Introduction
KEY TEACHING POINTS
|
Brugada syndrome (BrS) is characterized by ST-segment elevation in right precordial leads on 12-lead electrocardiogram (ECG) with associated sudden cardiac death because of ventricular fibrillation (VF).1 The ECG manifestations of this syndrome are often dynamic or concealed and may be unmasked or modulated by sodium channel blockers, febrile state, vagotonic agents, α-adrenergic agonists, β-adrenergic blockers, a combination of glucose and insulin, hypo- and hyperkalemia, alcohol or cocaine toxicity, and tricyclic antidepressants, as well as other medical drugs.2 In symptomatic patients the implantation of an implantable cardioverter-defibrillator (ICD) is recommended, with or without medical treatment, with the purpose of reduction of the ionic imbalance at the end of phase 1 of action potential, inhibitors of potassium outward currents (ito), or drugs that increase calcium currents (ICaL).3 In 2011 Nademanee et al4 published a case series of 9 symptomatic patients with BrS who had recurrent VF episodes and ICD discharges who underwent epicardial ablation of the right ventricular outflow tract (RVOT). As Sacher et al5 discovered later and then published by Boyle and Shivkumar6, in this location low voltages with prolonged duration and fractionated late potentials were found, and preliminary studies have shown an absence of clinical recurrence after the procedure of 78% in a 20-month follow-up.7, 8, 9
Case report
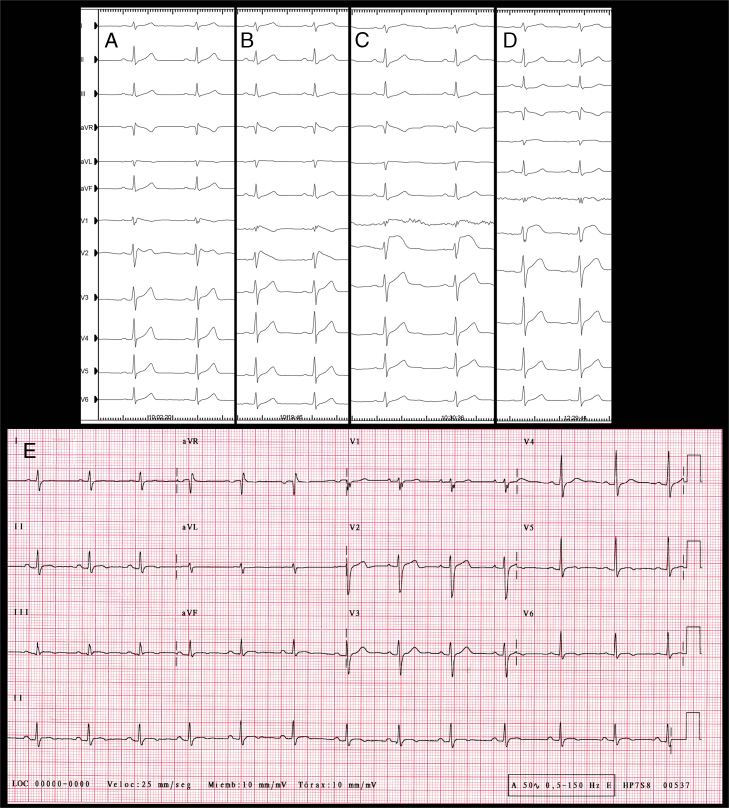
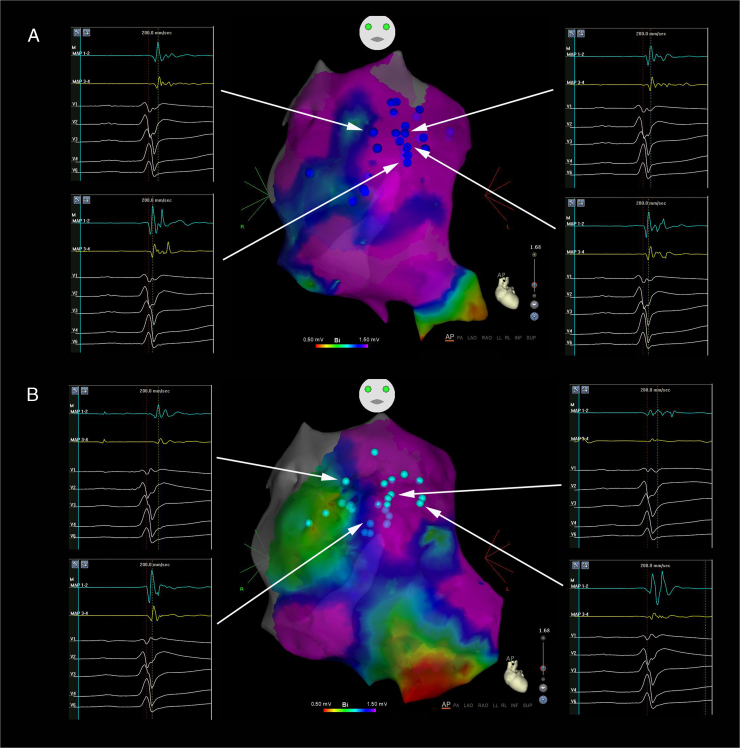
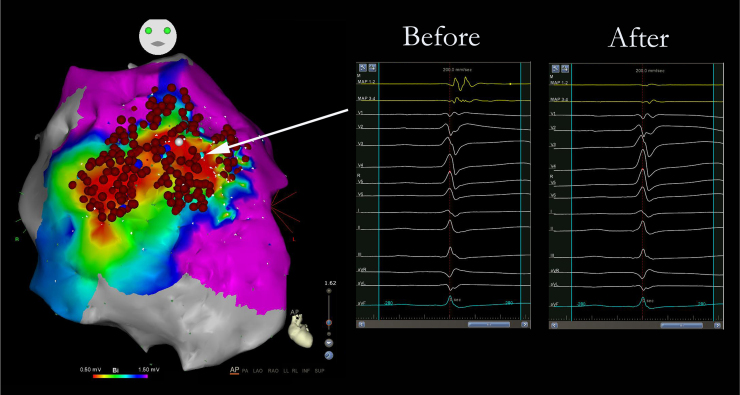
We present the case of a 33-year-old man, who in 2010 presented with recovered sudden cardiac death associated with VF and debut of BrS with type 1 transitory pattern. A VVI/ICD was implanted as secondary prevention and genetic testing for BrS susceptibility genes was performed, with negative results. The patient remained asymptomatic until 2013, when he presented with a VF episode with ICD appropriate discharge. In December 2014, 2 VF episodes were treated, also with ICD appropriate discharges. Medical treatment was initiated with quinidine sulfate 400 mg/8 hours but it was suspended 1 month later because of severe diarrhea, asthenia, and 7 kg weight loss. Two months later, the patient presented 2 VF episodes with ICD appropriate discharges, so epicardial ablation was proposed. Dynamic ECG manifestations of Brugada syndrome were clearly observed in this patient (Figure 1A–E). At the beginning of electrophysiological study, BrS type 2 pattern was evident (Figure 1A). Under general anesthesia, 2 femoral