Introduction
KEY TEACHING POINTS
|
Ventricular tachycardia (VT) is common in patients with advanced heart failure, and medical therapy alone may have limited success. Implantable cardioverter defibrillators (ICDs) have been widely accepted as standard of care for VT management and prevention of sudden cardiac death. However, this therapy does not in itself reduce episodes of clinical VT. For patients who do not respond well to medical therapy, percutaneous catheter ablation has been shown to reduce episodes of recurrent VT.1 However, patients undergoing catheter ablation with a severely decreased left ventricular ejection fraction (LVEF) may experience hemodynamic instability during the ablation procedure, requiring external hemodynamic support. One type of external hemodynamic support is a temporary percutaneous ventricular assist device (PVAD), which has been used to maintain perfusion enabling the electrophysiologist to perform long and complex procedures under more stable conditions.2 More specifically, the TandemHeart (TH) PVAD (CardiacAssist, Pittsburgh, PA) has been shown to provide adequate circulatory support during high-risk cardiac procedures.3
In typical catheter ablation procedures, the catheter is manually guided by the physician. The Stereotaxis magnetic navigation (SMN; St. Louis, MO) system utilizes 2 magnetic platforms, one on each side of the patient, to remotely guide the catheter from a computer interface. This approach has been utilized by many centers for VT ablation.4 However, it is not well established whether the SMN technology will exhibit electromagnetic interference (EMI) when used simultaneously with a PVAD. In this case study, we describe the combined use of SMN and the TH PVAD on a patient with severe cardiomyopathy and VT undergoing catheter ablation, which to our knowledge had not been previously performed in the United States at that time.
Case report
A 69-year-old male exhibited symptoms of dizziness and lightheadedness before being shocked 7 times by his ICD and was admitted to an outside hospital. The patient had a known history of chronic heart failure from ischemic cardiomyopathy dating back to 1996, when he was diagnosed with coronary artery disease and underwent multivessel bypass grafting. Upon his admission to an outside hospital, it was confirmed that the device had shocked the patient appropriately for VT, and he was started on mexiletine and ranolazine in addition to previously prescribed sotalol. He underwent a left heart catheterization and selective coronary angiogram demonstrating severe deterioration of the LVEF to 12%, as well as severe occlusive coronary disease without targets for revascularization. He was transferred to our center, and upon evaluation, it was recommended that a cardiac ablation procedure be performed.
The SMN technique was determined to be the most effective method to assist in the ablation procedure. However, because of the patient’s low ejection fraction, there was significant concern about the patient’s hemodynamic stability in the setting of anesthesia and prolonged procedural time. Based on this concern, the decision was made to use the TH PVAD. At the time, it was unknown whether the TH PVAD flow would be interrupted or if the wire-wound inflow and outflow cannulas would be displaced by the strong magnetic field (0.1 T) of the SMN machinery. Prior to the procedure, a mock trial of the TH PVAD with the SMN magnets was completed in the catheterization laboratory to observe any abnormal function. The mock trial included placing the TH PVAD at distances of 3 ft (0.91 m) and 6 ft (1.83 m). At each distance, the speed was adjusted to 2000 and 5000 rpm. When the 2 distances were compared at the same speed, there was no significant change in flow for the device. The cannulas were also advanced near the SMN magnets to determine whether there would be significant interference. No interaction was observed, and it was determined that there were no predictable risks involved while using the PVAD and SMN simultaneously.
The patient was intubated and placed under general anesthesia. After the induction of anesthesia at 8:30 AM, the mean arterial pressure (MAP) rapidly dropped from 103 mm Hg (preanesthesia) to 50 mm Hg and the epinephrine dosage was increased from 0.05 µg/kg/min to 0.2 µg/kg/min to maintain reasonable perfusion. Percutaneous femoral transseptal access was performed under the guidance of fluoroscopy and intracardiac echocardiography. A double-wire technique was used to advance both an 8.5F Mullins transseptal sheath to be used for mapping and ablation and a PVAD transseptal uptake cannula (21F) into the left atrium. The TH PVAD return cannula was placed via percutaneous femoral access per the usual protocol. The PVAD was started at 9:45 AM, and it provided, on average, 2.0 L/min flow at a speed of 5000 rpm. At this point, the hemodynamics improved (MAP of 75 mm Hg), and a lower dosage of epinephrine was needed (decreased to 0.04µg/kg/min) throughout the remainder of the procedure.
