Summary
Concerns have been raised regarding an increased risk of major adverse cardiovascular events (MACEs) (myocardial infarction, cerebrovascular accident or cardiovascular death) in patients treated with anti‐interleukin (IL)‐12/23 agents for moderate‐to‐severe psoriasis. We aimed to examine the risk of MACEs in adult patients with plaque psoriasis that are exposed to biologic therapies via a meta‐analysis of randomized controlled trials (RCTs). Data were obtained from systematic searches in the Cochrane Library, MEDLINE and Embase, U.S. Food and Drug Administration, European Medicines Agency, individual pharmaceutical companies online search platforms and five trials registers (up to 31 March 2016). We selected RCTs reporting adverse events in adults with plaque psoriasis receiving at least one licensed dose of biologic therapy, conventional systematic therapy or placebo. We calculated Peto odds ratios (ORs) with 95% confidence intervals (CIs) and calculated I 2 statistics to assess heterogeneity. Overall, 38 RCTs involving 18 024 patients were included. No MACEs were observed in 29 studies, while nine RCTs reported 10 patients experiencing MACEs. There was no statistically significant difference in risk of MACEs associated with the use of biologic therapies overall (OR 1·45, 95% CI 0·34–6·24); tumour necrosis factor‐α inhibitors (adalimumab, etanercept and infliximab) (OR 0·67, 95% CI 0·10–4·63); anti‐IL‐17A agents (secukinumab and ixekizumab) (OR 1·00, 95% CI 0·09–11·09) or ustekinumab (OR 4·48, 95% CI 0·24–84·77). No heterogeneity was observed in these comparisons. In conclusion, the limited existing evidence suggests that licensed biologic therapies are not associated with MACEs during the short randomized controlled periods in clinical trials.
Short abstract
What's already known about this topic?
The association between biologic therapies and the risk of major adverse cardiovascular events (MACEs) among patients with plaque psoriasis is unclear.
What does this study add?
This is the largest meta‐analysis to examine the risk of MACEs and biologic therapies; it includes 38 randomized controlled trials (18 024 patients with psoriasis) and considers ixekizumab, which has only recently been licensed for use in patients with plaque psoriasis.
The results of this systematic review and meta‐analysis of randomized controlled trials show there was no significant difference in risk of MACEs in patients with plaque psoriasis who were exposed to biologic therapies.
Linked Comment: Piaserico. Br J Dermatol 2017; 176:849–850
Several observational studies have suggested that patients with severe psoriasis and psoriatic arthritis (PsA) have a higher risk of cardiovascular events such as myocardial infarction (MI), stroke and cardiovascular death.1, 2, 3, 4 It is debated whether this represents a causal association or a predisposition due to the underlying risk factors exhibited by patients with severe psoriasis,5, 6, 7 but there is a hypothesis postulating that the inflammatory cascade activated in patients with severe psoriasis may contribute to the development of atherosclerosis. Thus, medications for psoriasis such as biologic therapies, which have anti‐inflammatory effects, could theoretically improve atherosclerosis and therefore modulate the risk of development of cardiovascular disease.8, 9, 10, 11, 12
Biologic therapies for the treatment of moderate‐severe plaque psoriasis include tumour necrosis factor‐α inhibitors (TNFi), such as infliximab, etanercept and adalimumab; an inhibitor of the p40 subunit common to interleukin (IL)12 and IL23, ustekinumab; and inhibitors of IL‐17A, secukinumab and ixekizumab. It is currently unclear whether any of these therapies could alter the risk of development of cardiovascular disease. However, a number of major adverse cardiovascular events (MACEs) (MI, cerebrovascular accident or cardiovascular death) were observed in psoriasis patients receiving briakinumab, another IL‐12/23 inhibitor, in randomized controlled trials (RCTs), and this has raised concern regarding whether IL‐12/23 inhibitors could be associated with an increased risk of cardiovascular disease.13, 14 This directly led to the discontinuation of the development programme of briakinumab.15 Despite the approval and licensing of several biologic therapies for the treatment of psoriasis by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in the last decade, the cardiovascular safety profile of these medicines is not well established. The aim of this systematic review of RCTs was to examine whether or not there is any association between currently licensed biologic therapies and risk of MACEs in adult patients with plaque psoriasis.
