Abstract
Background:
A large, multicenter, 10-year observational study is being conducted to compare the long-term safety and effectiveness of Natrelle silicone breast implants with saline implants or national norms. Study baseline data and surgical characteristics are reported here.
Methods:
Women seeking primary augmentation, revision-augmentation, primary reconstruction, or revision-reconstruction participated. Eligible subjects had completed surgery and received one implant or matching implants. Baseline demographics, health, lifestyle, and surgical characteristics were recorded. Data are presented here for subjects (≥22 years old) who underwent primary augmentation or revision-augmentation.
Results:
Of 50,979 subjects who underwent augmentation procedures, 35,756 received silicone implants and 15,223 received saline implants. Of these, 86.3 percent underwent primary augmentation, and 13.7 percent underwent revision-augmentation; nearly all subjects (99.3 percent) received bilateral implants. In the primary augmentation group, 67.6 percent of subjects received silicone implants versus 86.1 percent in the revision-augmentation group. Median age was lower in the primary augmentation group compared with the revision-augmentation group (33 versus 42 years old, respectively). Most subjects were white nonsmokers and had attended college. Hispanic subjects and subjects with a body mass index of 25 kg/m2 or greater were more likely to receive saline versus silicone implants. Across groups, the most common characteristics by procedure or implant type included inframammary incision site (54.6 percent), partial (58.2 percent) or complete (31.9 percent) submuscular placement, smooth surface implants (93.1 percent), and implant size of 300 to 399 cc. Incision size was larger for silicone versus saline implants.
Conclusion:
These data add to the body of knowledge on women undergoing augmentation procedures by providing an unprecedented look at a large number of subjects.
Breast augmentation is one of the most common cosmetic surgical procedures performed in the United States.1 The annual number of procedures has increased steadily since 2000, with nearly 300,000 procedures performed in 2013 alone.1 Despite these recent numbers and more than 50 years of experience with breast implants, the long-term safety of implants is still questioned.2 Thus, when the U.S. Food and Drug Administration approved silicone gel-filled breast implants in 2006, it required manufacturers to conduct postapproval studies to characterize the long-term performance and safety of the devices over 10 years.3
The Breast Implant Follow-up Study (BIFS-001) is a large, multicenter, 10-year observational study being conducted as part of this U.S. Food and Drug Administration requirement. This U.S. study is designed to compare the long-term safety and effectiveness of Natrelle silicone breast implants (Allergan, Inc., Irvine, Calif.) with those of saline implants or national norms. Natrelle silicone gel-filled breast implants are available worldwide in a range of implant options and are approved by the Food and Drug Administration for primary augmentation, revision-augmentation, primary reconstruction, and revision-reconstruction procedures.4 BIFS-001 is ongoing and has enrolled more than 50,000 subjects. Although primary outcome data comparing long-term safety for Natrelle silicone implants versus saline implants will continue to be collected over a 10-year period, baseline data are now available for analysis. The data set provides a unique look at an extensive population of women requesting breast augmentation. Further, because BIFS-001 is an observational study and the analysis of safety endpoints will entail comparisons between self-selected silicone and saline implant groups, it is critical to understand how characteristics that can affect safety outcomes differed at baseline between subjects who chose silicone implants and the comparator population who chose saline implants. This is particularly true for safety outcomes in the study and the demographic and lifestyle characteristics that may be associated with increased risk for those events.
The current analysis therefore describes demographic, health, lifestyle, and surgical characteristics of subjects enrolled in BIFS-001 who underwent either primary augmentation or revision-augmentation procedures, as well as baseline rates for some of the important safety outcomes. Results from a similar analysis of subjects who underwent primary reconstruction or revision-reconstruction surgery are reported separately. This analysis addresses two questions with important implications for the long-term safety of silicone gel-filled implants in clinical practice: How do women who undergo revision-augmentation differ from the primary augmentation population; and how do subjects who receive silicone implants differ at baseline from those who choose saline implants?
PATIENTS AND METHODS
Study Design
BIFS-001 is a long-term observational study comparing outcomes between women who received Natrelle silicone gel-filled breast implants and those who received saline-filled breast implants. Women seeking primary augmentation, revision-augmentation, reconstruction, or revision-reconstruction were invited to participate at the time they decided to undergo breast implantation. More than 1000 investigational sites participated in BIFS. Baseline information was collected at this time. After surgery, subjects must have received unilateral or bilateral silicone implants or saline implants. Subjects will complete follow-up questionnaires via Internet, phone interview, or mail annually for 10 years.
This study was approved by the institutional review board for each participating study site, was conducted in accordance with Good Clinical Practice guidelines, conformed with World Health Organization guidelines, and is registered at www.clinicaltrials.gov (NCT00443274). All participants provided written informed consent before enrollment.
Subjects
Only subjects who desired primary augmentation or revision-augmentation are included in the current analysis. Women 22 years of age or older were screened for study eligibility if they were fluent and literate in English or Spanish. Subjects were enrolled if they had completed surgery and had received one implant or matching implants (either both silicone or both saline). All silicone implants were required to be Natrelle devices. Subjects were ineligible for study inclusion if they were transgender or if they were deemed by the investigator to be unsuitable for long-term observation. Subjects who were currently implanted with saline implants were not eligible for the study if they had previously received silicone breast implants.
Assessments
Details of subject characteristics recorded at the baseline visit included demographics (age, race, weight, and height) and subject-reported health and lifestyle characteristics (marital status, education level, occupation, smoking status, alcohol use, and history of substance abuse). After surgery, data about the type of implant (silicone gel or saline), implant placement location, implant style and size, and incision size and site were documented by investigators. Long-term safety outcomes assessed at baseline included history of neurologic disease (multiple sclerosis), connective tissue disease (e.g., rheumatoid arthritis and fibromyalgia), cancer (brain, lung, breast, and cervical/vulvar), suicide attempt or suicidal ideation, and reproductive or lactation complications.
