KEY TEACHING POINTS
|
Introduction
Adult patients with congenital heart disease (CHD) are growing in number with the improvement in corrective techniques. Atrial arrhythmias are a common complication in the long-term follow-up of CHD patients, with a negative impact on morbidity and mortality.1, 2 Dextro-transposition of the great arteries (d-TGA) is one of the most life-threatening types of CHD, accounting for approximately 2 cases per 10,000 live births and 3% of all CHD. In d-TGA the aorta arises from the right ventricle (RV) and the pulmonary trunk from the left ventricle, with systemic and pulmonary circulations running in parallel. Therefore, a communication (shunt) between the 2 circulations is mandatory to support life. Since the early 1960s, atrial switch operation, which diverts the systemic venous return to the mitral valve and the pulmonary venous return to the tricuspid valve through baffles, has dramatically improved survival.3 This extensive atrial surgery creates a substrate for atrial arrhythmias, affecting nearly two-thirds of the population 25 years after surgery.4 As medical therapy is often limited owing to the concomitant presence of bradyarrhythmias and tachyarrhythmias, catheter ablation has been used in this setting, with excellent success rates.5 Atrial switch operation was replaced by arterial switch operation by the mid-1980s and is nowadays only seldom performed. Nevertheless, most adults today with d-TGA followed in adult specialized clinics have undergone an atrial switch operation with Mustard or Senning baffles. We report here a case of successful ablation of an atrial flutter in a patient cumulating several malformations, including a d-TGA with an atrial switch during childhood, a situs inversus, and an interruption of the inferior vena cava (IVC).
Case report
A 40-year-old female patient was regularly followed at the adult CHD outpatient clinic after an atrial switch operation performed at the age of 3 years for a d-TGA. She recently complained of intermittent rapid palpitations attributed to an atrial flutter (Figure 1A) alternating with sinus bradycardia and junctional rhythm (Figure 1B), which evolved rapidly into sustained palpitations. The atrial flutter had a cycle length of 220 ms with negative F-waves in the inferior leads and positive ones in lead V1, suggestive of a typical atrial flutter. Note the lack of R-wave progression in the left-sided precordial leads, suggestive of a right-sided heart. Importantly, lead V1 shows an R-wave of high magnitude with negative T waves and ST depression up to lead V4 that fits with RV strain in the context of a systemic RV following the atrial switch intervention. Figure 2A shows the situs inversus with the right-sided spleen, the left-sided liver, the right-sided heart, and the left-sided superior vena cava (SVC). Figure 2B shows a left lateral view with the interrupted IVC connecting with the left azygos vein in red, then with the SVC in green. Note the lack of any connection between the IVC and the heart. Figure 2C displays a posteroanterior view with a superior tilt. The systemic venous return through the SVC has been surgically directed to the systemic venous atrium (SVA) by the systemic venous pathway (SVP). The pulmonary venous atrium (PVA), in turn, is connected to the left ventricle, which functions as the subpulmonary ventricle. Figure 2D shows a left anterior oblique (LAO) view with a superior tilt. The pulmonary veins in purple have been surgically connected to the PVA through the pulmonary venous pathway, which directs the pulmonary venous return to the systemic RV.
Figure 1.
Electrocardiogram recordings. A: Atrial flutter characterized by negative F waves in inferior leads and positive ones in lead V1. B: Sinus rhythm in alternance with junctional rhythm.
Figure 2.
Magnetic resonance imaging–based segmentation. A: Anteroposterior (AP) view of internal organs. B: Left lateral view. C: Posteroanterior (PA) view with a superior tilt. D: Left anterior oblique (LAO) view with a superior tilt. IJV = internal jugular vein; IVC = inferior vena cava; LV = left ventricle; PVA = pulmonary venous atrium; PVP = pulmonary venous pathway; RV = right ventricle; SVA = systemic venous atrium; SVC = superior vena cava; SVP = systemic venous pathway.
