Introduction
Cyanide is a very potent mitochondrial inhibitor with a rapid onset of action. The majority of reported cyanide exposures are unintentional from sources such as smoke inhalation, especially following the combustion of materials that include wool, silk, synthetic rubber, and polyurethane. There is also potential for intentional cyanide poisoning that include suicide, homicide and an agent of terrorism.1-3
Cyanide poisoning should be considered in any patient with rapid collapse and metabolic acidosis. Common physical exam findings in cyanide poisoning include bright red venous blood or cherry-red skin.4,5 While whole blood cyanide testing is available there are limitations that include false negatives due to high volatility and the lack of timely availability of test results.6 Other biomarkers have been suggested as a surrogate marker of consequential cyanide exposure such as lactate and base deficit.7,8 This is also not reliable and a more sensitive assay is required.
Mitochondrial bioenergetics represents a growing field with the potential to predict severity of disease, response to treatment, and prognosis. Advances in relevant instruments have allowed the sensitive measurement of mitochondrial respiration in peripheral blood cells in clinical disease that include inherited mitochondrial disorders, diabetes and heart disease.9,10 The study of mitochondrial bioenergetics have also extended to areas of acute care that include sepsis and traumatic injuries. Whole blood cells such as peripheral blood mononuclear cells (PBMCs) and platelets have been explored for their potential to act as sensitive biomarkers for mitochondrial dysfunction in lieu of invasive and time consuming tissue biopsies.11-13
Measurement of mitochondrial respiration in cells isolated from human blood offers a more sensitive assay for mitochondrial poisons such as cyanide by directly measuring mitochondrial respiration. The objective of this study is to measure mitochondrial respiration using intact cells from whole blood exposed to cyanide as a new biomarker for mitochondrial inhibition.
Methods and Material
Study Design
This study was an ex vivo experiment using peripheral blood obtained from healthy human volunteers. The study was approved by the institutional review board of the University of Pennsylvania. Written informed consent was obtained from all participants.
Study Setting and Population
This study was conducted at a large, urban, academic emergency department. We recruited subjects from amongst the staff at an emergency medicine residency program. Participation was voluntary and free from cocercion. Exclusion criteria included patients less than 18 years of age, history of smoking and pregnancy. Subjects were enrolled between July 2015 and September 2015.
Blood collection and cell isolation
A single non-tourniqueted venous blood sample (2 tubes, 4.5 mL citrated and 2 VBG collection tubes) was collected from 10 healthy volunteers after informed consent. Venous lactate was measured immediately following blood collection (Radiometer ABL90 Series). Half of the remaining blood sample was then incubated with 100 mM of potassium cyanide (KCN) for five minutes and half of the sample remained unexposed. Repeat lactate measurements were performed from blood exposed and not exposed to KCN.
Peripheral blood mononuclear cells (PBMCs) were isolated from blood exposed to KCN and the unexposed blood for the measurement of mitochondrial respiration. PBMCs consisting of lymphocytes and monocytes were isolated from citrated whole blood by density gradient centrifugation in the following manner: Blood samples were diluted 1:1 using a balanced salt solution (anhydrous d-glucose 5.5mM, CaCl2 5mM, MgCl2 0.98mM, KCl 5.4mM, Tris 145mM, and NaCl 140mM with pH adjusted to 7.6) and layered on top of Ficoll-Paque PLUS (density 1.077g/mL; Amersham Biosciences, Piscataway, NJ). The sample was centrifuged at 400g for 40 minutes at 20°C. The layer containing the PBMCs at the interface was gently aspirated and centrifuged again at 1,800g for 5 minutes. The PBMC pellet was then resuspended in Hank balanced salt solution (pH 7.40) containing 5.5mM glucose, 1mM pyruvate, and 10mM 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid. Cell counts were performed using trypan blue exclusion (Countess II, Life Technologies, Grand Island, NY) with more than 95% viability.