Statistical Analysis
Statistical analysis of the 10-year safety data will include comparisons of adverse event rates between women who choose silicone breast implants and those who choose saline breast implants. Therefore, baseline and surgical differences between the two study populations were analyzed. Comparisons between the Natrelle silicone implant group and the saline group were based on a two-sided z test for continuous data and a two-sided chi-square test for categorical data. Comparisons were also made between the primary augmentation and revision-augmentation populations, but these analyses used descriptive statistics only.
RESULTS
Subjects
BIFS-001 enrolled 56,616 eligible subjects from February of 2007 through March of 2010 at 1116 sites. Of these, 50,979 subjects underwent augmentation procedures, with 35,756 (70.1 percent) receiving silicone gel implants and 15,223 (29.9 percent) receiving saline implants. Most subjects (n = 44,011; 86.3 percent) underwent primary augmentation; 13.7 percent of subjects (n = 6968) underwent revision-augmentation. In the primary augmentation group, 67.6 percent of subjects (n = 29,755) received silicone implants; in the revision-augmentation group, 86.1 percent of subjects (n = 6001) received silicone implants.
Demographics
Primary augmentation subjects were predominantly white (71 percent), with a median age of 33 years at implantation (range, 22 to 79 years; Table 1). More than 70 percent of subjects had a normal body mass index; 15 percent were overweight or obese. Within the primary augmentation group, silicone and saline groups differed statistically on a number of demographic characteristics of clinical interest. Subjects who received silicone implants were significantly older and had a lower mean body mass index compared with subjects who received saline implants (both p < 0.0001). Race distribution also differed significantly between implant groups (p < 0.0001); the percentage of white subjects was higher and the percentage of Hispanic subjects was lower in the silicone group compared with the saline group.
Table 1.
Subject Demographics by Procedure and Type of Implant
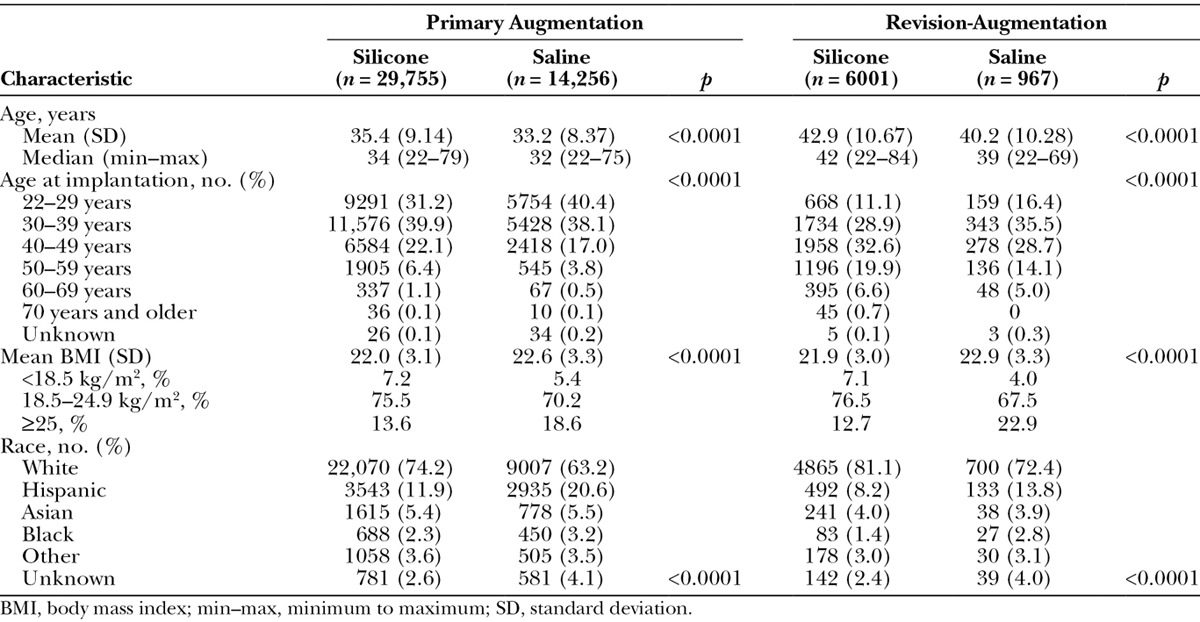
Subjects in the revision-augmentation group were older relative to those in the primary augmentation group, with a median age difference of approximately 9 years (42 versus 33 years). When divided by 10-year intervals, subjects who underwent primary augmentation procedures were most often between the ages of 22 and 39 years; in contrast, subjects who underwent revision-augmentation procedures were most often between 30 and 49 years of age (Fig. 1). Mean body mass index values and distribution were similar in the primary augmentation and revision-augmentation groups. Women who chose silicone implants for revision-augmentation procedures, similar to those who underwent primary augmentation, were significantly older and had a significantly lower mean body mass index than the women who selected saline implants (both p < 0.0001). The percentage of overweight/obese subjects was lower in subjects who received silicone implants compared with those who received saline implants. Most revision-augmentation subjects who received either silicone or saline implants were white, but a greater percentage of subjects in the saline group were black or Hispanic compared with the silicone group (p < 0.0001).
Fig. 1.
