Introduction
KEY TEACHING POINTS
|
We report the case of an 80-year-old woman with syncope and atrial tachycardia (AT) induced by swallowing. Pharmacologic therapy was ineffective, and she underwent catheter ablation of AT originating from the superior vena cava (SVC). Potential mechanisms of swallow-induced syncope including AT and parasympathetic stimulation are discussed. Electroanatomic correlation with the role for parasympathetic innervation from the aortocaval ganglionated plexus is provided.
Case report
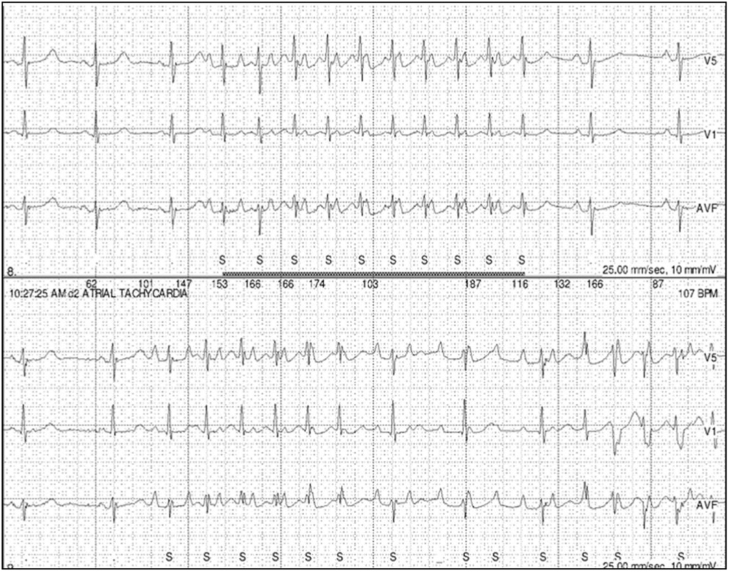
An 80-year-old woman presented with palpitations and facial flushing followed by pre-syncope or syncope associated with swallowing solid or liquid food for 2 months. Past medical history was significant for hypertension and hypothyroidism. Initial evaluation including electrocardiogram, transthoracic echocardiogram, cardiac stress test, neurologic examination, magnetic resonance imaging of head, esophageal swallow study, and barium esophagogram were unremarkable. A 24-hour Holter monitor and event recorder captured episodes of paroxysmal narrow complex tachycardia with variable atrioventricular delay at a rate of 200 bpm in association with her symptoms, suggestive of AT (Figure 1). Mild slowing of the sinus rhythm was observed before and immediately after the AT. Multiple premature atrial contractions (PAC) with the same P-wave morphology as the AT were also observed.
Figure 1.
Holter monitor showing correlation between narrow complex tachycardia, swallowing, and syncope.
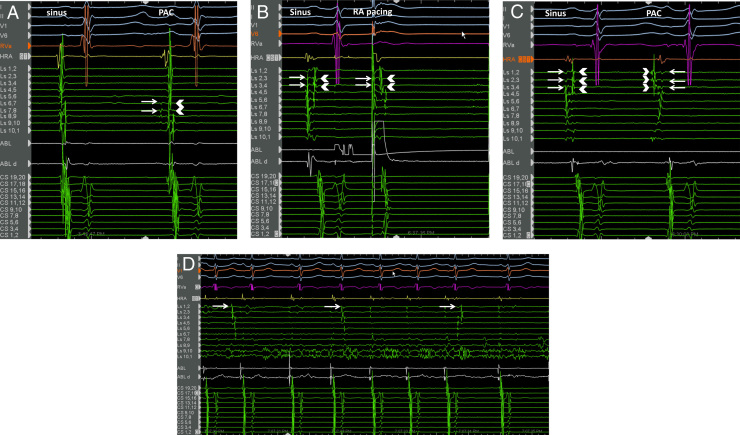
Metoprolol and flecainide therapy did not significantly affect the frequency or severity of her symptoms. Owing to persistent symptoms and fear of eating that resulted in 7 kg weight loss, she underwent an electrophysiology study. AT was not inducible with programmed atrial stimulation, isoproterenol infusion, and cold saline infusion into the esophagus. Activation mapping of the left and right atria during spontaneous PACs with P-wave morphology similar to that observed during deglutition in the awake state was performed using CARTO3 (Biosense Webster, Diamond Bar, CA). A circular mapping catheter was placed in the right superior pulmonary vein and showed early activation of far-field electrograms preceding onset of the P wave, by 48 ms (Figure 2A). This was suggestive of focal origin from the right atrium (RA) or SVC source. A circular mapping catheter placed in the SVC showed reversal of activation of the far- and near-field signals between sinus rhythm and PACs, with the SVC potential preceding RA activation during PAC (Figure 2B and C). This was diagnostic of PAC focus in the SVC, and further mapping showed earliest activation in the posterior SVC preceding P-wave onset by 65 ms (Figure 2C). Isolation of the SVC was then performed at the venoatrial junction using radiofrequency ablation. AT was induced during ablation near the posterior SVC-RA junction during ablation at sites away from the site of earliest activation during PAC (Figure 2D). Automaticity from the SVC with exit block to the atrium was seen following isolation. Multiple spontaneous episodes of hypotension independent of heart rate and AT were also observed, suggestive of the existence of a vasodepressor response, potentially autonomically mediated. At 11 months of follow-up, the patient remained free of swallow-induced symptoms.