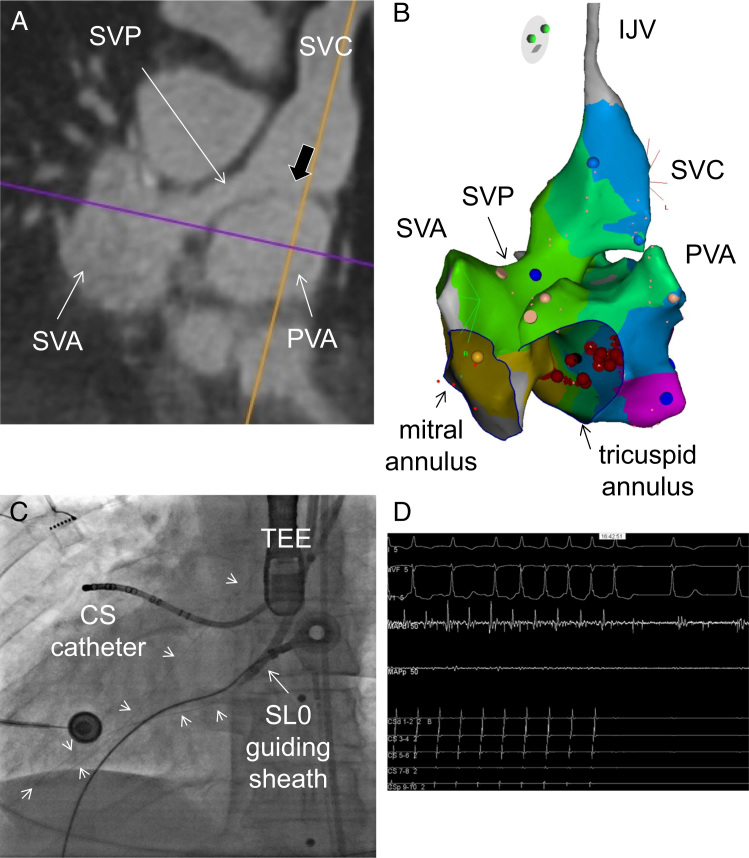
Catheter ablation of the atrial flutter was favored over medical therapy owing to the underlying sinus bradycardia and junctional rhythm. To plan the intervention, a high-resolution 3-dimensional (3D) image dataset of the heart and great vessels was acquired with cardiac magnetic resonance.6 Under general anesthesia we first accessed the SVP and the SVA by performing a puncture of the left internal jugular vein (IJV), which was the shortest path to the SVC, as shown Figures 2A and 3A. A 3D electroanatomical system (Carto3; Biosense Webster, Diamond Bar, CA) was used for mapping and reconstruction. Figure 3B shows the reconstructed IJV, SVC, SVP, and SVA. Importantly, potentials were recorded all along these structures, which fit with the use of atrial tissue for construction of the baffle during the Senning operation. Thirty percent of the flutter cycle length was recorded around the mitral annulus, but short postpacing intervals were only recorded on the septal side of the SVA and SVP (blue dot). We then accessed the PVA by performing a transbaffle puncture at the floor of the SVC overlying the roof of the PVA. The black arrow in Figure 3A shows a thin septum separating both structures in direct continuity of the SVC. Under transesophageal echocardiography guidance, a transbaffle puncture was successfully performed with an SL0 guiding sheath (St Jude, Minneapolis, MN, USA) using a radiofrequency system (Baylis Medical Company Inc, Montreal, Canada). Figure 3C shows an anteroposterior radiograph view with the radiofrequency guidewire advanced within the PVA across the systemic RV into the aorta (short arrows) to improve support. The SL0 guiding sheath was then advanced within the PVA. Figure 3B shows the reconstructed PVA. Activation times covered 100% of the atrial flutter cycle length in a clockwise fashion around the tricuspid annulus of the PVA with short periannular postpacing intervals suggestive of a peritricuspid flutter. The delivery of ablation on the septal side (red dots) of the tricuspid annulus abruptly stopped the flutter (Figure 3D), which is suggestive of boundaries made of the tricuspid annulus on one side and the suture lines connecting the tricuspid annulus to the PVA on the other side.
Figure 3.
Intracardiac access and reconstruction. A: Magnetic resonance imaging slice depicting the superior vena cava (SVC) in direct continuity with the systemic venous pathway (SVP) and the systemic venous atrium (SVA). B: Image shows on the left-hand side the systemic venous structures made of the SVC, SVP, and SVA, and on the right-hand side the pulmonary venous atrium (PVA), reconstructed using a 3-dimensional electroanatomic system. C: Anteroposterior radiograph view after the transbaffle puncture with the SL0 guiding sheath within the PVA and the radiofrequency guidewire (arrows) advanced across the left ventricle into the aorta. D: Termination of the atrial flutter after several seconds of radiofrequency application on the septal and basal interatrial septum of the PVA, as shown by the red dots in panel B. CS = coronary sinus; IJV = internal jugular vein; TEE = transesophageal probe.