Measurement of mitochondrial respiration
Intact PBMCs were placed in a 2-mL chamber at a final concentration of 2-3×106 cells/mL. Measurement of oxygen consumption were performed at 37°C in a high-resolution oxygraph (Oxygraph-2k Oroboros Instruments, Innsbruck, Austria). Oxygen flux (in pmol O2/s/106 cells), which is directly proportional to oxygen consumption, was recorded continuously using DatLab software 6 (Oroboros Instruments).
After routine oxygen consumption was recorded for 10–20 minutes, the following sequential injections of select compounds are carried out in what is referred to as a SUIT (substrate-uncoupler-inhibitor-titration) protocol that provides a wealth of information on the key parameters of mitochondrial respiration. Figure 1 demonstrates the compound used for our study and the sequence given along with a typical oxygraph tracing. The following terms are important in the interpretation of mitochondrial respiration:.
Figure 1. Mitochondrial Respiration High-resolution respirometry and coupling control protocol with intact cells.
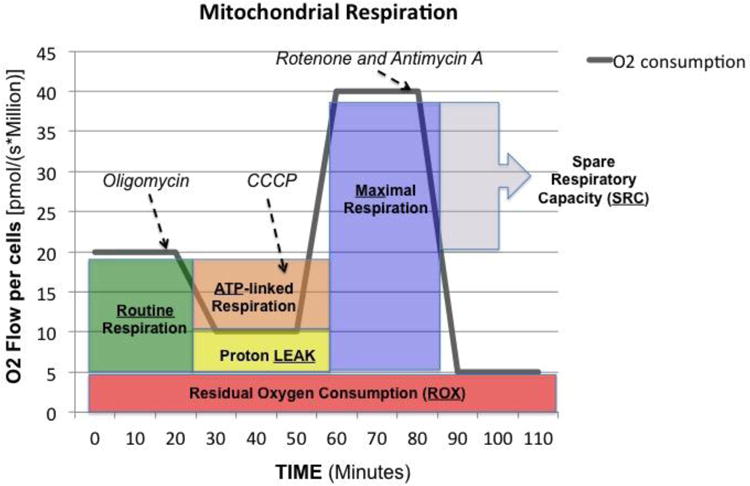
Representative tracing of oxygen consumption measured in intact peripheral blood mononuclear cells. The solid grey line shows the rate of oxygen consumption. After measuring routine oxygen consumption, the adenosine triphosphate (ATP)-synthase inhibitor oligomycin is added to obtain proton LEAK. The uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) allows the measurement of maximal oxygen consumption (Max) stimulating maximal respiration assuming all required substrates are present. Finally, rotenone (complex I inhibitor) and antimycin A (complex III inhibitor) will completely prevent oxygen consumption through the electron respiratory chain. The remaining residual oxygen consumption (ROX) is attributed from other non-mitochondrial sources such as oxidases. ATP-linked oxygen consumption is calculated as routine respiration minus LEAK and spare respiratory capacity (SRC) is calculated as maximal respiration minus routine oxygen consumption.
Routine respiration (ROUTINE): Also known as basal respiration is the oxygen consumption due to the combination of ATP production and proton leak. This represents energy demand under steady state conditions.14 Changes in routine respiration can be from a change in leak and/or ATP-linked respiration.
Proton LEAK: The remaining mitochondrial respiration after the injection of oligomycin (complex V inhibitor) is due to proton leak. A degree of proton leak is expected under normal mitochondrial respiration. Significant proton leak can be an indication of mitochondrial damage from direct injury.
Maximal respiration (Max): The addition of a mitochondrial uncoupler such as dinitrophenol (DNP) or Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (CCP) stimulates maximal respiration by mimicking a physiological energy demand leading to a increase in oxygen consumption. This assumes that all the required substrates are present in adequate amounts to support maximal respiration.
Residual oxygen consumption (ROX): The addition of mitochondrial inhibitors such as the combination of rotenone (complex I) and antimycin (complex III) will completely shut down the electron transport chain with complex V already inhibited. The remaining oxygen consumption is due to non-mitochondrial respiration such as oxidases and other cellular enzymes.