Figure 2.
Electrophysiology study findings. A: Circular mapping catheter (Ls) in the right superior pulmonary vein (RSPV). The first beat is a sinus beat followed by the premature atrial complex (PAC). The RSPV recorded early far-field atrial electrograms (arrow) followed by recording of pulmonary vein potential (arrowhead) during the PAC. This is suggestive of origin of tachycardia in an adjacent chamber—in this case, the right atrium (RA) or the superior vena cava (SVC). B: Circular mapping catheter (Ls) in the SVC. The first beat is a sinus beat showing 2 sets of signals on the Ls catheter. In order to determine the origin of these signals, pacing is performed from the high RA using the ABL catheter (second beat). The first set of signals (arrow) are “pulled into” the pacing spike with capture of the RA, demonstrating that this represents right atrial signal. The remaining electrogram is thus identified as the SVC potential (arrowhead). C: Circular mapping catheter in the SVC. The first beat is a sinus beat followed by a PAC. The right atrium (arrow) is activated first in sinus rhythm, followed by the SVC potential (arrowhead). This relationship is reversed during PAC, with activation of the SVC potential occurring prior to activation of the right atrial signal. The phenomenon of “reversal of the near-field and far-field electrogram” proves that the “chamber of origin” of the PAC is the SVC. The origin of the PAC was mapped to the posterior SVC. D: SVC automaticity (arrows) during ablation at the SVC–right atrial venoatrial junction immediately following isolation of the SVC. Entrance block into the SVC is established at this point.
Discussion
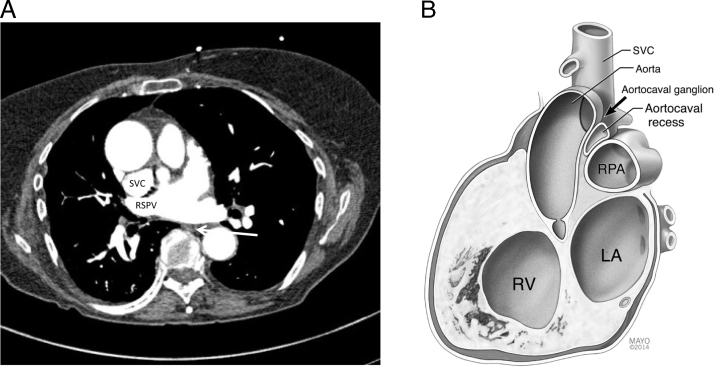
We present a case of deglutition-induced syncope associated with AT originating from the SVC. Swallow-induced tachyarrhythmia is rare, and cases described in the literature have reported arrhythmia originating from the atria and pulmonary vein.1 This is the first report, to our knowledge, of deglutition-induced AT from the SVC. Proposed mechanisms of swallow-induced tachyarrhythmia include autonomic reflex (parasympathetic or sympathetic mediated) and mechanical stimulation of the atrium by food bolus.2 Computed tomography scan of the chest, shown in Figure 3A, revealed that the esophagus was not in proximity to the SVC, the site of origin of the AT. The lack of correlation of severity of symptoms with the type of food bolus, the lack of physical proximity of the focus of the AT to the esophagus, and the presence of other autonomic features such as vasodepressor symptoms make a vagally mediated reflex the most likely mechanism in this case.
