Introduction
KEY TEACHING POINTS
|
Transcatheter valve-in-valve replacement is an attractive alternative to redo open-heart surgery for failing bioprosthetic valves and is increasingly being performed in patients with high surgical risk.1 Unlike the robust data on aortic valve-in-valve replacement, limited experience with tricuspid valve-in-valve replacement has been reported.2 Moreover, the feasibility and safety of tricuspid valve-in-valve implantation in patients with a transvenous permanent pacemaker are largely unexplored.
Case description
A 53-year-old female patient was referred to our interdisciplinary transcatheter heart valve clinic for progressive worsening of a tricuspid valve bioprosthesis (Carpentier-Edwards, porcine, 31 mm; Edwards Lifesciences, Irvine, CA), which was implanted 23 years before in the setting of infective endocarditis. The degenerating tricuspid bioprosthetic valve showed severe regurgitation and moderate stenosis, with a mean transvalvular gradient of 8.4 mm Hg (Figure 1). The patient’s clinical course was characterized by right heart failure.
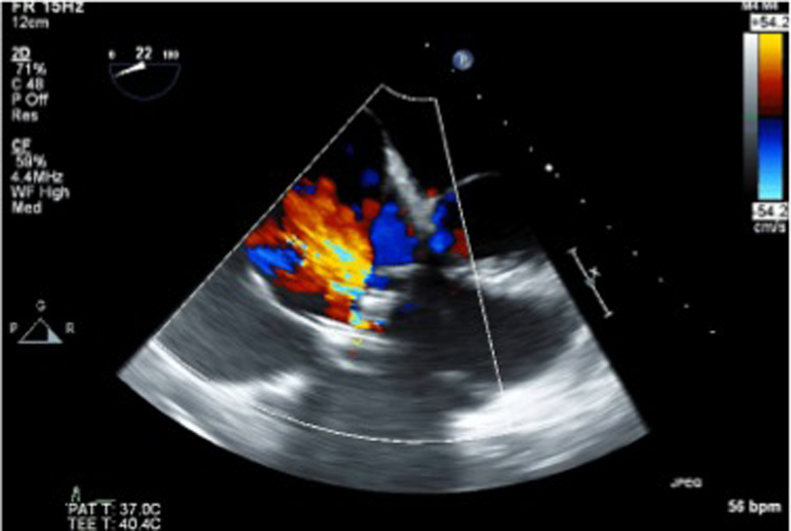
Figure 1.
Preprocedure transesophageal echocardiogram. A transesophageal echocardiography long-axis view of the failing bioprosthetic tricuspid valve with evidence of severe regurgitation.
Her medical history was otherwise significant for sinus node dysfunction with atrial flutter/atrial fibrillation and tachycardia–bradycardia syndrome for which a right-sided dual-chamber pacemaker system had been implanted 2 years previously (Medtronic Advisa DR MRI A3DR01; Medtronic, St Paul, MN; atrial and ventricular lead: St. Jude Medical Tendril STS 2088TC, active fixation; St Jude Medical, St Paul, MN). Redo open heart surgery was not considered a preferred option, because of significant comorbidities including advanced liver cirrhosis, significant pulmonary hypertension, and active hepatitis-C infection. Therefore, the patient was referred for a less-invasive transcatheter tricuspid valve-in-valve replacement.
Procedure
The preoperative pacemaker interrogation showed programming to a dual-chamber non-tracking mode with rate response (DDIR) mode (55–120 beats per minute) with paced AV delay of 340 milliseconds and a total of 48% of right ventricular pacing over time. There was no evidence of prior lead or generator malfunction. Her underlying rhythm was sinus bradycardia competing with a low atrial or junctional rhythm and intermittent isorhythmic dissociation.
The valve-in-valve procedure was performed under general anesthesia, and the device implantation was guided by transesophageal echocardiography (TEE). A 20F sheath for the delivery system was inserted in the right femoral vein. A 29-mm Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA) transcatheter valve was implanted with fluoroscopic and TEE guidance inside the failed tricuspid bioprosthesis (Figures 2A and 2B and Online Data Supplement 1A). Valve deployment was performed under rapid right ventricular pacing at a rate of 180 beats per minute by controlling the previously implanted pacemaker (Figure 2B). Postdeployment fluoroscopy and TEE results showed good positioning. There was an excellent hemodynamic result (Figure 2E) with a residual transvalvular gradient of 2 mm Hg and no evidence of any intra- or paravalvular leak (Figure 2E). The total fluoroscopy time was 34 minutes.
