Introduction
KEY TEACHING POINTS
|
Sarcoidosis is a multisystemic granulomatous disease of unknown etiology. All body organs may be involved by sarcoidosis; thus, magnetic resonance imaging (MRI) is an important imaging modality. However, in the past, it was impossible to perform magnetic resonance (MR) scanning for extracardiac lesions in patients with cardiac sarcoidosis requiring the implantation of traditional cardiac devices, including pacemaker, implantable cardioverter-defibrillator (ICD), and cardiac resynchronization therapy defibrillator (CRT-D). Recently, MR-conditional devices have become available; however, the experience of using MR-conditional CRT-D is rare. We describe a patient with cardiac sarcoidosis involving a cervical extradural lesion in whom efficacy of corticosteroid treatment for spinal lesion could be clearly assessed by MRI after implanting of an MR-conditional CRT-D.
Case report
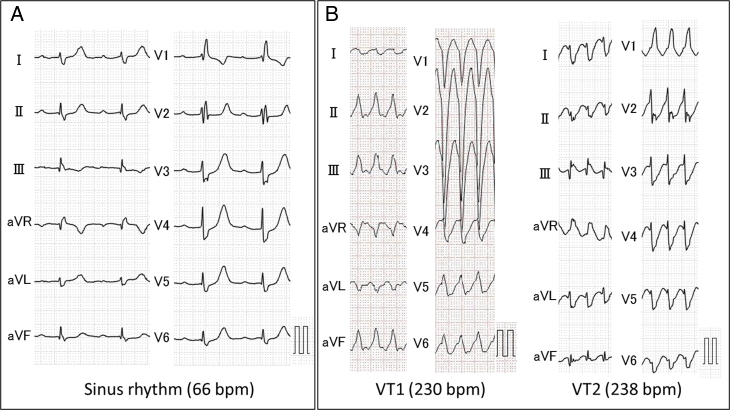
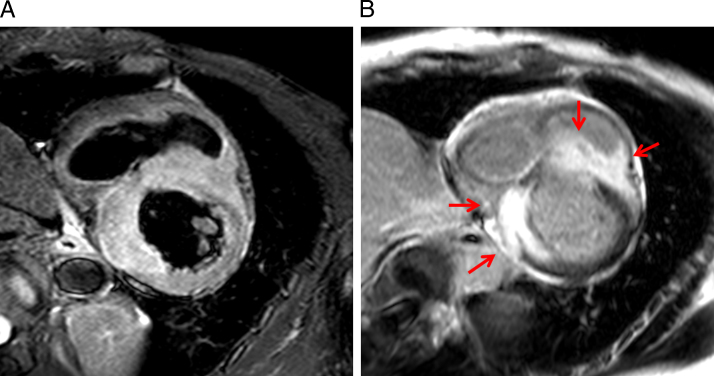
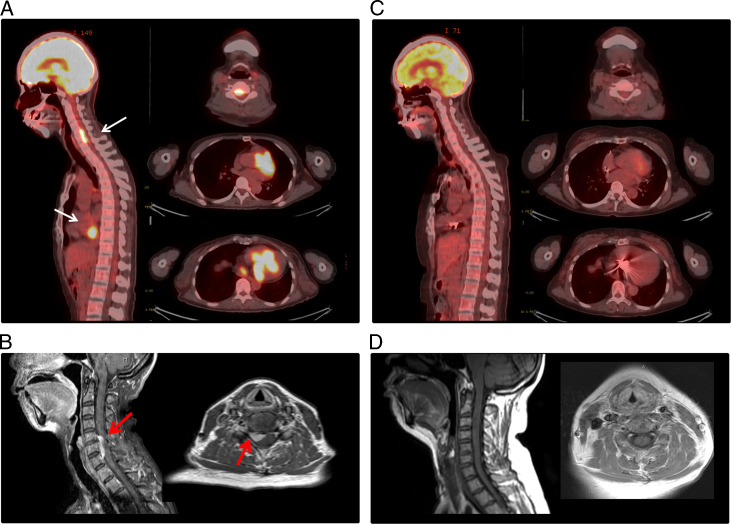
A 56-year-old woman was admitted to our hospital for hemodynamically unstable ventricular tachycardia (VT) treatment. She had no familial history of cardiovascular disease or sudden cardiac death. The 12-lead electrocardiograms during sinus rhythm and VTs are shown in Figure 1. The 12-lead electrocardiogram during sinus rhythm showed a first-degree atrioventricular block and right bundle branch block (Figure 1A). Two VTs were observed after admission to our hospital (Figure 1B); VTs were suppressed by a combination therapy of antiarrhythmic drugs (amiodarone 200 mg, carvedilol 5 mg, and mexiletine 300 mg). Her transthoracic echocardiogram demonstrated a decreased left ventricular ejection fraction (LVEF) of 42% and focal thinning of the basal anterior septum and some focal intracardiac mass in the ventricular septum and left ventricular inferior wall. Cardiac MRI confirmed myocardial edema evidenced by increased signal intensity on T2-weighted images, and late gadolinium enhancement was confirmed to match the intracardiac mass in the ventricular septum and inferior wall (Figure 2). Fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission tomography / computed tomography revealed a strong 18F-FDG uptake in the myocardium as focal or diffuse pattern and in the cervical spinal canal (Figure 3A). Although the patient had no neurologic symptoms, spinal gadolinium-enhanced MRI showed a focal extradural lesion with high signal intensity at the C5-7 level (Figure 3B). Sarcoidosis was strongly suspected by multimodality imaging. Ocular examination was normal. Serum levels of angiotensin-converting enzyme, lysozyme, and soluble interleukin-2 receptor were within normal limits. The tuberculin reaction was negative. Angiography indicated normal coronary arteries.