Figure 3.
A: Anatomic relationship of the superior vena cava (SVC), right superior pulmonary vein (RSPV), and the esophagus (arrow). B: Anatomic relationships of the SVC and aortocaval ganglion. The aortocaval ganglion is located in the aortocaval recess of the pericardium, which is bound anterolaterally by the SVC and posteromedially by the ascending aorta. The right pulmonary artery (RPA) forms the floor of the recess. The aortocaval ganglion receives parasympathetic preganglionic neurons from the vagus nerve. Postganglionic neurons then project to the sinus node, atrial muscle, and muscle sleeve of the SVC. LA = left atrium; RV = right ventricle.
Activation of esophageal mechanoreceptors may activate afferent vagal inputs to autonomic centers including the nucleus tractus solitarius, which in turn provide vagal efferent output to the cardiac ganglionated plexi (GP). Correlating anatomy with electrophysiology findings of this case, we draw attention to the potential role of the aortocaval GP located in the aortocaval recess between the posteromedial wall of the SVC and the anterolateral wall of the ascending aorta (Figure 3B). The aortocaval GP receives preganglionic parasympathetic neurons from the vagus nerve. The postganglionic neurons from the GP innervate the atrium and SVC. Stimulation of the GP has been shown to shorten action potential duration and induce rapid firing in the thoracic veins, triggering AT.3 The possible role of the GP in initiating AT in this case is supported by (1) the consistency of location of aortocaval ganglion with the site of ectopy, (2) sinus rate slowing observed prior to onset of AT, and (3) induction of AT by radiofrequency ablation at the SVC-RA junction, far removed from the AT focus but in close proximity to the GP. We, however, did not observe ventricular rate slowing or PR interval prolongation during the AT. Selective innervation of the sinus node and atrioventricular node by certain GP is well described. Hou et al4 described sinus rate slowing with anterior right GP stimulation with no effect on the AV node. In our own experience (unpublished data), high-frequency stimulation of the aortocaval GP in dogs predominantly results in shortening of the atrial effective refractory period with minimal effect on sinus rate. Hence, we propose that potential factors contributing to syncope in this case include vagal-mediated atrial tachyarrhythmia and vasodepressor response. Ablation of the parasympathetic aortocaval GP located in close proximity to the SVC at the time of SVC isolation has the potential to address both mechanisms, leading to the clinical resolution of symptoms in this patient.
Conclusions
Swallow-induced tachyarrhythmia is rare, may arise from the atria or thoracic veins, and is frequently mediated by autonomic reflex. In the case of SVC ectopy, the proximity of the aortocaval ganglion suggests the role GP may play in triggering tachycardia and syncope. Swallow-induced tachyarrhythmia and syncope can be disabling, and radiofrequency ablation provides a definitive cure for select patients who do not respond to pharmacologic therapy.
References
- 1.Undavia M., Sinha S., Mehta D. Radiofrequency ablation of swallowing-induced atrial tachycardia: case report and review of literature. Heart Rhythm. 2006;3(8):971–974. doi: 10.1016/j.hrthm.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Tada H., Kaseno K., Kubota S., Naito S., Yokokawa M., Hiramatsu S., Goto K., Nogami A., Oshima S., Taniguchi K. Swallowing-induced atrial tachyarrhythmias: prevalence, characteristics, and the results of the radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2007;30(10):1224–1232. doi: 10.1111/j.1540-8159.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 3.Lu Z., Scherlag B.J., Niu G., Lin J., Fung K.M., Zhao L., Yu L., Jackman W.M., Lazzara R., Jiang H., Po S.S. Functional properties of the superior vena cava (SVC)-aorta ganglionated plexus: evidence suggesting an autonomic basis for rapid SVC firing. J Cardiovasc Electrophysiol. 2010;21(12):1392–1399. doi: 10.1111/j.1540-8167.2010.01787.x. [DOI] [PubMed] [Google Scholar]
- 4.Hou Y., Scherlag B., Lin J., Zhang Y., Lu Z., Truong K., Patterson E., Lazzara R., Jackman W., Po S.S. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input. J Am Coll Cardiol. 2007;50(1):61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]