Methods
A systematic review and meta‐analysis was conducted and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.16
Eligibility criteria
We included RCTs that reported adverse events (AEs) in adult patients with plaque psoriasis receiving at least one licensed dose of biologic therapy compared with conventional systematic therapy or placebo/no treatment during the randomized controlled phase. The doses of biologic therapies and conventional systemic therapies assigned had to be approved by the U.S. FDA, the EMA or any European country. The outcomes of interest were MACEs [MI, cerebrovascular accident (including ischaemic and haemorrhagic strokes) or cardiovascular death].
Data sources and search strategy
The Cochrane Library, MEDLINE and Embase were independently searched without language restrictions from their inception dates to 31 March 2016. The search term sets, which consisted of psoriasis, biologic therapies (individual drug names, trade names and drug classes) and study design, were tailored for each database. An example search strategy is provided in Appendix S1 (see Supporting Information). MEDLINE and Embase databases were searched using all search term sets while the Cochrane Library was searched using only search term sets covering psoriasis and biologic therapies. The Cochrane handbook for systematic reviews of interventions recommends that study design should not be used as a search term set to identify RCTs in the Cochrane Library (unlike MEDLINE or Embase).17 Both MeSH and free text terms were used to identify relevant trials. In addition, the U.S. FDA, EMA, five trial registries [the U.S. National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov); the EU Clinical Trials Register (www.clinicaltrialsregister.eu/); the World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/); the Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au); and the International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.isrctn.com)] and pharmaceutical company websites [AbbVie marketing Humira® (adalimumab), Pfizer marketing Enbrel® (etanercept), Janssen and Merck marketing Remicade® (infliximab), Janssen marketing Stelara® (ustekinumab), Eli Lilly and Company marketing Taltz® (ixekizumab), and Novartis Pharmaceutical Corporation marketing Cosentyx® (secukinumab)] were searched for additional details of clinical trials. Furthermore, we screened the reference lists of all included studies to determine whether they mentioned any other eligible trials.
Study process
All abstracts and full‐text articles were read by one investigator (W.R.) in order to screen for the relevant trials. Two investigators (W.R. and Z.Z.N.Y.) double extracted information from eligible RCTs. Three additional authors (D.M.A., C.E.M.G. and R.B.W.) provided advice on the included studies in case any decision was unclear.
Data extraction and quality assessment
Data relating to the relevant trial comparisons (biologic therapies, conventional systemic therapies, placebo or no treatment) were extracted that included information on study characteristics (number of study sites, blinding, length of the randomized controlled phase and rate of missing patient data (defined as percentage of patients withdrawing during the study period or excluded from the analysis)); patient characteristics [age, sex, history of PsA, weight, duration of psoriasis, Psoriasis Area and Severity Index (PASI) score, and percentage of body surface area (BSA) affected by psoriasis]; interventions (regimens of biologic therapies, conventional systemic therapies and placebo) and the numbers of participants receiving at least one dose of study drug/placebo/no treatment and separate AEs [MI, cerebrovascular accident (ischaemic and haemorrhagic strokes) and cardiovascular death] or MACEs in each intervention group. If the RCTs did not report the number of separate AEs or MACEs, they were recorded as zero events.
For extension RCTs in which treatment assignments were switched (for instance, patients who were initially treated with placebo switched to a biologic therapy), only MACEs before that point were documented. For multiple reports on the same RCT, all data were collated and aligned to a single RCT. If MACEs were reported at multiple follow‐up points, data from the longest randomized follow‐up were selected provided there was a continuation of the control arm. The overall number of MACEs during the randomized controlled phase in the treatment and control groups of the individual RCTs was extracted for patients who received at least one dose of study agent or placebo or did not receive any treatment.
The Cochrane quality assessment tool for RCTs18 was used for assessing risk of bias. Eight domains including sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data (defined as missing outcome data owing to patients dropping out during the study period or excluded from the analysis), selective outcome reporting, adjudication of MACEs and baseline imbalance were considered.