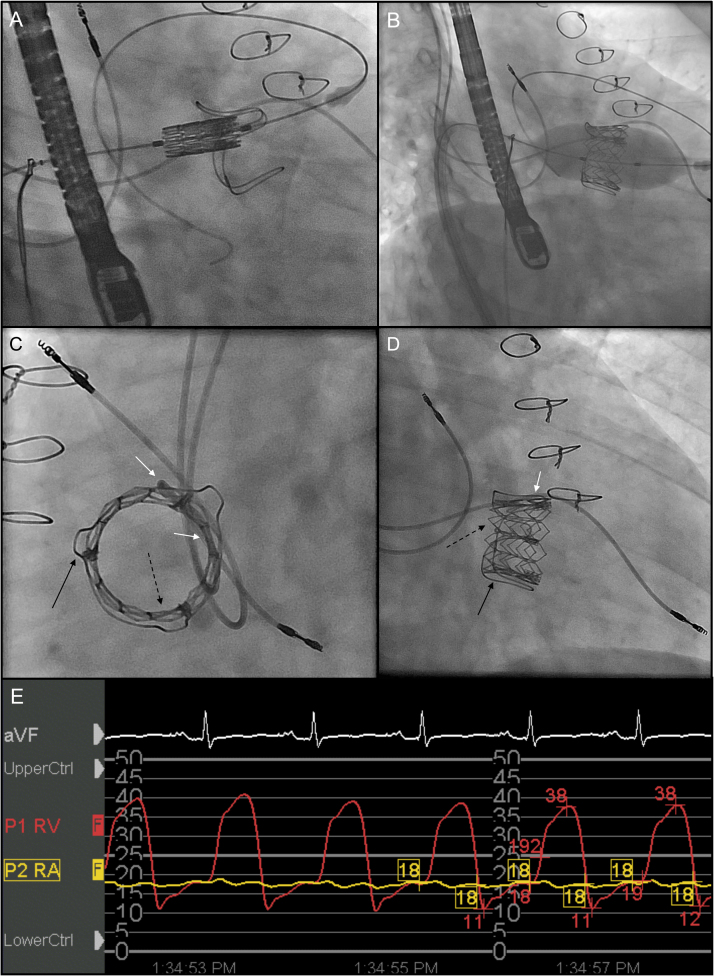
Figure 2.
Valve-in-valve tricuspid valve replacement. A, B: Right anterior oblique (RAO) views during valve-in-valve implantation. A 29-mm SAPIEN XT valve implanted inside a Carpentier-Edwards, porcine, 31-mm valve. C, D: Post–valve deployment cinefluoroscopy demonstrates that the RV pacing lead is now sandwiched (white arrows) between the 2 bioprosthetic valves (black arrows: old Carpentier-Edwards valve; dashed arrows: new Edwards SAPIEN XT valve); C: Left anterior oblique view (LAO). D: Right anterior oblique view (RAO). E: Postimplantation hemodynamic recordings show disappearance of the prominent right atrial v-wave and only a minimal residual diastolic transvalvular gradient of 2–3 mmHg.