Figure 1.
Twelve-lead electrocardiography (ECG). A: ECG during sinus rhythm on admission. B: ECG during ventricular tachycardias (VTs). Two QRS morphologies of VTs in the 12-lead ECG were observed; 1 VT exhibited a QRS complex having a right bundle branch block–type morphology and the other exhibited a left bundle branch block–type morphology.
Figure 2.
Cardiac magnetic resonance imaging (MRI). A: Cardiac MRI showing myocardial inflammation and edema. B: Late gadolinium enhancement was confirmed to match the intracardiac mass (red arrows).
Figure 3.
Fluorine-18-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) / computed tomography (CT) and spinal magnetic resonance imaging (MRI) obtained before and after corticosteroid treatment and the implantation of a cardiac resynchronization therapy defibrillator (CRT-D) (the left panel in each figure shows sagittal view, and the right panel shows transaxial view). A: Abnormal uptake of 18F-FDG was registered in the heart and cervical spinal canal before treatment (white arrows). B: Gadolinium-enhanced MRI showed a cervical extradural lesion with high signal intensity at the C5 -7 level (red arrow). C: Follow-up 18F-FDG PET/CT showed a disappearance of abnormal 18F-FDG uptake in the heart and cervical spinal canal 5 months after treatment. D: The follow-up MRI 5 months after treatment showing a further decline of the epidural lesion. Very clear images were obtained despite CRT-D implantation.
An endomyocardial biopsy was performed, and specimens were obtained from the portion of the right side of the ventricular septum. No obvious noncaseous granuloma with giant cells was observed; however, a small inflammatory lesion that was formed by lymphocytes and macrophages was found in a part of a specimen, and it appeared to be a granulomatous lesion. There was no finding of malignancy. On the basis of the above findings, the patient was clinically diagnosed with cardiac sarcoidosis involving cervical extradural lesion by the Japanese Ministry of Health and Welfare criteria modified in 2006, including the clinical/imaging pathway.1 She underwent implantation of a CRT-D because hemodynamically unstable VTs, progression of conduction abnormality, and reduced LVEF were observed. The device selected was an MR-conditional CRT-D (Ilesto7HF-T DF-4Pro; BIOTRONIC, Berlin, Germany) and leads (left ventricular lead, Corox ProMRI OTW-L 85BP; right ventricular lead, Protego ProMRI S65; atrial lead, Solia S53; BIOTRONIC, Berlin, Germany).