At the first postablation follow-up visit, the patient complained of symptomatic bradycardia. Electrocardiogram and 24-hour Holter recording revealed slow junctional rhythm and sinus bradycardia with a mean heart rate of 35 beats/min. Digoxin was discontinued and implantation of an epicardial pacemaker was discussed. On the next follow-up visit, she was asymptomatic with a sinus rhythm at 50 beats/min on the electrocardiogram; she has remained asymptomatic ever since.
Discussion
Malposition of the heart is one of the first described cardiac congenital malformations, mentioned as early as 1606 by Hieronymus Fabricius.7 Its estimated prevalence is 0.1% of live births, and the incidence of situs inversus with right thoracic heart is about 1 in 8000 in the general population.7 It usually occurs with a structurally normal heart. Congenital anomalies of the IVC, such as interrupted IVC with azygos continuation, are rare and their prevalence ranges between 0.6% and 2% in the CHD population. Some isolated cases addressed for catheter ablation have recently been described.8
d-TGA represents 5%–7% of CHD defects. Mustard or Senning atrial redirection with construction of atrial baffles has dramatically increased survival of d-TGA, but extensive atrial surgery predisposes to atrial arrhythmias such as sinus node dysfunction and atrial tachyarrhythmias. The most common form of intraatrial reentrant tachycardia in this population is an atrial flutter whose circuit rotates around the tricuspid valve, similar to typical cavotricuspid isthmus-dependent atrial flutter.9 In d-TGA patients, the cavotricuspid isthmus is divided by the baffle placement and the tricuspid annulus lies on the pulmonary venous side of the septum, connected to the systemic RV. Catheter ablation is often the therapy of choice, given its low morbidity and mortality rates. Catheter ablation appears safe and efficient,9 even when involving transbaffle puncture.9, 10, 11 Complex cases of ablation have been described in the adult CHD population.12
Venous access might be challenging in patients with interrupted IVC and in complex congenital patients with extensive atrial surgery, such as our patient. Different routes have been advocated for accessing the left atrium or the SVA. Lim et al13 and Kato et al14 both accessed the left atrium by performing a transseptal puncture using a right IJV approach. Recently, Singh et al15 proposed an alternative route using a transhepatic venous access that was suitable for patients with interruption of the IVC. The first case was successfully ablated for an atrial flutter in the setting of a Fontan procedure with a total cavopulmonary connection for a single ventricle and atrium with d-TGA, and the second case underwent pulmonary vein isolation for atrial fibrillation. This route was favored over the right IJV access for the first case owing to the disconnection of the SVC from the single atrium, preventing any access to an isthmus-dependent flutter defined by the atrioventricular ring and the confluence of the hepatic veins. A retrograde access has been described10, 11 but is challenging, owing to the crossing of systemic semi-lunar and atrioventricular valves, with some risk of injury because of the tortuous retrograde course and torque imposed to the catheter. In our case, a left IJV with transbaffle puncture was favored over transhepatic or retrograde access. A transhepatic access was not considered, as the hepatic veins drained into the SVA, opposite to the PVA, as shown in Figure 2A. This acute angle would have impeded transbaffle puncture and access to the area of interest. As for the retrograde access, we estimate that the tortuous access might have precluded a detailed mapping of the tricuspid annulus and precise delivery of ablation. One alternative would be using the Stereotaxis system with magnetically driven soft catheter. This system, however, is not available at our institution.
Conclusion
In summary, we describe an atrial flutter ablation in a d-TGA patient combining an atrial switch, an interrupted IVC, and a situs inversus. This case outlines the importance of pre- and intraprocedural imaging combining transesophageal echocardiography and cardiac magnetic resonance merged with 3D reconstruction modalities for the selection of the safest access to arrhythmic circuits in complex cardiac congenital patients.