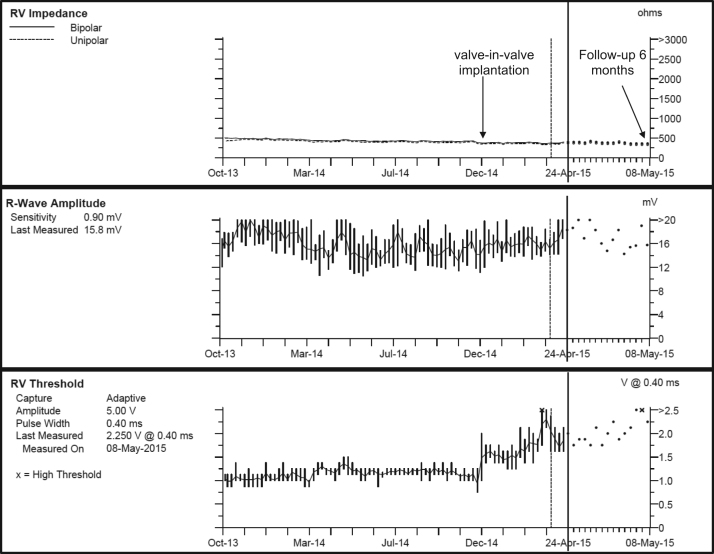
Results of the patient’s pacemaker interrogation before and after the procedure are outlined in Table 1. All parameters remained unaffected by the valve implantation. There was a very mild, transient decrease of ventricular sensing immediately after the valve deployment, without safety concerns. Fluoroscopy showed a marked reduction in the heart-synchronous swinging movements of the right ventricular pacing lead (Figures 2C and 2D and Online Data Supplements 1B and 1C). The clinical course post intervention was favorable, and the patient was discharged 24 hours later. Results of the device interrogation at 6 weeks and 6 months post procedure are outlined in Table 1 and Figure 3. There was a slight increase in the ventricular pacing threshold at 6 months. Sensing and lead impedances remained excellent. A control cinefluoroscopy study conducted 6 weeks post implantation demonstrated a stable valve position. After more than 6 months of follow-up, there is still no evidence of paravalvular regurgitation.
Table 1.
Pacemaker performance
| Preprocedure | Immediately after deployment | 6 hours post procedure | 6 weeks post implantation | 6 months post implantation | |
|---|---|---|---|---|---|
| Lead impedances, Ω | |||||
| Atrial | 418 | 361 | 380 | 456 | 456 |
| Ventricular | 418 | 361 | 380 | 418 | 399 |
| Sensing, mV | |||||
| P waves | 1.6 | 2.8 | 3.3 | 2.8 | 2.4 |
| R waves | 17.4 | 9.9 | 13.5 | 15.3 | 18.3 |
| Pacing threshold, V/ms | |||||
| Atrial | 0.75/0.4 | 0.50/0.4 | 0.50/0.4 | 1.0/0.4 | 0.75/0.4 |
| Ventricular | 1.25/0.4 | 1.0/0.4 | 0.75/0.4 | 1.25/0.4 | 2.0/0.4 |
Figure 3.
Results of pacemaker interrogation 6 months post valve-in-valve replacement.
Discussion
Transcatheter valve-in-valve replacements for failing or degenerating bioprosthetic valves are increasingly being performed, with most procedures targeting transcatheter aortic valve replacement.1 The feasibility for transcatheter tricuspid valve-in-valve replacement was shown previously,3 but experience is still limited to case reports and small series.2, 4 The limitations of tricuspid valve-in-valve procedures may include sizing problems, since many of these devices are too large for available transcatheter devices, and malposition. Additional concerns in patients with pacemakers include postimplant regurgitation and lead failure. None of the previous reports described patients with transvenous permanent pacemakers, which is a relatively common condition in patients with tricuspid valvular disease.
This report demonstrates the feasibility and short-term safety of tricuspid valve-in-valve replacement in a patient with a permanent transvenous pacemaker. A multidisciplinary approach between interventional cardiology and cardiac electrophysiology was chosen to address procedural steps with potential for acute iatrogenic lead damage. Although the risk is projected to be small, there is a risk of lead displacement during insertion of the guide wires and the delivery system across the tricuspid valve, despite fluoroscopy guidance. In the hands of experienced operators and in the presence of active fixation leads with remote implantation (>12 months), as was the case was the case in our patient, the risk of having lead dislodgement during positioning of the delivery system should probably be low. Acute failure of the right ventricular lead is a possible complication during valve deployment, which could crush the ventricular lead between both valves and significantly impair the lead movements. The feared complications of acute mechanical lead damage (fracture or major insulation defect) or acute lead displacement did not occur in our patient. Nevertheless, a transvascular temporary pacemaker system should always be available for backup pacing, especially in pacemaker-dependent patients. Alternatives to tricuspid valve-in-valve procedures include redo open heart surgery or medical treatment alone. Alternative pacemaker management could include lead removal prior to the valve-in-valve procedure and subsequent reimplantation, or left ventricular pacing through a coronary sinus lead. Those two options might be challenging in pacemaker-dependent patients, and it is also associated with an increased risk of infection or lead dislodgement.