Data analysis
Extracted data were combined for the meta‐analysis using Review Manager (RevMan) 5·3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Peto odd ratios (ORs) were calculated as an effect measure to quantify the risk of MACEs in patients receiving biologic therapies compared with placebo/no treatment or the same biologic with different dosing. The Peto OR has been reported to perform better than other meta‐analytical methods for rare event rates (lower than 1%).19 There were six main comparisons, which included: (i) any biologic therapies (TNFi, anti‐IL‐17A agents and anti‐IL‐12/23 agent) vs. placebo/no treatment; (ii) TNFi vs. placebo; (iii) anti‐IL‐17A agents (secukinumab and ixekizumab) vs. placebo; (iv) anti‐IL‐12/23 agent (ustekinumab) vs. placebo; (v) ustekinumab 45 mg vs. 90 mg; and (vi) secukinumab 150 mg vs. 300 mg. In the first four comparisons, all licensed doses of biologic therapies were considered. A sensitivity analysis was also undertaken using the Mantel–Haenszel risk difference (RD) to explore whether analysis methods had influence on the results of the comparisons. This method (unlike the Peto OR) does not exclude RCTs without MACEs in both comparison groups.19 Heterogeneity between studies was assessed using the χ2‐test (P < 0·05 was considered statistically significant) and I 2 statistics (significant heterogeneity, I 2 > 50%; insignificant heterogeneity, I 2 < 40%). Funnel plot analysis was used for detection of potential publication bias.
Results
Study selection
In all, 38 RCTs (identified in 38 reports)20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 met the eligibility criteria and were included, as shown in Figure 1. These trials involved a total of 18 024 patients with plaque psoriasis. The 38 RCTs were conducted across a range of 1–231 (median 47) study sites. Thirty‐five RCTs (92·1%) were double‐blind studies. The length of the randomized controlled phase ranged from 10 to 30 (median 12) weeks. The included studies involved from 20 to 1303 patients with plaque psoriasis, with the percentage of male patients ranging from 53% to 90%, the percentage with PsA from 3% to 37%, mean age range 39·2–55·7 years, mean duration of psoriasis range 11·9–21·5 years, mean PASI range 11·5–30·3 (Table S1; see Supporting Information).
Figure 1.
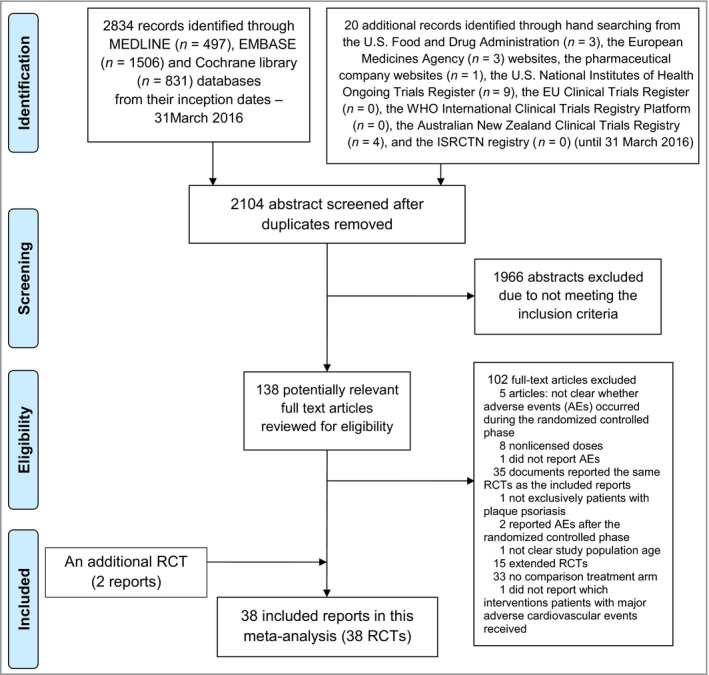
PRISMA flowchart of included randomized controlled trials (RCTs).
