki A, Yonekura Y, et al. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: acetate metabolism in tumor cells. Nucl Med Biol. 2001;28:117–122. doi: 10.1016/s0969-8051(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 17.Janardhan S, Srivani P, Sastry GN. Choline kinase: an important target for cancer. Curr Med Chem. 2006;13:1169–1186. doi: 10.2174/092986706776360923. [DOI] [PubMed] [Google Scholar]
- 18.Mohsen B, Giorgio T, Rasoul ZS, et al. Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU Int. 2013;112:1062–1072. doi: 10.1111/bju.12279. [DOI] [PubMed] [Google Scholar]
- 19.Mena E, Turkbey B, Mani H, et al. 11C-acetate PET/CT in localized prostate cancer: a study with MRI and histopathologic correlation. J Nucl Med. 2012;53:538–545. doi: 10.2967/jnumed.111.096032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haseebuddin M, Dehdashti F, Siegel BA, et al. 11C-acetate PET/CT before radical prostatectomy: nodal staging and treatment failure prediction. J Nucl Med. 2013;54:699–706. doi: 10.2967/jnumed.112.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strandberg S, Karlsson CT, Sundström T, et al. (11)C-acetate PET/CT in pre-therapeutic lymph node staging in high-risk prostate cancer patients and its influence on disease management—a retrospective study. EJNMMI Res. 2014;4:55. doi: 10.1186/s13550-014-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Souvatzoglou M, Weirich G, Schwarzenboeck S, et al. The sensitivity of [11C]choline PET/CT to localize prostate cancer depends on the tumor configuration. Clin Cancer Res. 2011;17:3751–3759. doi: 10.1158/1078-0432.CCR-10-2093. [DOI] [PubMed] [Google Scholar]
- 23.Grosu AL, Weirich G, Wendl C, et al. 11C-Choline PET/pathology image coregistration in primary localized prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:2242–2248. doi: 10.1007/s00259-014-2861-0. [DOI] [PubMed] [Google Scholar]
- 24.Scher B, Seitz M, Albinger W, et al. Value of 11C-choline PET and PET-CT in patients with suspected prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34:45–53. doi: 10.1007/s00259-006-0190-7. [DOI] [PubMed] [Google Scholar]
- 25.Farsad M, Schiavina R, Castellucci P, et al. Detection and localization of prostate cancer: correlation of (11C) C-choline PET/CTPET-CT with histopathologic step-section analysis. J Nucl Med. 2005;46:1642–1649. [PubMed] [Google Scholar]
- 26.Martorana G, Schiavina R, Cort B, et al. 11C-choline positron emission tomography/computed tomography for tumor localization of primary prostate cancer in comparison with 12-core biopsy. J Urol. 2006;176:954–960. doi: 10.1016/j.juro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Eschmann SM, Pfannenberg AC, Rieger A, et al. Comparison of 11C-choline PET-CT and whole body MRI for staging of prostate cancer. Nuklearmedizin. 2007;46:161–168. doi: 10.1160/nukmed-0075. [DOI] [PubMed] [Google Scholar]
- 28.Eiber M, Nekolla SG, Maurer T, et al. 68Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom Imaging. 2015;40:1769–1771. doi: 10.1007/s00261-014-0301-z. [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty PS, Tripathi M, Agarwal KK, et al. Metastatic poorly differentiated prostatic carcinoma with neuroendocrine differentiation: negative on 68Ga-PSMA PET/CT. Clin Nucl Med. 2015;40:e163–e166. doi: 10.1097/RLU.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 30.Jadvar H, Chen K, Ukimura O. Targeted prostate gland biopsy with combined transrectal ultrasound, mpMRI, and 18F-FMAU PET/CT. Clin Nucl Med. 2015;40:e426–428. doi: 10.1097/RLU.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruce JY, Lang JM, McNeel DG, et al. Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol. 2012;10:716–722. [PubMed] [Google Scholar]