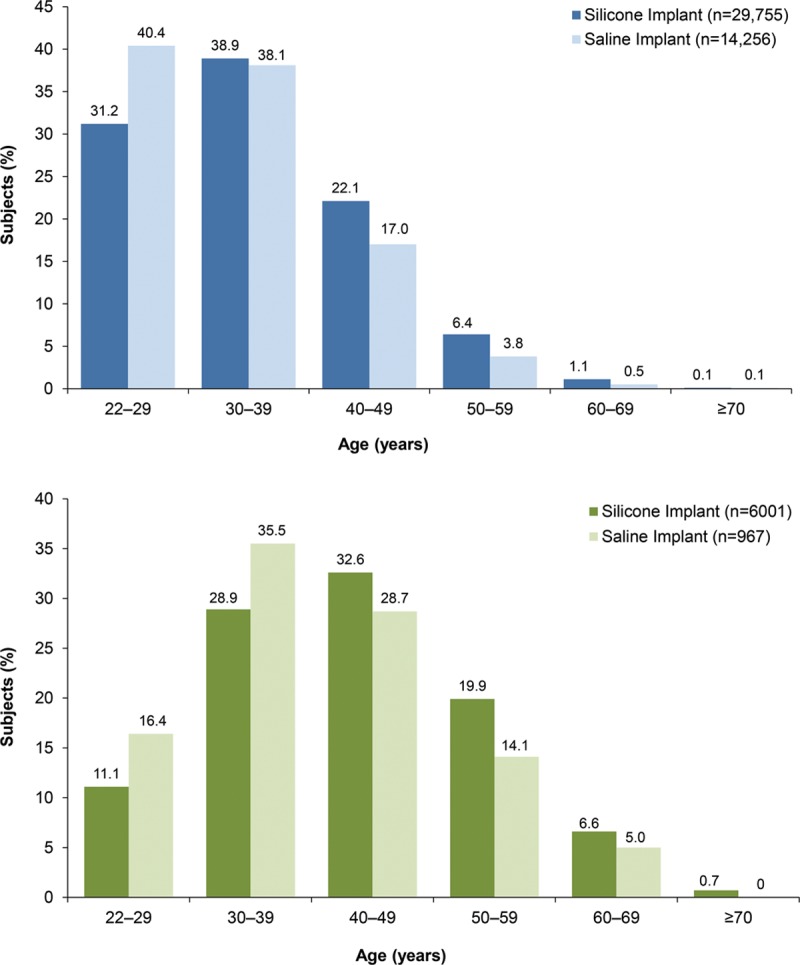
Age distribution for primary augmentation (above) and revision-augmentation (below) subjects. Percentages of women who selected silicone implants were higher than the percentages who selected saline implants in age groups 30 years and older for primary augmentation and in age groups 40 years and older for revision-augmentation (both p < 0.0001).
Lifestyle Characteristics
Lifestyle characteristics were generally consistent between the primary augmentation and revision-augmentation groups (Table 2). Approximately 70 percent to 80 percent of subjects had at least some college, and more than half of the subjects in both groups were married (with a higher rate in the older, revision-augmentation group). Approximately half of the subjects in both groups reported having a professional occupation. In both the primary augmentation and revision-augmentation groups, a greater percentage of subjects who received silicone implants were married (both p < 0.0001), had a college degree or greater (both p ≤ 0.0007), and were professionals or homemakers compared with subjects who chose saline implants (both p < 0.0001).
Table 2.
Lifestyle Characteristics of Subjects by Procedure and Type of Implant
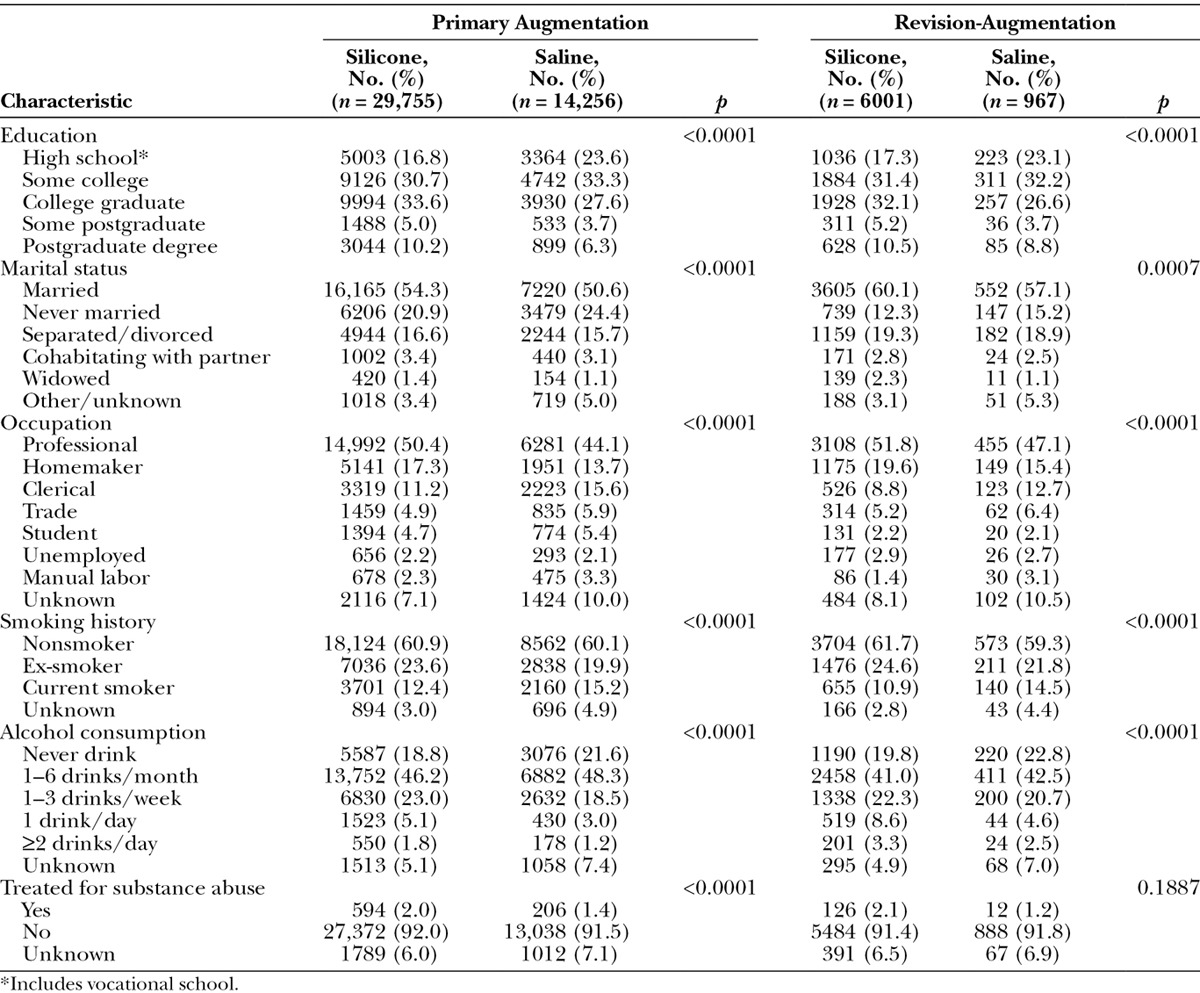
Approximately 60 percent of subjects in both the primary augmentation and revision-augmentation groups were nonsmokers, and roughly one-fourth of all subjects were ex-smokers. More than 80 percent of subjects in both groups consumed three or fewer alcoholic beverages per week, including approximately 20 percent of subjects who do not drink at all, and fewer than 9 percent of all subjects had ever been treated for substance abuse. In both the primary augmentation and revision-augmentation groups, the percentage of current smokers was lower and alcohol consumption was higher in the subjects who received silicone versus saline implants (all p < 0.0001).