ATP-linked production: The difference between ROUTINE and LEAK, it is oxygen consumption related to ATP production. The decrease in oxygen consumption with the injection of oligomycin represents the oxygen consumption utilized for ATP production and often referred to as ATP-linked respiration.
Spare respiratory capacity (SRC): The difference between maximal respiration and routine respiration represents the cell's SRC. The SRC is thought to indicate the ability of the cell to respond to the energetic demand and thus a measure of a cell's fitness. A decrease in the SRC may limit the cell's ability to handle a stress response resulting in mitochondrial dysfunction.
Statistics
We designed the study to have 80% power to detect a 25% change in the key parameters of mitchondrial respiration from baseline. To assess differences in between control/cyanide pairs, the paired t-test was used for the all parameters. To account for multiple statistical testing (7 tests) on same data, an adjusted p–value of .007 using a Bonferroni correction was considered statistically significant. All analyses were performed using SAS statistical software (Version 9.4, SAS Institute, Cary NC).
Results
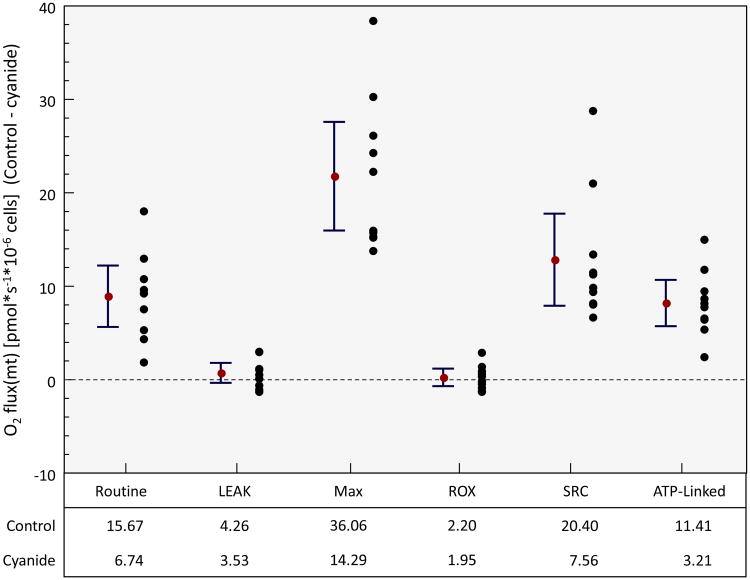
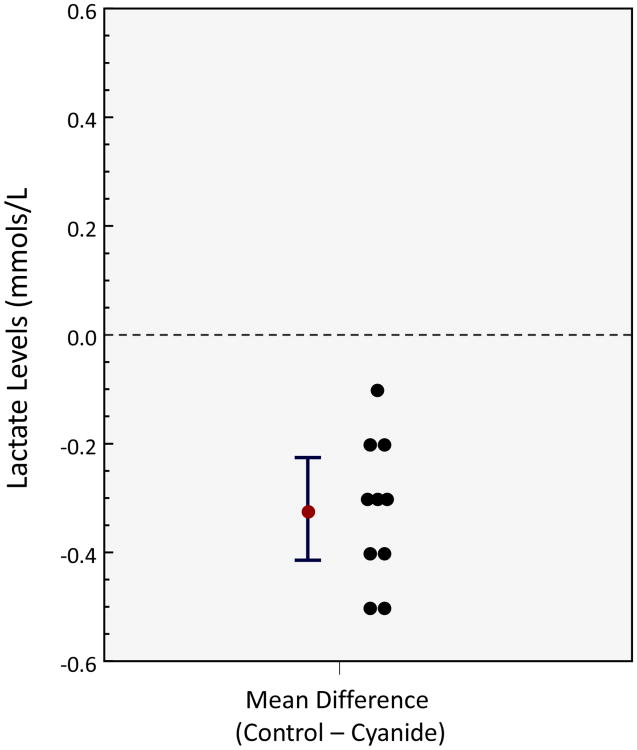
Participants included 10 healthy subjects: 5 males and 5 females, median age of 28 (interquartile range = 26-31). Of the parameters measuring mitochondrial respiration, 4 of the 6 demonstrated a statistically significant mean difference between control and cyanide (Figure 2): For routine (Mean difference (control-cyanide): 8.9 pmol O2/s/106 cells; 95% CI 5.6 to 12.2, p<.0001); LEAK (Mean difference: 0.73 pmol O2/s/106 cells; 95% CI -0.33 to 1.79, p=.157); Max (Mean difference: 21.7 pmol O2/s/106 cells; 95% CI 16.0 to 27.6, p<.0001); ROX (Mean difference 0.25 pmol O2/s/106 cells; 95% CI -0.68 to 1.18, p=.557). There was a significant difference in SRC and ATP-linked respiration with the control samples demonstrating a higher SRC and ATP-linked respiration. Finally there is a statistically significant difference in lactate (mean difference -.32, 95% CI: -0.41 to -.23, p<.0001), though clinically similar level, with a higher lactate concentration in the cyanide samples (Figure 3).