- 32.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 33.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Chang CH, Wu HU, Tsai JJ, et al. Detecting metastatic pelvic lymph nodes by 18F-2-deoxyglucose positron emission tomography in patients with prostate specific antigen relapse after treatment for localized prostate cancer. Urol Int. 2003;70:311–315. doi: 10.1159/000070141. [DOI] [PubMed] [Google Scholar]
- 35.Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18FNaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umbehr MH, Muntener M, Hany T, et al. The role of choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64:106–117. doi: 10.1016/j.eururo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 37.von Eyben FE, Kairemo K. Meta-analysis of 11C-choline and 18F-choline PET/CT for management of patients with prostate cancer. Nucl Med Commun. 2014;35:221–230. doi: 10.1097/MNM.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 38.Evangelista L, Zattoni F, Guittilla A, et al. Choline PET and PET/CT and biochemical relapse of prostate cancer. Clin Nucl Med. 2013;38:305–314. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 39.Treglia G, Ceriani L, Sadeghi R, et al. Relationship between prostate-specific antigen kinetics and detection rate of radiolabeled choline PET/CT in restaging prostate cancer patients: a meta-analysis. Clin Chem Lab Med. 2014;52:725–733. doi: 10.1515/cclm-2013-0675. [DOI] [PubMed] [Google Scholar]
- 40.Rodado-Marina S, Coronado-Poggio M, Garcia-Vicente AM, et al. Clinical utility of (18) F-fluorocholine positron emission tomography/computed tomography (PET/CT) in biochemical relapse of prostate cancer after radical treatment: results of a multi-center study. BJU Int. 2015;115:874–883. doi: 10.1111/bju.12953. [DOI] [PubMed] [Google Scholar]
- 41.Castellucci P, Picchio M. 11C-choline PET/CT and PSA kinetics. Eur J Nucl Med Mol Imaging. 2013;40(Suppl 1):S36–S40. doi: 10.1007/s00259-013-2377-z. [DOI] [PubMed] [Google Scholar]
- 42.Yu CY, Desai B, Ji L, Groshen S, et al. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging. 2014;4:580–601. [PMC free article] [PubMed] [Google Scholar]
- 43.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 44.Jadvar H, Desai B, Ji L, et al. RECIST 1.0, PERCIST 1.0 and PSA treatment response criteria in metastatic castrate-resistant prostate cancer. Radiological Society of North America annual meeting; Chicago, IL. 2013. [Abstract] [Google Scholar]
- 45.Jadvar H, Desai B, Ji L, et al. Comparison of RECIST 1.0, PERCIST 1.0 and PCWG2 treatment response criteria in meta-static castrate-sensitive prostate cancer. Society of nuclear medicine and molecular imaging annual meeting; Baltimore, MD. 2015. [Abstract] [Google Scholar]
- 46.Doroudinia A, Desai B, Yoon J, et al. Treatment response assessment in metastatic prostate cancer with 18F-NaF PET/CT. Society of Nuclear Medicine and Molecular Imaging annual meeting; Baltimore, MD. 2015. [Abstract] [Google Scholar]
- 47.Yu EY, Muzi M, Hackenbracht JA, Rezvani BB, et al. C11-acetate and F-18 FDG PET for men with prostate cancer bone metastases: elative finding and response to therapy. Clin Nucl Med. 2011;36:192–198. doi: 10.1097/RLU.0b013e318208f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu EY, Duan F, Muzi M, et al. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: results from American College of Radiology Imaging Network 6687. J Nucl Med. 2015;56:354–360. doi: 10.2967/jnumed.114.146936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook G, Jr, Parker C, Chua S, et al. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin) EJNMMI Res. 2011;1:4. doi: 10.1186/2191-219X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyazaki KS, Kuano Y, Kwee SA. Changes in skeletal tumor activity on (18)F-choline PET/CT in patients receiving (223)Radium radionuclide therapy for metastatic prostate cancer. Nucl Med Mol Imaging. 