Medical History
Baseline rates of selected medical diagnoses that are safety endpoints in BIFS-001 are listed for primary augmentation and revision-augmentation groups in Table 3. Among primary augmentation subjects, the silicone versus the saline group had a significantly higher overall rate of previous cancer diagnoses (p < 0.0001), with significantly greater rates of basal cell carcinoma or squamous cell carcinoma (p < 0.0001) and breast cancer (p < 0.0001) at baseline. No differences between groups were observed in previous brain, lung, or cervical/vulvar cancer rates. The number of women who reported previous suicide attempts or thoughts about suicide was also significantly higher in the silicone group. In each implant group, 79.4 percent of subjects had ever been pregnant, and the proportion of women who had tried to breast-feed and of those who experienced difficulties with breast-feeding were significantly higher at baseline among women who chose silicone versus saline implants (both p < 0.0001). The infertility rate was also significantly higher in the silicone group.
Table 3.
Selected Medical History by Procedure and Type of Implant*
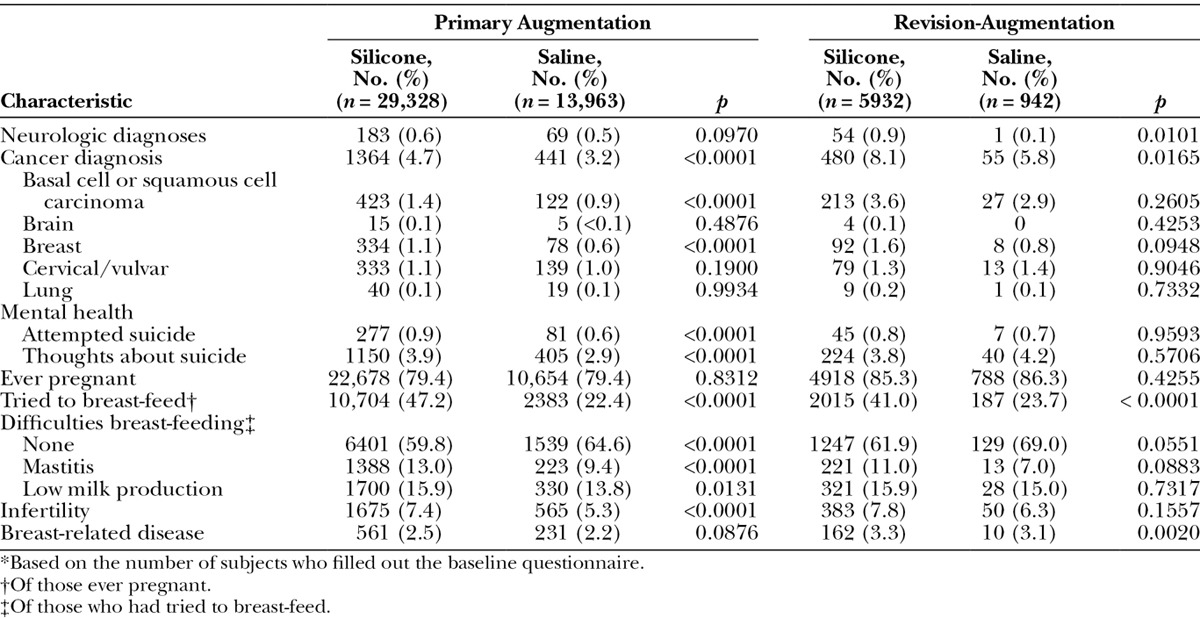
Among revision-augmentation subjects, a significantly higher rate of neurologic diagnoses was noted in the silicone group at baseline compared with the saline group (p = 0.0101), although rates were not significantly higher for any specific diagnosis. A higher rate of previous cancer diagnoses was observed in subjects who chose silicone versus saline implants (p = 0.0165; Table 3), but again, no specific cancer of interest in this study was reported at a statistically higher rate at baseline. There was no difference between implant groups in the number of subjects who reported previous suicide attempts or thoughts about suicide. For the revision-augmentation indication, 85.3 percent of the silicone group and 86.3 percent of the saline group had ever been pregnant. Significantly more women in the silicone group had tried to breast-feed (p = 0.0001); no difference in the baseline rate of difficulties in breast-feeding was observed. Infertility rates were similar for revision-augmentation subjects who chose silicone versus saline implants; the baseline rate of breast-related disease was higher in the silicone group (p = 0.0020).
Surgical Characteristics
Nearly all subjects received bilateral breast implants (Table 4). Among subjects who underwent primary augmentation, 99.5 percent of those with silicone gel implants and 99.7 percent of those with saline implants received bilateral implants (p = 0.0015). For subjects who underwent revision-augmentation procedures, the percentage of subjects who received bilateral implants was significantly higher for those who chose silicone gel compared with saline implants (98.4 percent versus 90.3 percent; p < 0.0001). In both the primary augmentation and revision-augmentation groups, the majority of implants were placed submuscularly, with partial submuscular placement favored over complete submuscular placement for both implant types. However, in both the primary augmentation and revision-augmentation procedures, the distribution of implant locations differed significantly by implant type (both p < 0.0001; Table 4). Complete submuscular placement was less common and subglandular placement was more common for silicone versus saline implants.