Figure 2. Mitochondrial Respiration in control samples versus cyanide samples in key parameters in mitochondrial respiration.
There is a significant difference in both routine respiration, maximal respiration (Max), spare respiratory capacity (SRC) and ATP-linked respiration that is higher in the control samples when compared to cyanide samples. There was no difference in proton LEAK or residual oxygen consumption (ROX). Routine (Mean difference (control-cyanide): 8.9 pmol O2/s/106 cells; 95% CI 5.6 to 12.2, p<.0001); LEAK (Mean difference: 0.73 pmol O2/s/106 cells; 95% CI -0.33 to 1.79, p=.157); Max (Mean difference: 21.7 pmol O2/s/106 cells; 95% CI 16.0 to 27.6, p<.0001); ROX (Mean difference 0.25 pmol O2/s/106 cells; 95% CI -0.68 to 1.18, p=.557).
Figure 3. Lactate in control samples versus cyanide samples in key parameters in mitochondrial respiration.
There is a statistically significant difference in lactate, though clinically similar level, with a higher lactate concentration in the cyanide samples with a mean difference -.32, 95% CI: -0.41 to -.23, p<.0001.
Discussion
There are several imitations to consider in this study in addition that this is an ex vivo study and may not mimic the complex interactions that is found in vivo. One of the limitations is whether whole blood cells represent mitochondrial function in key organs such as the brain and the heart. The correlation of PMBCs with organ mitochondrial function has been studied in animal models of traumatic shock with variable correlation. However, it is clear PBMCs develop significant mitochondrial dysfunction following hemorrhagic shock. Impaired mitochondrial bioenergetics may also contribute to the immunosuppression that may contribute to the overall clinical picture of patients.11
Another limitation of this study is the use of intact PBMCs to study mitochondrial respiration. While it is clear in our study that cyanide ex vivo causes a change in mitochondrial respiration another limitation with the use of intact cells is the inability to ascertain the involvement of other complexes. There are methods to permeabilize cells with the use of a mild detergent such as digitonin. Permeabilized cells allow the examination of specific complex-linked respiration. Another important factor is the role of radical oxygen species (ROS) which this study does not address but may play an important role.15 There are methods to perform simultaneous mitochondrial respiration and ROS measurements with the Oroboros O2K utilizing a fluorophore such as amplex red to measure hydrogen peroxide.16
A third limitation, with the assumption whole cells reflect mitochondrial function of organs, is the cell type to monitor mitochondrial respiration. In our study we used PBMCs that are a mixed population of both monocytes and lymphocytes. There are studies that are able to demonstrate differences in the bioenergetic profile in various cell types such as platelets and neutrophils. A fourth limitation is the use of healthy young volunteers with no medical history; we may have observed different results with a more heterogeneous population with medical comorbidities and a history of smoking. While our ex vivo study does not fully reflect the action of cyanide in vivo, this study would not be possible in vivo for obvious ethical concerns.