venous punctures were performed, for a quadripolar catheter and an ablation catheter (Navistar ThermoCool Biosense Webster, Diamond Bar, CA) 8F irrigated curve D, in the right ventricle (RV). Endocardial RV voltage mapping was performed with an electroanatomic mapping system (CARTO 3; Biosense Webster, Diamond Bar, CA), where normal electrograms were found. Epicardial access was attempted using a subxiphoid percutaneous approach and through a steerable introducer an Agilis EPI (St Jude Medical, Minnetonka, MN) 9F ablation catheter was advanced. RV epicardial electroanatomic voltage mapping was then performed. Late, fragmented, double, and triple potentials were localized in the RV anterior region (Figure 2A). Flecainide acetate was infused intravenously over 10 minutes (dose of 100 mg) under ECG monitoring, and type 1 BrS was unmasked with a 3 mm elevation of ST segment in V1-V2 (Figure 1B). A new epicardial map was performed, and a wider area of target potentials was localized (a total area of approximately 42.56 cm2), with manifest double and triple potentials (Figure 2B). Radiofrequency (RF) ablation with 30 W was performed, with progressive slowing of potentials until disappearance (Figure 3), presenting transient ST ascendant elevation in V1-V2 (Figure 1C). After RF ablation, flecainide acetate was infused at the same dose and endocardial RV with 2 extrastimuli decremented stimulation for ventricular tachycardia (VT)/VF induction protocol was performed up to 500/310/270 msec, in which the patient presented VF requiring electric defibrillation. RV epicardial mapping was continued and substrate RF ablation was again performed. A new VT/VF induction protocol was performed up to 500/310/250 msec, and VF was induced, requiring electric defibrillation. Epicardial mapping was continued, including RVOT, where slow, fragmented, and double potentials were also observed, and RF was delivered. A new VT/VF induction protocol was performed with 1 and 2 extrastimuli until RV refractory effective period: 500/290/200 msec, with no VT/VF induction. Total radiofrequency duration required was 32 minutes, 30 seconds. Electrophysiological study was concluded. The following day, the patient presented with acute mild pericarditis with typical pericardial pain and ST-segment elevation in inferior leads, so ibuprofen and colchicine was prescribed, with good response. After 4 days the patient was discharged with no BrS ECG pattern (Figure 1D). Five months after the electrophysiological study, the patient remained asymptomatic, with no VT/VF episodes and no BrS ECG pattern persisting (Figure 1E).