Table 4.
Surgical Characteristics by Procedure and Type of Implant
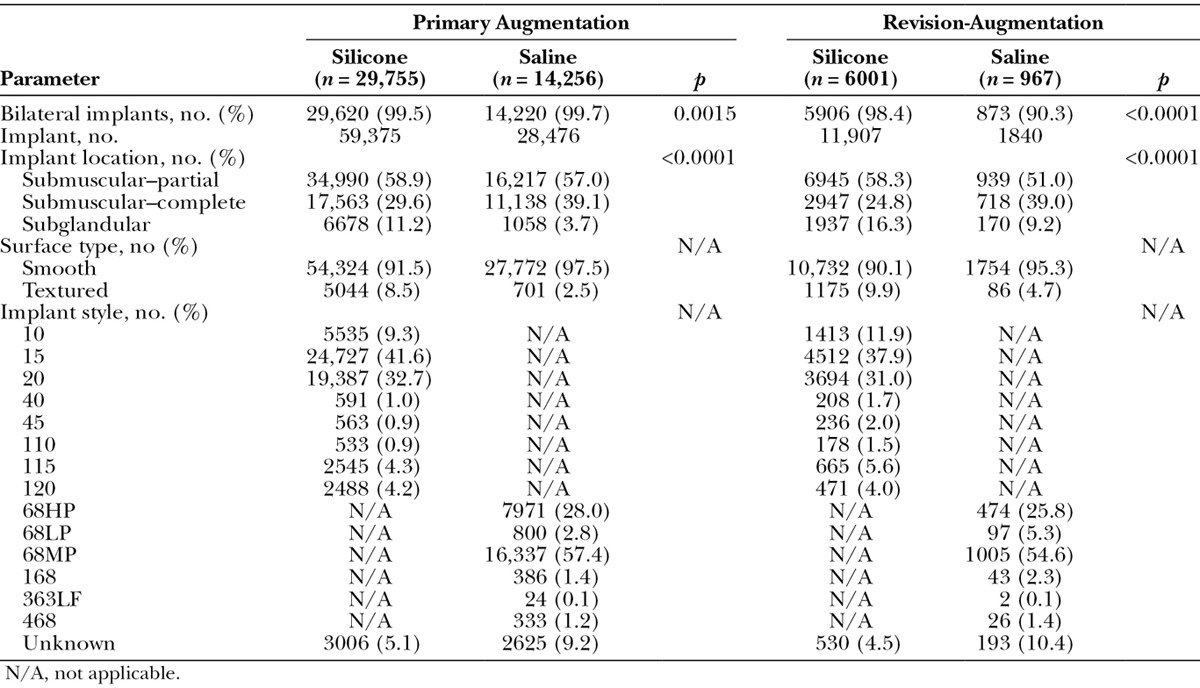
Implant Styles and Sizes
The most frequently used silicone implants for the primary augmentation and revision-augmentation groups, respectively, were Natrelle styles 15 (41.6 percent and 37.9 percent) and 20 (32.7 percent and 31.0 percent), which are considered midrange profile and high profile, respectively (Table 4). The most frequently used saline implants in the primary augmentation and revision-augmentation groups, respectively, were the moderate and high-profile Natrelle styles 68MP (57.4 percent and 54.6 percent) and 68HP (28.0 percent and 25.8 percent). More than 90 percent of silicone and saline implants used in both the primary augmentation and revision-augmentation procedures had a smooth surface.
The most common implant size range was 300 to 399 cc, followed by 400 to 499 cc, for both silicone and saline implants, regardless of the augmentation procedure. More specifically, the most common implant sizes for both indications and both implant types were 350 to 374 cc. For both indications, however, size distributions differed between the silicone and saline groups (both p < 0.0001; Fig. 2). For primary augmentation, the next most common sizes were 325 to 349 cc, followed by 400 to 424 cc for silicone, and 400 to 424 cc, followed by 300 to 324 cc for saline. For revision-augmentation, the next most common sizes for both implant types was 400 to 424 cc, followed by 300 to 324 cc for silicone and 500 to 524 cc for saline. Percentages of women who selected saline implants were higher than that for those who selected silicone for implant sizes 400 cc and up for primary augmentation, and for implant sizes 500 cc and up for revision-augmentation. A greater percentage of subjects who underwent revision-augmentation procedures received larger-volume implants (500 to 599 cc and 600 to 699 cc) compared with subjects who underwent primary augmentation procedures. When subjects were divided by 10-year age intervals, the majority of subjects received 300 to 399 cc silicone or saline implants, regardless of age interval or implantation procedure, with one exception: subjects 22 to 29 years of age who underwent revision-augmentation procedures more frequently received 400 to 499 cc silicone gel or saline implants. Similarly, when subjects were assessed by their BMI category, the 300- to 399-cc silicone and saline implant size was most frequently used, regardless of body size for both augmentation procedures; however, overweight subjects were slightly more likely to receive the 400- to 499-cc implants, and obese subjects were just as likely to receive the 300- to 399-cc implants as the 400- to 499-cc implants for primary augmentation.
Fig. 2.
