Introduction
KEY TEACHING POINTS
|
Clinical trials investigating the safety and effectiveness of transcatheter leadless pacemakers in humans are ongoing.1, 2 These devices offer the benefits of cardiac pacing with the potential for a significant decrease in many of the risks associated with conventional pacing systems, including hematoma formation, pneumothorax, lead-related complications, and vascular obstruction.3
Human evaluation of the Medtronic Micra Transcatheter Pacing System (Medtronic, Minneapolis, MN) began in 2013, and the device is currently undergoing clinical investigation.1 The devices are implanted via a femoral venous approach and use a novel tined system for fixation to the right ventricular endocardium. The pacemakers can be readily retrieved at the time of implantation, but no data exist regarding the ability to remove these devices in humans after the initial implantation procedure. We report the first successful extraction of a Micra Transcatheter Pacing System, 3 weeks after initial device implantation.
Case report
A 61-year-old man developed dizziness and near syncope in the setting of permanent atrial fibrillation. Evaluation revealed a persistently slow ventricular response (30–40 beats per minute). He subsequently underwent transcatheter leadless pacemaker implantation, as part of the Micra transcatheter pacing study. The implantation was uncomplicated and he was discharged from the hospital with stable pacing (sensing at 8 mV, pacing threshold of 0.63 V at 0.24 ms and impedance of 650 ohms). He returned to the clinic 15 days later, noting a several-day history of dizziness and fatigue. An electrocardiogram demonstrated atrial fibrillation with a slow ventricular response as well as noncaptured pacing impulses. His device was interrogated and an elevated capture threshold was noted (5 V at 1.0 ms). The automated capture management algorithm had appropriately monitored the threshold and increased the pacing output significantly (5.0 V at 1.0 ms). Sensing and impedance parameters were stable. A chest radiograph demonstrated stable device position. The pacing output was increased and he was admitted to the hospital. As battery longevity would be curtailed at high pacing outputs, the decision was made to replace the pacing system.
He was subsequently brought to the Electrophysiology laboratory for device extraction and replacement. The initial plan was to place a second transcatheter leadless pacemaker at a remote site in the right ventricle (RV) and use the delivery system for the second device to recapture and remove the first device. The Medtronic Micra sheath (Medtronic, Minneapolis, MN) was placed in the right femoral vein and the Micra delivery system was used to advance a new transcatheter leadless pacemaker to the RV. It was difficult to find a suitable right ventricular site for the second pacemaker that elicited acceptable electrical values and was sufficiently removed from the initial implantation site. After a number of attempts, this approach was abandoned and the delivery system was removed.
A 6F sheath was placed in the left femoral vein and a 5F quadripolar catheter (Bard, Lowell, MA) was advanced to the RV, to provide backup pacing once the initial device was removed. Attention was then turned to extraction of the transcatheter leadless pacemaker.
An 8.5F 28 mm medium-curve steerable sheath (Agilis NxT; St Jude Medical, Minnetonka, MN) was advanced via the 23F Medtronic Micra sheath to the right atrium. The deflectable sheath was used to advance a foreign body retrieval device (20 mm EN snare; Merit Medical Systems, South Jordan, UT) with multiple loops to the RV. The proximal retrieval feature of the device could not be engaged with this system, but 1 of the fixation tines was snared (Figure 1). The device was removed to the right atrium but became detached from the snare. The multiple-loop snare was removed and a single-loop snare (Amplatz GooseNeck Snare; Covidien/Medtronic, Plymouth, MN) was used to attempt to capture the retrieval feature on the device. Ultimately, 1 of the fixation tines of the device was captured with the snare. With only 1 of the 4 fixation tines attached, the device could not be properly aligned and retracted into the introducer. A second snare (20 mm EN Snare; Merit Medical Systems) was then advanced to the right atrium and a second tine was snared (Figure 2). The 2 snares were used to align the pacemaker and it was retracted into the introducer and subsequently removed from the body (Figure 3). The new transcatheter leadless pacemaker was then successfully implanted to the RV.
Figure 1.
The Micra pacemaker was snared in the right ventricle using a multilobed gooseneck snare, which captured 1 of the fixation tines of the device.
Figure 2.

Two snares were used to capture 2 separate fixation tines on the Micra pacemaker. This allowed the device to be properly aligned so that it could be retracted into the Micra introducer.
Figure 3.
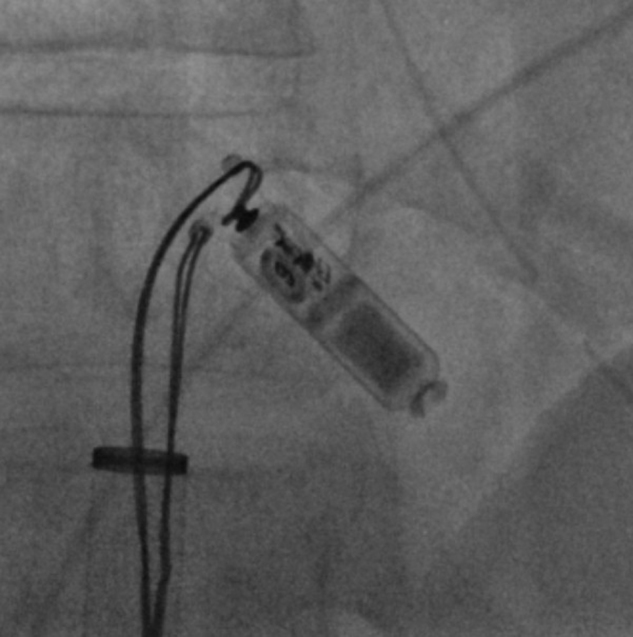
The snares were used in tandem to properly orient the device and allow its retraction into the Micra introducer and subsequent removal from the body.
Discussion
Implantable transcatheter leadless pacemakers offer many potential advantages over conventional pacing systems, including decreased risk of infection, pneumothorax, pocket-related issues, and transvenous lead–related issues. However, other risks associated with pacemaker implantation will likely remain, some of which may necessitate device removal or revision.
Currently available delivery systems allow for the device to be repositioned multiple times during the initial implant procedure. However, once the device has been fully delivered, device recapture is much more challenging. The Micra device has been designed with a proximal retrieval feature to aid in recapture. Any fixation tines that have not engaged the endocardium are also a potential target for retrieval. We have demonstrated that it is possible to capture the device and successfully remove it several weeks after implantation, using readily available foreign body removal snares.
Conclusion
Despite their theoretical advantages, transcatheter leadless pacemakers will still occasionally require device repositioning or removal. Few data are available regarding the ease or feasibility of device retrieval after implantation. Though the device is designed with a proximal retrieval feature, we have demonstrated that it can be safely removed with an alternate method, using routinely available foreign body retrieval equipment to capture the fixation tines of the device.
Footnotes
Dr Grubman serves as an investigator for the MICRA Clinical Study.
References
- 1.Steinwender C., Ritter P., Duray G.Z. Early electrical performance of a novel leadless transcatheter pacemaker system: data from the MICRA clinical study. Heart Rhythm. 2015;12:S417. [Google Scholar]
- 2.Reddy V.Y., Knops R.E., Sperzel J. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129(14):1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 3.Udo E.O., Zuithoff N.P., van Hemel N.M. Incidence and predictors of short- and long-term complications in pacemaker therapy; the FOLLOWPACE study. Heart Rhythm. 2012;9:728–735. doi: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]