After implantation of the CRT-D, prednisolone (30 mg) was administered daily. 18F-FDG positron emission tomography / computed tomography was performed to evaluate the corticosteroid treatment after 5 months; abnormal uptake of FDG at heart and spine completely disappeared (Figure 3C). Spinal MRI was performed to evaluate the cervical extradural lesion at the C5–7 level; however, it had completely disappeared (Figure 3D). The patient had no cardiac events such as recurrence of VT or heart failure during 10 months after corticosteroid treatment, and her LVEF did not decrease under all biventricular pacing.
Discussion
The prognosis of sarcoidosis is affected by the presence of myocardial lesions. However, diagnosis of cardiac sarcoidosis is difficult because endomyocardial biopsy is limited by sampling and technical errors and has a low sensitivity. The Heart Rhythm Society published a new criterion for the diagnosis of cardiac sarcoidosis in 2014.2 Unfortunately, no obvious noncaseous granuloma with giant cells was observed in this case. Therefore, the patient was clinically diagnosed with cardiac sarcoidosis using the Japanese Ministry of Health and Welfare criteria.1 Although useful, this criterion is limited by low diagnostic specificity. Therefore, we tried to exclude other inflammatory and malignant diseases before establishing the clinical diagnosis of cardiac sarcoidosis.
Cardiac and neurologic sarcoidosis are rare; however, the prevalence of subclinical cardiac and neurologic sarcoidosis may be much higher. Baughman et al3 described that approximately half of 736 sarcoidosis patients had multiple organ involvement. Therefore, the patients with cardiac sarcoidosis involving neurologic lesions may be more common than documented. Additionally, sarcoidosis is a progressive disease. If the sarcoidosis patients have single-area involvement at the time of first diagnosis, multiple organ involvement may develop during follow-up. Thus, MRI must be an essential imaging modality in patients with cardiac sarcoidosis because extracardiac lesions, particularly neurologic lesions such as spinal sarcoidosis, should be evaluated by MRI.4 Recently, MR-conditional devices have become available. However, there is no report that MRI was performed for extracardiac lesions after implantation of MR-conditional devices. To the best of our knowledge, this is the first report on detailed evaluation and assessment of corticosteroid treatment for a cervical extradural lesion performed in a patient with cardiac sarcoidosis and a CRT-D implant.
In the present case, the patient had hemodynamically unstable VTs; thus ICD was essential. We implanted a CRT-D, not an ICD, because progressive conduction abnormalities, including a first-degree atrioventricular block, were observed and LVEF was moderately decreased. Some studies described that high percentages of right ventricular pacing may promote left ventricular systolic dysfunction.5, 6 Curtis et al7 proposed that biventricular pacing was superior to right ventricular pacing in patients with atrioventricular block. There is a possibility of atrioventricular block progressing along with the progress of disease. Currently, atrioventricular block is progressive despite corticosteroid treatment, and the patient required biventricular pacing during follow-up; however, her LVEF has not reduced, and she has not developed any cardiac events.
References
- 1.Diagnostic standard and guidelines for sarcoidosis. Jpn J., Sarcoidosis Granulomatous D.i.s. 2007;27:89–102. [Google Scholar]
- 2.Birnie D.H., Sauer W.H., Bogun F. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1304–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 3.Baughman R.P., Teirstein A.S., Judson M.A. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.11.2106001. [DOI] [PubMed] [Google Scholar]
- 4.Joseph F.G., Scolding N.J. Sarcoidosis of the nervous system. Pract Neurol. 2007;7:234–244. doi: 10.1136/jnnp.2007.124263. [DOI] [PubMed] [Google Scholar]
- 5.Olshansky B., Day J.D., Lerew D.R., Brown S., Stolen K.Q., INTRINSIC RV Study Investigators Eliminating right ventricular pacing may not be best for patients requiring implantable cardioverter-defibrillators. Heart Rhythm. 2007;4:886–891. doi: 10.1016/j.hrthm.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Udo E.O., van Hemel N.M., Zuithoff N.P., Doevendans P.A., Moons K.G. Risk of heart failure- and cardiac death gradually increases with more right ventricular pacing. Int J Cardiol. 2015;185:95–100. doi: 10.1016/j.ijcard.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 7.Curtis A.B., Worley S.J., Adamson P.B., Chung E.S., Niazi I., Sherfesee L., Shinn T., Sutton M.S. Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]