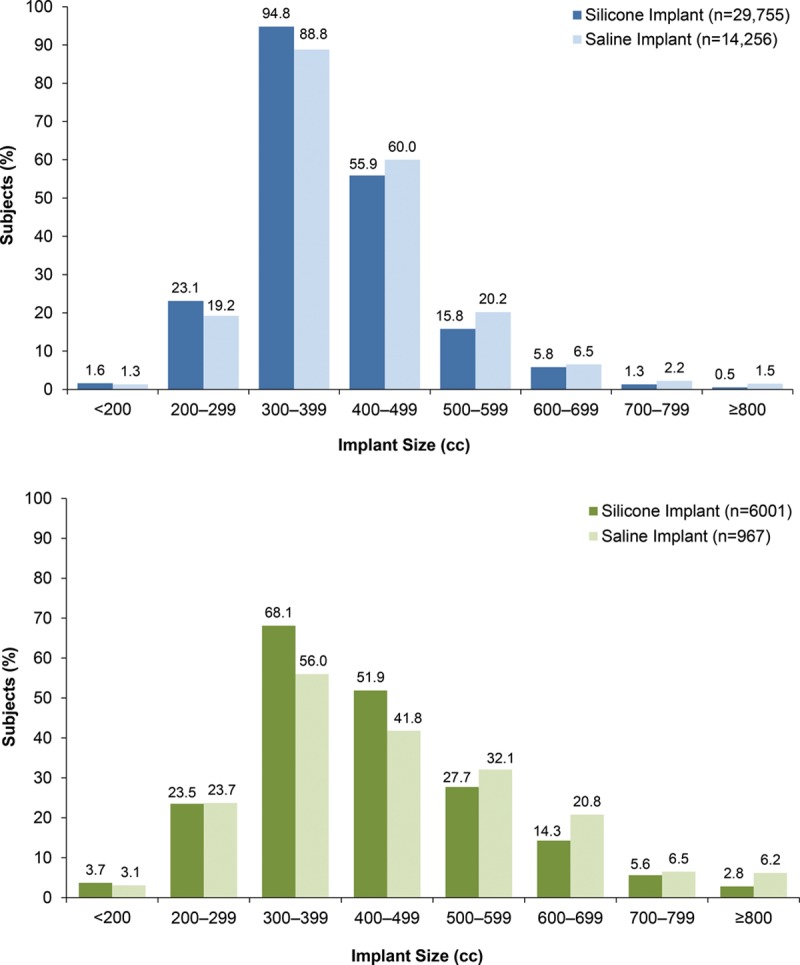
Implant size for primary augmentation (above) and revision-augmentation (below) subjects. Size distributions differed between the silicone and saline groups in both indications (both p < 0.0001).
Incision Sizes and Sites
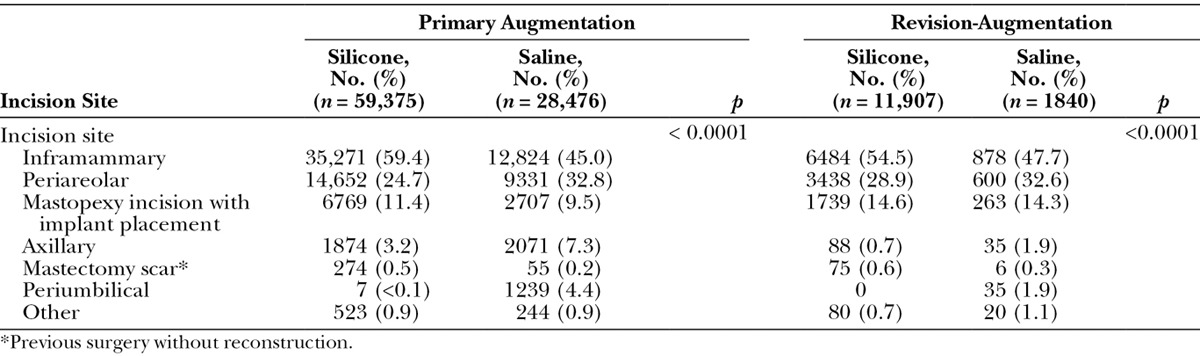
In the primary augmentation group, the majority of silicone implants required incision sizes of 4.0 to 4.9 cm. In contrast, saline implants typically required slightly smaller incision sizes of 3.0 to 3.9 cm (Fig. 3). This trend was also evident in the revision-augmentation group, wherein silicone implants more often required incision sizes of 4.0 to 4.9 cm or 5.0 to 5.9 cm compared with 3.0 to 3.9 cm for saline implants. The most frequently used anatomical incision site for both the primary and revision-augmentation subjects was the inframammary incision, followed by periareolar incision. Although the percentage of subjects with each incision site differed significantly by implant type for both primary and revision-augmentation procedures (both p < 0.0001; Table 5), these were the two most common incision sites for both implant types.
Fig. 3.
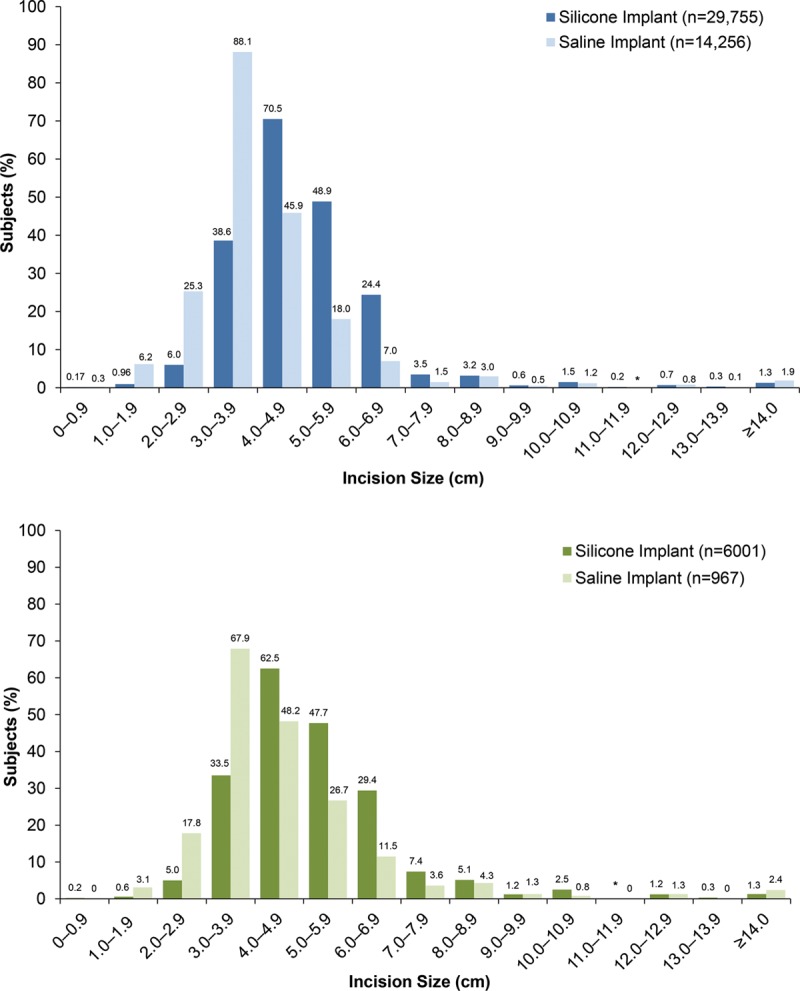
Incision size for primary augmentation (above) and revision-augmentation (below) subjects. *Percentage was <0.1 percent.