Eighteen RCTs compared TNFi (four adalimumab,20, 21, 22, 23, 24 nine etanercept,26, 27, 28, 29, 30, 31, 32, 34, 35 five infliximab36, 37, 38, 39, 40) with placebo, with three studies reporting MACEs; four RCTs compared ustekinumab (anti‐IL‐12/23) with placebo46, 47, 48 with no MACEs reported. One RCT compared ixekizumab with placebo without MACEs reported.56, 57 Six RCTs compared different dose regimens of ustekinumab (three RCTs)49, 50, 51 or secukinumab (anti‐IL‐17A, three RCTs)43, 44, 45 with placebo, with four MACEs reported from three RCTs. One RCT compared ustekinumab 45 mg and 90 mg with etanercept but no MACEs were observed.52 Secukinumab 150 mg was compared with 300 mg in one RCT and one patient experienced a MACE in the 300‐mg dose group.42 Etanercept (TNFi) was compared with ustekinumab,53 secukinumab,43 ixekizumab33 and placebo/no treatment in four RCTs but only two of them reported two MACEs. One RCT compared adalimumab (TNFi) with placebo and methotrexate (MTX),25 one RCT compared adalimumab with MTX54 and one RCT compared infliximab (TNFi) with MTX;41 no patients in these studies experienced a MACE (Table 1). The overall MACE rates were 0·06% (n = 8) for any biologic therapies (total patients 12 596), 0·05% (n = 3) for TNFi (total patients 6216), 0·09% (n = 3) for anti‐IL‐17A agents (secukinumab and ixekizumab) (total patients 3514), 0·07% (n = 2) for ustekinumab (total patients 2866), 0·04% (n = 2) for placebo (total patients 5092) and 0% (n = 0) for MTX (total patients 336). Seventeen RCTs reported the outcomes using an aggregate MACE definition (this included a study by Papp et al. 2008,49 which used the term ‘cardiovascular events’ instead of MACEs but its definition was the same as the definition of MACEs in our manuscript) while 21 RCTs reported AEs separately.
Table 1.
Rates of major adverse cardiovascular events (MACEs) in included randomized controlled trials
| First author | Interventions | Number of participants receiving treatment | MACEs | Randomized controlled phase (weeks) |
|---|---|---|---|---|
| Adalimumab vs. placebo | ||||
| Menter 2008 (REVEAL)20 | Adalimumab 80 mg SC at week 0 followed by 40 mg SC every other week starting at week 1 | 814 | 0 | 16 |
| Placebo at week 0 then every other week starting at week 1 | 398 | 0 | ||
| Maari 201421 | Adalimumab 80 mg followed by 40 mg at week 1 and then 40 mg every other week for 7 weeks | 10 | 0 | 12 |
| Placebo for 7 weeks | 10 | 0 | ||
| Gordon 2015 (X‐PLORE)22 | Adalimumab 80 mg SC at week 0 and then 40 mg every other week starting at week 1 | 43 | 0 | 16 |
| Placebo SC | 42 | 0 | ||
| AbbVie 2015, NCT01646073, clinicaltrials.gov23 | Adalimumab 80 mg SC at week followed by 40 mg SC every other week starting at week 124 | 338 | 1 | 12 |
| Placebo at week 0 and every other week starting at week 124 | 87 | 0 | ||
| Adalimumab vs. methotrexate | ||||
| Goldminz 201554 | Adalimumab 80 mg SC at week 0 followed by 40 mg SC every other week | 15 | 0 | 16 |
| Methotrexate 7·5–25 mg per week orally | 15 | 0 | ||
| Adalimumab vs. methotrexate vs. placebo | ||||
| Saurat 2008 (CHAMPION)25 | Adalimumab 80 mg SC at week 0 followed by 40 mg SC every other week starting at week 1 | 107 | 0 | 16 |
| Methotrexate 7·5–25 mg per week orally | 110 | 0 | ||
| Placebo | 53 | 0 | ||
| Etanercept vs. placebo | ||||
| Gottlieb 200326 | Etanercept 25 mg SC twice weekly | 57 | 0 | 24 |