Long-term concerns for the patient’s ventricular lead include chronic insulation defects caused by fine shear stress at the level of lead fixation between the 2 valve bodies as well as chronic traction on the lead with the possibility of late-onset lead displacement. The fact that her leads were implanted 2 years prior to the procedure might suggest that lead displacement should be of less concern. Tendril STS 2088TC leads have an Optim insulation, which is composed of a copolymer of silicone and polyurethane that has been shown to provide excellent long-term durability and lead survival.5 Another theoretical issue might be noise oversensing on the ventricular lead through microfriction between the 2 valves. To date, no episode of noise or far-field sensing on the ventricular lead has been recorded in our patient. Frequent device follow-up will be the key element for early detection of lead issues that might arise from the procedure. Late onset of paravalvular regurgitation is an additional concern and is not predictable at this stage. So far, our patient has not developed paravalvular regurgitation. A close follow-up with repeat imaging is recommended.
A potential advantage for patients with permanent pacemakers might be the fact that rapid pacing for the valve deployment can be performed through the indwelling device. Alternatively, the tricuspid valve deployment could also be supported by pacing through a coronary sinus catheter or a left ventricular pacing wire, which would however require additional hardware.2
Limitations
Although our report describes the feasibility of sandwiching a pacemaker lead during a valve-in-valve procedure, this is a single case report with limited device follow-up after tricuspid valve-in-valve implantation. Longer follow-up periods and more patient data are warranted before general conclusions can be drawn on the long-term safety of this procedure in patients with pacemakers. Our approach cannot be extrapolated to pacemaker-dependent patients in the absence of robust long-term data. In addition, the findings of our case might not apply to patients with an implantable cardioverter defibrillator or patients with multiple right ventricular leads.
Transvenous lead extraction might be impossible if the right ventricular lead is sandwiched between 2 prosthetic valves or might carry a high risk of valve damage. However, in a given intracardiac device–related infection, removal of all prosthetic material is warranted, and lead extraction alone would be unlikely to achieve infection control. Also, the overall high procedural risk disqualifies patients with such infections for lead extraction in other situations, such as debulking or lead failure.
Conclusion
This case demonstrates the feasibility and short-term safety of transcatheter tricuspid valve-in-valve implantation of an Edwards SAPIEN XT valve in a patient with permanent right ventricular pacemaker.
Online Data Supplement 1
Valve-in-valve implantation and sandwiching of the right ventricular pacing lead. A: Balloon inflation and valve deployment. B, C: Cinefluoroscopy views post valve-in-valve implantation of the Edwards SAPIEN XT valve demonstrate a marked reduction of the RV lead swinging. Shown are B: a right anterior oblique view (RAO) and C: a left anterior oblique (LAO) view.
Footnotes
Conflicts of interest: The authors have no conflict of interest to declare.
Dr Steinberg and Dr Dvir contributed equally to this article.
Supplementary material cited in this article is available online at doi:10.1016/j.hrcr.2015.05.004.
Appendix. Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1.Dvir D., Webb J.G., Bleiziffer S. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–170. doi: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
- 2.Godart F., Baruteau A.E., Petit J., Riou J.Y., Sassolas F., Lusson J.R., Fraisse A., Boudjemline Y. Transcatheter tricuspid valve implantation: A multicentre French study. Arch Cardiovasc Dis. 2014;107:583–591. doi: 10.1016/j.acvd.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Hon J.K., Cheung A., Ye J., Carere R.G., Munt B., Josan K., Lichtenstein S.V., Webb J. Transatrial transcatheter tricuspid valve-in-valve implantation of balloon expandable bioprosthesis. Ann Thorac Surg. 2010;90:1696–1697. doi: 10.1016/j.athoracsur.2010.04.101. [DOI] [PubMed] [Google Scholar]
- 4.Hoendermis E.S., Douglas Y.L., van den Heuvel A.F. Percutaneous Edwards SAPIEN valve implantation in the tricuspid position: Case report and review of literature. EuroIntervention. 2012;8:628–633. doi: 10.4244/EIJV8I5A95. [DOI] [PubMed] [Google Scholar]
- 5.St Jude Medical. Product performance report 2014: https://professional.sjm.com/resources/product-performance/product-performance-reports/current.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material