References
- 1.Walsh E.P., Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534–545. doi: 10.1161/CIRCULATIONAHA.105.592410. [DOI] [PubMed] [Google Scholar]
- 2.Bouchardy J., Therrien J., Pilote L., Ionescu-Ittu R., Martucci G., Bottega N., Marelli A.J. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 3.Warnes C.A. Transposition of the great arteries. Circulation. 2006;114:2699–2709. doi: 10.1161/CIRCULATIONAHA.105.592352. [DOI] [PubMed] [Google Scholar]
- 4.Moons P., Gewillig M., Sluysmans T., Verhaaren H., Viart P., Massin M., Suys B., Budts W., Pasquet A., De Wolf D., Vliers A. Long term outcome up to 30 years after the mustard or senning operation: a nationwide multicentre study in Belgium. Heart. 2004;90:307–313. doi: 10.1136/hrt.2002.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanter R.J., Papagiannis J., Carboni M.P., Ungerleider R.M., Sanders W.E., Wharton J.M. Radiofrequency catheter ablation of supraventricular tachycardia substrates after mustard and senning operations for d-transposition of the great arteries. J Am Coll Cardiol. 2000;35:428–441. doi: 10.1016/s0735-1097(99)00557-4. [DOI] [PubMed] [Google Scholar]
- 6.Monney P., Piccini D., Rutz T., Vincenti G., Coppo S., Koestner S.C., Sekarski N., Di Bernardo S., Bouchardy J., Stuber M., Schwitter J. Single centre experience of the application of self navigated 3d whole heart cardiovascular magnetic resonance for the assessment of cardiac anatomy in congenital heart disease. J Cardiovasc Magn Reson. 2015;17:55. doi: 10.1186/s12968-015-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perloff J.K. The cardiac malpositions. Am J Cardiol. 2011;108:1352–1361. doi: 10.1016/j.amjcard.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Forleo G.B., Pappalardo A., Avella A., Visigalli L., Dello Russo A., Tondo C. Real-time integration of intracardiac echocardiography and 3d electroanatomical mapping to guide catheter ablation of isthmus-dependent atrial flutter in a patient with complete situs inversus and interruption of the inferior vena cava with azygos continuation. J Interv Card Electrophysiol. 2011;30:273–277. doi: 10.1007/s10840-009-9427-2. [DOI] [PubMed] [Google Scholar]
- 9.Khairy P., Van Hare G.F. Catheter ablation in transposition of the great arteries with mustard or senning baffles. Heart Rhythm. 2009;6:283–289. doi: 10.1016/j.hrthm.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Jones D.G., Jarman J.W., Lyne J.C., Markides V., Gatzoulis M.A., Wong T. The safety and efficacy of trans-baffle puncture to enable catheter ablation of atrial tachycardias following the mustard procedure: a single centre experience and literature review. Int J Cardiol. 2013;168:1115–1120. doi: 10.1016/j.ijcard.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Correa R., Walsh E.P., Alexander M.E., Mah D.Y., Cecchin F., Abrams D.J., Triedman J.K. Transbaffle mapping and ablation for atrial tachycardias after mustard, senning, or fontan operations. J Am Heart Assoc. 2013;2:e000325. doi: 10.1161/JAHA.113.000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bargas R.L., Sauer W.H., Lowery C.M. Typical atrial flutter in an atypical patient. Congenit Heart Dis. 2011;6:665–667. doi: 10.1111/j.1747-0803.2011.00535.x. [DOI] [PubMed] [Google Scholar]
- 13.Lim H.E., Pak H.N., Tse H.F., Lau C.P., Hwang C., Kim Y.H. Catheter ablation of atrial fibrillation via superior approach in patients with interruption of the inferior vena cava. Heart Rhythm. 2009;6:174–179. doi: 10.1016/j.hrthm.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Kato H., Kubota S., Yamada Y., Kumamoto T., Takasawa Y., Takahashi N., Yamamoto M. Circumferential pulmonary vein ablation of atrial fibrillation via superior vena cava approach in a patient with interruption of the inferior vena cava. Europace. 2010;12:746–748. doi: 10.1093/europace/eup449. [DOI] [PubMed] [Google Scholar]
- 15.Singh S.M., Neuzil P., Skoka J., Kriz R., Popelova J., Love B.A., Mittnacht A.J., Reddy V.Y. Percutaneous transhepatic venous access for catheter ablation procedures in patients with interruption of the inferior vena cava. Circ Arrhythm Electrophysiol. 2011;4:235–241. doi: 10.1161/CIRCEP.110.960856. [DOI] [PubMed] [Google Scholar]