Table 5.
Incision Site by Procedure and Type of Implant
DISCUSSION
These data provide an in-depth look at the demographics, lifestyle, health, and surgical characteristics of a large number of subjects who underwent primary augmentation and revision-augmentation breast implant procedures. Most subjects in this population received smooth silicone or saline implants, and nearly all subjects underwent bilateral procedures. Baseline demographics were generally similar between primary and revision-augmentation groups, except that subjects who underwent primary augmentation were, on average, younger than those who underwent revision-augmentation. Hispanic subjects were the second-most common racial group to undergo breast augmentation procedures and were more likely to receive saline implants regardless of the augmentation procedure. Partial submuscular placement of breast implants using an inframammary incision was most common, regardless of augmentation procedure. Relative to subjects who underwent primary augmentation procedures, subjects who underwent revision-augmentation had, on average, larger implant sizes but similar incision sites, regardless of implant type. Subjects who received saline implants for either primary augmentation or revision-augmentation generally had smaller incisions than those who silicone implants, most likely because saline implants are filled after insertion3,5
The baseline comparison between subjects who chose silicone versus saline implants is of particular importance in assessing the long-term safety of silicone gel-filled implants. As an observational study, subjects were not randomly assigned; rather, they self-selected into the silicone or saline implant group. Therefore, in examining the long-term safety data, it will be critical to understand any differences between populations who choose silicone versus saline implants and control for those differences statistically in the safety analyses. Indeed, a number of statistically significant differences were observed between silicone and saline groups at baseline that could influence the 10-year findings. Women who chose silicone implants were significantly older, more educated, and more likely to be married at baseline than were women who chose saline implants. They also had a lower mean BMI, were less likely to smoke, and had a greater mean alcohol consumption compared with the saline group. Demographic and lifestyle characteristics such as these may be risk factors for, or associated with different rates of, BIFS-001 safety outcomes, including cancer,6–8 rheumatic and neurologic diseases,9,10 attempted suicide,11,12 and infertility.13 Statistically significant differences between silicone and saline groups were also found in baseline rates of BIFS-001 outcomes, including previous cancer diagnoses, attempted suicide and thoughts of suicide, breast-feeding difficulties, and infertility.
The baseline data from BIFS-001 also afford a comparison between a substantial cross-section of real-world augmentation patients and the limited populations previously enrolled in clinical trials for silicone and saline implants. Smaller studies (n = 183 to 4412) that reported demographic information and surgical characteristics for subjects who underwent breast augmentation show similar baseline characteristics.14–21 For example, several studies reported that subjects who underwent primary breast augmentation were primarily in their mid-30s, with the majority of subjects receiving implant volumes between 300 and 399 cc and implants inserted mainly through inframammary incisions.14–21 In those studies, the majority of subjects were white, married, and had attended college.14,17,19–21 However, implant type, surface, and shape varied between these studies, with two describing round silicone gel implants,14,20 others describing anatomic silicone gel implants or both types,17,18,21 and one study directly comparing silicone with saline implants.16 Of the studies that solely used silicone implants, nearly all (99 percent) subjects underwent bilateral primary augmentation or revision-augmentation procedures. Several of these findings are in agreement with the results of a 2009 survey of plastic surgeons, in which the average size of breast implants in 81 percent of respondents was 300 to 400 cc, with smooth implants most frequently chosen for augmentation.5 Although approximately 40 percent of surgeons reported using saline implants in 75 percent of primary breast augmentation procedures, 80 percent reported increased use of silicone implants after Food and Drug Administration approval of the devices for primary breast augmentation in November of 2006. While the current study confirms many of the findings reported previously, it is substantially more robust with regard to sample size, thereby providing a more comprehensive, well-defined picture of the population of women who have undergone breast augmentation procedures with silicone or saline implants.
The strength of this study primarily relies on its multicenter design because this allowed for the inclusion of a vast array of real-life patients who received silicone or saline implants for either primary augmentation or revision-augmentation procedures. The subject demographics, lifestyle, and surgical characteristics are obtained from more than 50,000 subjects and include categories not described in recent augmentation studies, including medical history, history of substance abuse, distribution of implant sizes by age and BMI, and distribution of incision sizes.
An important limitation of BIFS-001 is its observational design with a biased sample. A primary comparison for long-term safety endpoints will be conducted between the silicone and saline groups. However, it is clear that women who elect to receive silicone-filled versus saline breast implants may differ in clinically important ways that may impact safety outcomes. This analysis of the baseline characteristics of the two groups in the primary augmentation and revision-augmentation indications addresses this limitation by highlighting demographic and clinical differences between the groups that must be addressed in all forthcoming safety analyses. An additional limitation of this study is that some data, such as lifestyle information, were self-reported. However, any reporting bias may be mitigated by the large sample size.
This analysis of BIFS-001 provides valuable information to better characterize subjects who have undergone breast augmentation procedures, together with data regarding surgical characteristics associated with augmentation procedures and implant types. Elucidation of differences between women who choose silicone implants and those who choose saline can inform practicing physicians in counseling augmentation patients. Further, these results outline baseline comparisons that will be critical in analyzing and interpreting BIFS-001 long-term safety outcomes for silicone gel-filled implants.