Finally another limitation is the ideal method to normalize mitochondrial function. In our study cell number was used but there are a variety of ways to normalize mitochondrial function which include but are not limited to mitochondrial mass, number, or activity. Citrate synthase is commonly used as well. Mitochondrial DNA has also been used and the ratio of mitochondrial to nuclear DNA is also another method currently being explored.17
This study demonstrates the use of mitochondrial respiration as a potential sensitive assay for cellular dysfunction from acute poisoning. We found that samples exposed to cyanide showed a significant decrease in key parameters of mitochondrial respiration including routine and maximal respiration. We also found that SRC, which represents the mitochondrial bioenergetic reserve, was significantly decreased as well as ATP-linked respiration in the cyanide group.
The use of circulating blood cells such as white blood cells and platelets potentially serve as an early warning or “canary in the coal mine” for mitochondrial dysfunction that may occur in conditions of metabolic stress.18 Once isolation of PBMCs have occurred, the time to obtain key mitochondrial respiration parameters (Routine, LEAK, Max, ROX) is typically less than an hour which may have relevance for time-sensitive clinically problems such as acute poisoning or sepsis. It may be less useful in cases of severe cyanide poisoning where death may occur in a short period of time. Prior to the development of sensitive instruments to measure mitochondrial oxygen consumption the most common approach has been to use isolated mitochondria from biopsies that is invasive, painful and time-consuming. The advantage of our described method is the non-invasive method (phlebotomy), ability to perform multiple measurements, monitor response to treatment, and prognosis.
In control samples versus samples that were exposed to cyanide our results demonstrate lower routine and maximal respiration. This indicates that exposure to cyanide results in a decrease in ATP-linked respiration resulting in lower routine respiration. This is consistent with cyanide's mechanism of action with inhibition of complex IV. There was no significant difference in both the LEAK and the ROX between control and cyanide samples that may support that cyanide does not directly damage mitochondrial membranes which would result in an increase in LEAK.
One of the mitochondrial respiration parameters that can be calculated is the spare respiratory capacity. It is obtained by the difference of the maximal and routine respiration (Figure 1). The SRC represents the energetic reserve of the mitochondria. A decrease in SRC may indicate dysfunction of the mitochondria and the inability to meet an increase in bioenergetic demand that occur in a stress response from sepsis or acute poisoning. Our ex vivo study demonstrates a decrease both in the maximal respiration of the cell and hence the SRC. Studies in the bioenergetic function of immune cells in sepsis and traumatic injuries often show a decreased SRC. When a stress response requires an energetic demand beyond that of the cell's SRC, cellular dysfunction may occur leading to organ dysfunction.18
Another finding in our study was a significant but not necessarily a clinically significant increase in lactate with cyanide samples. Lactate is often used as a surrogate marker for cyanide poisoning and while there is an association between cyanide and lactate concentrations, elevated lactate can be seen in a number of other critical conditions and is therefore non-specific. Also lactate may only be mildly elevated in cyanide poisoning due mixed exposures that occur in fires (CO and H2S) and also the timing of measurement and the effect of treatments provided.7,8 Some of the reasons why lactate was only mildly elevated in our study is the ex vivo nature of our study and also the dose of cyanide used may be less than what would be encountered in the clinical setting.
The change in mitochondrial respiration was much more profound than the change in lactate. Reliance on lactate may miss an important exposure as a small increase may be disregarded in real practice.
Our study demonstrates that measuring key parameters in mitochondrial respiration may be a more sensitive measure of cellular function when compared to lactate. The use of mitochondrial bioenergetics represents a potential new avenue for diagnostics and the ability to measure response to therapy for other poisonings that affect the mitochondria such as carbon monoxide and hydrogen sulfide where these poisonings may not be as time sensitive as cyanide making the measurement of mitochondrial respiration feasible.
The project described was supported by award K12 HL109009 from the National Heart, Lung, and Blood Institute.
Acknowledgments
We thank Prasanth Potluri, PhD (The Children's Hospital of Philadelphia-Center of Mitochondrial and Epigenomic Medicine) in assisting with the preparation of the cyanide used in this study.