Figure 1.
Twelve-lead electrocardiogram (ECG). A: At the beginning of electrophysiological study, BrS type 2 pattern. B: After flecainide acetate intravenous infusion. C: During radiofrequency onset. D: Final ECG at the end of electrophysiological study. E: Twelve-lead ECG 5 months after epicardial ablation.
Figure 2.
Anteroposterior view of right ventricle epicardial voltage map. Different late electrograms are shown before ablation, located predominantly in the anterior wall and outflow tract. A: Before flecainide acetate infusion. B: After flecainide acetate infusion.
Figure 3.
Anteroposterior view of the right ventricle epicardial voltage map after radiofrequency (RF) ablation. Electrograms displayed before and after RF delivery.
Conclusion
Epicardial ablation has been done on the basis of studies that demonstrate histologic changes and abnormal activation of RVOT.7 As in previous studies, this case supports the hypothesis that patients with BrS have a depolarization disorder8, 9 in a “current-to-load mismatch fashion,”10 in a relatively delimitated area of subepicardial origin5 in accordance with late activation in epicardial RVOT. This could be observed more clearly after flecainide was infused and double potentials and late depolarization were mapped in a wider area of the RV epicardial wall. But additionally, as described by Yan and Antzelevitch,11 we consider that it could also be a repolarization substrate disorder.12 According to this hypothesis, after flecainide was infused, to account for the negative T wave in coved-type ST elevation, an epicardial dispersion of repolarization was generated. This dispersion creates a vulnerable window, which allows phase 2 reentry to cause a premature impulse, which triggers VT/VF based on reentry between transmural layers. In our case, inducibility persisted until epicardium double and late potentials were eliminated, but also after BrS pattern and typical repolarization (T-wave alteration) disappeared. Further studies should be done with a longer follow-up, especially in BrS patients with higher risk.
References
- 1.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Antzelevitch C., Brugada P., Borggrefe M. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 3.Brugada B., Brugada J., Brugada R., Brugada P. Síndrome de Brugada. Rev Esp Cardiol. 2009;62(11):1297–1315. doi: 10.1016/s1885-5857(09)73357-2. [DOI] [PubMed] [Google Scholar]
- 4.Nademanee K., Veerakul G., Chandanamattha P., Chaothawee L., Ariyachaipanich A., Jirasirirojanakorn K., Likittanasombat K., Bhuripanyo K., Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 5.Sacher F., Jesel L., Jais P., Haissaguerre M. Insight into the mechanism of Brugada syndrome: epicardial substrate and modification during ajmaline testing. Heat Rhythm. 2014;11(4):732–734. doi: 10.1016/j.hrthm.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Boyle N., Shivkumar K. Epicardial interventions in electrophysiology. Circulation. 2012;126:1752–1769. doi: 10.1161/CIRCULATIONAHA.111.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronel R., Casini S., Koopmann T. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome. Circulation. 2005;112:2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 8.Brugada P., Brugada J., Roy D. Brugada syndrome 1992-2012: 20 years of scientific excitement, and more. Eur Heart J. 2013;34(47):3610–3615. doi: 10.1093/eurheartj/eht113. [DOI] [PubMed] [Google Scholar]
- 9.Hoogendijk M., Opthof T., Postema P., Wilde A., Bakker J., Coronel R. The Brugada ECG pattern. Circ Arrhythm Electrophysiol. 2010;3:283–290. doi: 10.1161/CIRCEP.110.937029. [DOI] [PubMed] [Google Scholar]
- 10.Nagase S., Fukushima K., Morita H., Fujimoto Y., Kakishita M., Nakamura K., Emori T., Matsubara H., Ohe T. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome. J Am Coll Cardiol. 2002;39(12):1992–1995. doi: 10.1016/s0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]
- 11.Yan G.X., Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu W., Aiba T., Kurita T., Kamakura S. Paradoxic abbreviation of repolarization in epicardium of the right ventricular outflow tract during augmentation of Brugada type ST segment elevation. J Cardiovasc Electrophysiol. 2001;12(12):1418–1422. doi: 10.1046/j.1540-8167.2001.01418.x. [DOI] [PubMed] [Google Scholar]