ACKNOWLEDGMENTS
The authors thank Kristin E. Larsen, Ph.D., and Kathleen Dorries, Ph.D., of Peloton Advantage, Parsippany, N.J., for writing and editorial assistance, which were provided and funded by Allergan, Inc. (Irvine, Calif.).
Footnotes
This trial is registered under the name “Safety Evaluation in Subjects with Silicone and Saline Breast Implants (Breast Implant Follow-Up Study; BIFS),” Clinical Trials.gov identification number NCT00443274 (https://www.clinicaltrials.gov/ct2/show/NCT00443274?term=NCT00443274&rank=1).
Disclosure: This study was sponsored by Allergan, Inc., Irvine, Calif. Dr. Singh serves as the chair of the Data and Safety Monitoring Board (DSMB) for the Allergan BIFS-001 study. Dr. Picha is an employee of American Medical Technology, Applied Medical Technology, and Abeon Medical; serves as a consultant and advisory board member for Allergan, Inc.; is a member of the DSMB for BIFS-001; serves as an advisor for Intellirod and Mutual Capital Partners; and is an advisory board member for Intellirod. Ms. Murphy is an employee of Allergan, Inc., and holds stock and stock options in that company. Neither honoraria nor other forms of payment were made for authorship.
REFERENCES
- 1.American Society of Plastic Surgeons. 2013 Plastic Surgery Statistics Report. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2013-statistics/plastic-surgery-statistics-full-report-2013.pdf. Accessed November 9, 2015.
- 2.Jewell ML. Silicone gel breast implants at 50: The state of the science. Aesthet Surg J. 2012;32:1031–1034. doi: 10.1177/1090820X12461649. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Devices and Radiological Health; U.S. Food and Drug Administration. FDA update on the safety of silicone gel-filled breast implants; June 2011. Available at: http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM260090.pdf. Accessed November 9, 2015.
- 4.Allergan, Inc. Irvine, Calif.: Allergan, Inc.; 2009. Natrelle Silicone-Filled Breast Implants [directions for use]. [Google Scholar]
- 5.Reece EM, Ghavami A, Hoxworth RE, et al. Primary breast augmentation today: A survey of current breast augmentation practice patterns. Aesthet Surg J. 2009;29:116–121. doi: 10.1016/j.asj.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Zhang J, Wu AH, Pike MC, Deapen D. Invasive breast cancer incidence trends by detailed race/ethnicity and age. Int J Cancer. 2012;130:395–404. doi: 10.1002/ijc.26004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia X, Chen W, Li J, et al. Body mass index and risk of breast cancer: A nonlinear dose-response meta-analysis of prospective studies. Sci Rep. 2014;4:7480. doi: 10.1038/srep07480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med. 2011;171:125–133. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernatsky S, Joseph L, Pineau CA, et al. Scleroderma prevalence: Demographic variations in a population-based sample. Arthritis Rheum. 2009;61:400–404. doi: 10.1002/art.24339. [DOI] [PubMed] [Google Scholar]
- 10.Handel AE, Williamson AJ, Disanto G, et al. Smoking and multiple sclerosis: An updated meta-analysis. PLoS One. 2011;6:e16149. doi: 10.1371/journal.pone.0016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosby AE, Han B, Ortega LA, Parks SE, Gfroerer J Centers for Disease Control and Prevention (CDC) Suicidal thoughts and behaviors among adults aged ≥18 years–United States, 2008-2009. MMWR Surveill Summ. 2011;60:1–22. [PubMed] [Google Scholar]
- 12.Handley TE, Inder KJ, Kay-Lambkin FJ, et al. Contributors to suicidality in rural communities: Beyond the effects of depression. BMC Psychiatry. 2012;12:105. doi: 10.1186/1471-244X-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augood C, Duckitt K, Templeton AA. Smoking and female infertility: A systematic review and meta-analysis. Hum Reprod. 1998;13:1532–1539. doi: 10.1093/humrep/13.6.1532. [DOI] [PubMed] [Google Scholar]
- 14.Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: A 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;132:1115–1123. doi: 10.1097/01.prs.0000435317.76381.68. [DOI] [PubMed] [Google Scholar]
- 15.Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66:1165–1172. doi: 10.1016/j.bjps.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson JM, Gatti ME, Schaffner AD, Hill LM, Spear SL. Effect of incision choice on outcomes in primary breast augmentation. Aesthet Surg J. 2012;32:456–462. doi: 10.1177/1090820X12444267. [DOI] [PubMed] [Google Scholar]
- 17.Hammond DC, Migliori MM, Caplin DA, Garcia ME, Phillips CA. Mentor Contour Profile Gel implants: Clinical outcomes at 6 years. Plast Reconstr Surg. 2012;129:1381–1391. doi: 10.1097/PRS.0b013e31824ecbf0. [DOI] [PubMed] [Google Scholar]
- 18.Lista F, Tutino R, Khan A, Ahmad J. Subglandular breast augmentation with textured, anatomic, cohesive silicone implants: A review of 440 consecutive patients. Plast Reconstr Surg. 2013;132:295–303. doi: 10.1097/PRS.0b013e3182958a6d. [DOI] [PubMed] [Google Scholar]
- 19.Bengtson BP, Van Natta BW, Murphy DK, Slicton A, Maxwell GP Style 410 U.S. Core Clinical Study Group. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):40S–48S. doi: 10.1097/01.prs.0000286666.29101.11. [DOI] [PubMed] [Google Scholar]
- 20.Spear SL, Murphy DK, Slicton A, Walker PS Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):8S–16S. doi: 10.1097/01.prs.0000286580.93214.df. discussion 17S. [DOI] [PubMed] [Google Scholar]
- 21.Gladfelter J, Murphy D. Breast augmentation motivations and satisfaction: A prospective study of more than 3000 silicone implantations. Plast Surg Nurs. 2008;28:170–174. doi: 10.1097/PSN.0b013e31818ea7e0. quiz 175. [DOI] [PubMed] [Google Scholar]