Contributor Information
David H. Jang, University of Pennsylvania Perelman School of Medicine, Department of Emergency Medicine, National Heart, Lung, and Blood Institute (NHLBI) K12 Scholar.
Frances S. Shofer, University of Pennsylvania Perelman School of Medicine, Department of Emergency Medicine.
Scott L. Weiss, Division of Critical Care Medicine, Department of Anesthesia and Critical Care, The Children's Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Lance B. Becker, University of Pennsylvania Perelman School of Medicine, Department of Emergency Medicine, Director, Center for Resuscitation Science and Division of Critical Care.
References
- 1.Jett DA, Yeung DT. The CounterACT Research Network: basic mechanisms and practical applications. Proc Am Thorac Soc. 2010;7:254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002 Jul;32(4):259–89. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- 3.Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991 Dec 19;325(25):1761–6. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 4.Fortin JL, Desmettre T, Manzon C, et al. Cyanide poisoning and cardiac disorders: 161 cases. J Emerg Med. 2010;38:467–476. doi: 10.1016/j.jemermed.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RP, Mellors JW. Arteriolization of venous blood gases: a clue to the diagnosis of cyanide poisoning. J Emerg Med. 1988;6:401–404. doi: 10.1016/0736-4679(88)90014-5. [DOI] [PubMed] [Google Scholar]
- 6.Bechtel L, Holstege C. Forensic analysis of potassium cyanide stored in gelatin capsules. Clin Toxicol. 2010;48:614. [Google Scholar]
- 7.Baud FJ, Borron SW, Megarbane B, et al. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30:2044–2050. doi: 10.1097/00003246-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Baud FJ, Borron SW, Bavoux E, et al. Relation between plasma lactate and blood cyanide concentrations in acute cyanide poisoning. BMJ. 1996;312:26–27. doi: 10.1136/bmj.312.7022.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake R, Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta. 2014 Apr;1840(4):1404–12. doi: 10.1016/j.bbagen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Chacko BK, Kramer PA, Ravi S, et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013 Jun;93(6):690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villarroel JP, Guan Y, Werlin E, et al. Hemorrhagic shock and resuscitation are associated with peripheral blood mononuclear cell mitochondrial dysfunction and immunosuppression. J Trauma Acute Care Surg. 2013 Jul;75(1):24–31. doi: 10.1097/TA.0b013e3182988b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss SL, Selak MA, Tuluc F, et al. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med. 2015 Jan;16(1):e4–e12. doi: 10.1097/PCC.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japiassú AM, Santiago AP, d'Avila JC, et al. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5′-triphosphate synthase activity. Crit Care Med. 2011 May;39(5):1056–63. doi: 10.1097/CCM.0b013e31820eda5c. [DOI] [PubMed] [Google Scholar]
- 14.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 15.Kanthasamy AG, Ardelt B, Malave A, et al. Reactive oxygen species generated by cyanide mediate toxicity in rat pheochromocytoma cells. Toxicol Lett. 1997;93:47–54. doi: 10.1016/s0378-4274(97)00068-4. [DOI] [PubMed] [Google Scholar]
- 16.Makrecka-Kuka M, Krumschnabel G, Gnaiger E. High-Resolution Respirometry for Simultaneous Measurement of Oxygen and Hydrogen Peroxide Fluxes in Permeabilized Cells, Tissue Homogenate and Isolated Mitochondria. Biomolecules. 2015 Jun 29;5(3):1319–38. doi: 10.3390/biom5031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilbaugh TJ, Lvova M, Karlsson M, et al. Peripheral Blood Mitochondrial DNA as a Biomarker of Cerebral Mitochondrial Dysfunction following Traumatic Brain Injury in a Porcine Model. PLoS One. 2015 Jun 22;10(6):e0130927. doi: 10.1371/journal.pone.0130927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chacko BK, Kramer PA, Ravi S, et al. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci (Lond) 2014 Sep;127(6):367–73. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]