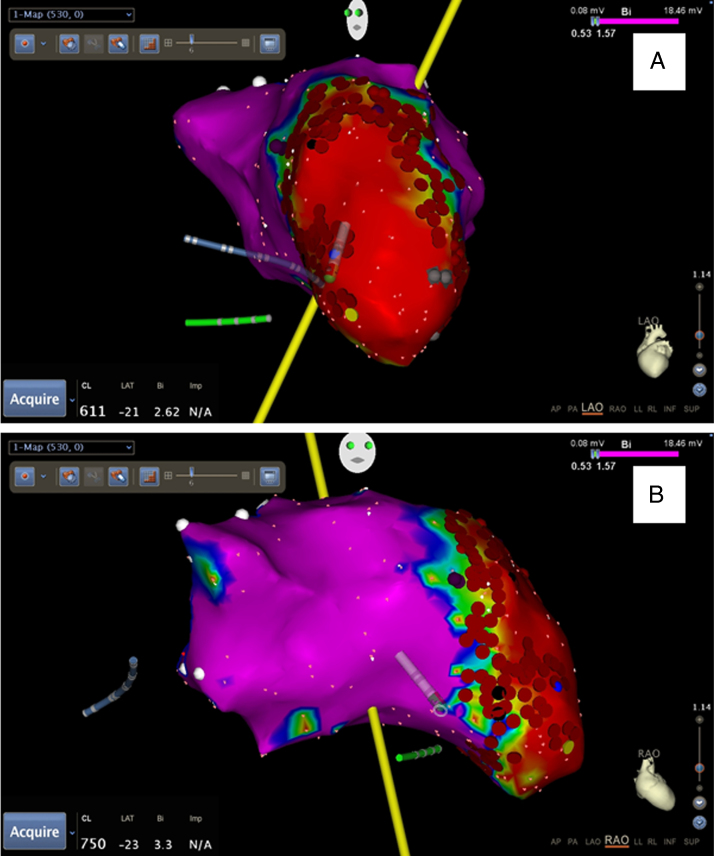
A stable MAP was maintained while using the PVAD, which allowed for detailed mapping and ablation using SMN. Substrate mapping of the left ventricle revealed an extremely large apical aneurysmal scar extending up the anterior wall and along the inferior wall and septum. More than 8 different VT morphologies were identified with brief induction, and extensive ablation was performed with an emphasis on substrate modification (Figure 1). At the end of the 7-hour procedure, it proved difficult to induce VT with triple extrastimuli. The patient was successfully weaned from the TH PVAD, which was removed in the electrophysiology laboratory before extubation. A total of 11.4 minutes of fluoroscopy was utilized for the entire procedure. The patient tolerated the procedure well, and there were no clinically evident peripheral vascular complications. Clinical follow-up has shown the patient to be free of ventricular arrhythmias for more than 18 months.
Figure 1.
Cardiac isochronal map of the left ventricle. Both the A: left anterior oblique (LAO) view and B: right anterior oblique (RAO) view of the patient’s heart demonstrate extensive ablation performed during the procedure
Discussion
Stable hemodynamic support for this critically ill patient was successfully provided using the TH PVAD while minimizing the need for pharmacologic support within the SMN environment. MAP values were maintained in the presence of extensive VT mapping and ablation, including several episodes of sustained VT. In our view, the presence of the TH PVAD facilitated the weaning of epinephrine and mitigated the significant risk of hemodynamic collapse in this critically ill patient.
A primary concern when using SMN in conjunction with other technologies is the potential for EMI. There are 2 different interactions that are generally a concern: the interaction between the PVAD on the electro anatomic mapping system and the interaction between the SMN system and the PVAD motor. The interaction between the PVAD and the mapping system has been observed by Vaidya et. al.,4 who looked at the EMI produced when magnet-based electroanatomic mapping was performed in close proximity to the PVAD Impella 2.5 (Abiomed, Danvers, MA). They found that severe EMI was observed using Carto3 (Biosense Webster, Diamond Bar, CA) for 9.4% of all points attempted at the maximum performance level (P8).5 The lack of EMI observed in our case study for both types of interactions is likely due to the location of the pump and drive mechanics. The TH PVAD motor is located externally, in this case near the patient’s leg, in contrast to the Impella motor, which is located internally near the left ventricular outflow tract. In order to confirm these findings, a multicenter investigation of PVAD-assisted cardiac ablations with multiple types of PVAD with SMN mapping should be considered. Further study would yield important results concerning the outcomes of this treatment option, verify the lack of EMI observed in this case study, and establish this approach as a reasonable option for this difficult clinical circumstance.
Footnotes
Source of Funding: There were not external sources of funding provided for the following research.
Conflicts of Interest: Dr Revenaugh receives royalties from Stereotaxis, Inc., for a joint patent. Dr Weiss has received payment from Stereotaxis, Inc., for consulting, lectures, and development of educational presentations. There are no other conflicts of interest to disclose.
References
- 1.Stevenson W.G., Wilber D.J., Natale A. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: The multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118:2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 2.Bunch Jared T., Mahapatra S., Madhu Reddy Y., Lakkireddy D. The role of percutaneous left ventricular assist devices during ventricular tachycardia ablation. Europace. 2012;14:ii26–ii32. doi: 10.1093/europace/eus210. [DOI] [PubMed] [Google Scholar]
- 3.Alli O.O., Singh I.M., Holmes D.R., Pulido J.N., Park S.J., Rihal C.S. Percutaneous left ventricular assist device with TandemHeart for high-risk percutaneous coronary intervention: The Mayo Clinic experience. Catheter Cardiovasc Interv. 2012;80:728–734. doi: 10.1002/ccd.23465. [DOI] [PubMed] [Google Scholar]
- 4.Bradfield Jason, Tung Roderick, Mandapati Ravi. Catheter Ablation Utilizing Remote Magnetic Navigation: A Review of Applications and Outcomes. Pacing Clin Electrophysiol. 2012;8:1021–1034. doi: 10.1111/j.1540-8159.2012.03382.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya V.R., Desimone C.V., Madhavan M., Noheria A., Shahid M., Walters J., Ladewig D.J., Mikell S.B., Johnson S.B., Suddendorf S.H., Asirvatham S.J. Compatibility of electroanatomical mapping systems with a concurrent percutaneous axial flow ventricular assist device: electromagnetic interference with ventricular assist device. J Cardiovasc Electrophysiol. 2014;25:781–786. doi: 10.1111/jce.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]