2015;49:160–164. doi: 10.1007/s13139-014-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Challapalli A, Barwick T, Tomasi G, et al. Exploring the potential of [11C]choline PET/CT as a novel imaging biomarker for predicting early treatment response in prostate cancer. Nucl Med Commun. 2014;35:20–29. doi: 10.1097/MNM.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 52.Amanie J, Jane HS, Wuest M, et al. Analysis of intraprostatic therapeutic effects in prostate cancer patients using [(11)C]-choline PET/CT after external beam radiation therapy. Curr Oncol. 2013;20:104–110. doi: 10.3747/co.20.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oyama N, Akino H, Suzuki Y, et al. Prognostic value of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography imaging for patients with prostate cancer. Mol Imaging Biol. 2002;4:99–104. doi: 10.1016/s1095-0397(01)00065-6. [DOI] [PubMed] [Google Scholar]
- 54.Meirelles GS, Schoder H, Ravizzini GC, et al. Prognostic value of baseline [18F]fluorodeoxyglucose positron emission tomography and 99mTc-MDP bone scan in progressing metastatic prostate cancer. Clin Cancer Res. 2010;16:6093–6096. doi: 10.1158/1078-0432.CCR-10-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jadvar H, Desai B, Ji L, et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schiavina R, Scattoni V, Castellucci P, et al. 11c Choline positron emission tomography/computed tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol. 2008;54:392–410. doi: 10.1016/j.eururo.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 57.Gacci M, Cai T, Siena G, et al. Prostate-specific antigen kinetics parameters are predictive of positron emission tomography features worsening in patients with biochemical relapse after prostate cancer treatment with radical intent: results from a longitudinal cohort study. Scand J Urol. 2014;48:259–267. doi: 10.3109/21681805.2013.846936. [DOI] [PubMed] [Google Scholar]
- 58.Breeuwsma AJ, Rybalov M, Leliveld AM, et al. Correlation of [11C]choline PET/CT with time to treatment and disease-specific survival in men with recurrent prostate cancer after radical prostatectomy. Q J Nucl Med Mol Imaging. 2012;56:440–446. [PubMed] [Google Scholar]
- 59.Giovacchini G, Picchio M, Garcia-Parra R, et al. 11C-Choline PET/CT predicts cancer-specific survival in patients with biochemical failure during androgen-deprivation therapy. J Nucl Med. 2014;55:233–241. doi: 10.2967/jnumed.113.123380. [DOI] [PubMed] [Google Scholar]
- 60.Kwee SA, Lim J, Watanabe A, et al. Prognosis related to metastatic burden measured by 18F-fluorocholine PET/CT in castration-resistant prostate cancer. J Nucl Med. 2014;55:905–910. doi: 10.2967/jnumed.113.135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mease RC, Foss CA, Pomper MG. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr Top Med Chem. 2013;13:951–962. doi: 10.2174/1568026611313080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jadvar H. PSMA PET in prostate cancer. J Nucl Med. 2015;56:1131–1132. doi: 10.2967/jnumed.115.157339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okudaira H, Shikano N, Nishii R, et al. Putative transport mechanism and intracellular fate of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med. 2011;52:822–829. doi: 10.2967/jnumed.110.086074. [DOI] [PubMed] [Google Scholar]
- 64.Ren J, Yuan L, Wen G, et al. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta Radiol. 2015 doi: 10.1177/0284185115581541. [DOI] [PubMed] [Google Scholar]
- 65.Nanni C, Schiavina R, Brunocilla E, et al. 18F-Fluciclovine PET/CT for the detection of prostate cancer relapse: a comparison to 11C-choline PET/CT. Clin Nucl Med. 2015;40:e386–e391. doi: 10.1097/RLU.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 66.Beheshti M, Haim S, Zakavi R, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: influence of androgen deprivation therapy and correlation with PSA kinetics. J Nucl Med. 2013;54:833–840. doi: 10.2967/jnumed.112.110148. [DOI] [PubMed] [Google Scholar]