| Placebo SC twice weekly | 55 | 1 | ||
| Tyring 200627 | Etanercept 50 mg SC twice weekly | 312 | 0 | 12 |
| Placebo SC twice weekly | 306 | 0 | ||
| van de Kerkhof 200828 | Etanercept 50 mg SC weekly | 96 | 0 | 12 |
| Placebo SC weekly | 46 | 0 | ||
| Gottlieb 201129 | Etanercept 50 mg SC twice weekly week 0–11 | 141 | 0 | 12 |
| Placebo SC matching active treatment | 68 | 0 | ||
| Strober 201130 | Etanercept 50 mg SC twice weekly week 0–11 | 139 | 0 | 12 |
| Placebo SC matching active treatment | 72 | 0 | ||
| Bagel 201231 | Etanercept 50 mg SC twice weekly | 59 | 0 | 12 |
| Placebo SC twice weekly | 62 | 0 | ||
| Bachelez 201532 | Etanercept 50 mg SC twice weekly | 335 | 1 | 12 |
| Placebo | 107 | 0 | ||
| Etanercept (different strengths) vs. placebo | ||||
| Leonardi 200334 | Etanercept 25 mg SC weekly | 160 | 0 | 12 |
| Etanercept 25 mg SC twice weekly | 162 | 0 | ||
| Etanercept 50 mg SC twice weekly | 164 | 0 | ||
| Placebo | 166 | 0 | ||
| Papp 200535 | Etanercept 25 mg SC twice weekly | 196 | 0 | 12 |
| Etanercept 50 mg SC twice weekly | 194 | 0 | ||
| Placebo SC twice weekly | 193 | 0 | ||
| Etanercept vs. ixekizumab vs. placebo | ||||
| Griffiths 2015 (UNCOVER‐2)33 | Etanercept 50 mg SC twice weekly | 357 | 1 | 12 |
| Ixekizumab 160 mg SC week 0 then 80 mg SC every 2 weeks | 350 | 0 | ||
| Placebo | 167 | 0 | ||
| Griffiths 2015 (UNCOVER‐3)33 | Etanercept 50 mg SC twice weekly | 382 | 0 | 12 |
| Ixekizumab 160 mg SC week 0 then 80 mg SC every 2 weeks | 384 | 0 | ||
| Placebo | 193 | 1 | ||
| Infliximab vs. placebo | ||||
| Chaudhari 200136 | Infliximab 5 mg ml−1 IV at week 0, 2 and 6 | 11 | 0 | 10 |
| Placebo IV at week 0, 2 and 6 | 11 | 0 | ||
| Gottlieb 2004 (SPIRIT)37 | Infliximab 5 mg kg−1 IV infusion at week 0, 2 and 6. At week 26, if patients had a static Physician's Global Assessment of moderate to severe disease, they received a single additional IV infusion of infliximab 5 mg kg−1 | 99 | 0 | 30 |
| Placebo IV infusion at week 0, 2 and 6. At week 26, if patients had a static Physician's Global Assessment of moderate to severe disease, they received a single additional IV infusion of placebo | 51 | 0 | ||
| Reich 2005 (EXPRESS)38 | Infliximab 5 mg kg−1 IV at week 0, 2 and 6 and every 8 weeks | 298 | 0 | 24 |
| Placebo at week 0, 2, 6, 14 and 22 | 76 | 0 | ||
| Menter 2007 (EXPRESS II)39 | Infliximab 5 mg kg−1 infusion at week 0, 2 and 6 | 314 | 0 | 14 |
| Placebo infusion at week 0, 2 and 6 | 207 | 0 | ||
| Yang 201240 | Infliximab 5 mg kg−1 IV drip infusion week 0, 2 and 6 | 84 | 0 | 10 |
| Placebo IV drip infusion week 0, 2 and 6 | 45 | 0 | ||
| Infliximab vs. methotrexate | ||||
| Barker 2011 (RESTORE1)41 | Infliximab 5 mg kg−1 at week 0, 2, 6, 14 and 22 | 649 | 0 | 16 |
| Methotrexate 15 mg weekly with a dose increase to 20 mg weekly at week 6 if Psoriasis Area and Severity Index response < 25% | 211 | 0 | ||
| Ixekizumab vs. placebo | ||||
| Gordon 2016 (UNCOVER‐1)57 | Ixekizumab 160 mg SC week 0 then 80 mg SC every 2 weeks | 433 | 0 | 12 |
| Placebo SC week 0 then every 2 weeks | 431 | 0 | ||
| Secukinumab 150 mg vs. secukinumab 300 mg | ||||
| Mrowietz 2015 (SCULPTURE)42 | Secukinumab 150 mg SC at week 0, 1, 2, 3, 4 and 8 | 482 | 0 | 12 |
| Secukinumab 300 mg SC at week 0, 1, 2, 3, 4 and 8 | 483 | 1 | ||
| Secukinumab 150 mg vs. secukinumab 300 mg vs. placebo | ||||
| Langley 2014 (ERASURE)43 | Secukinumab 150 mg SC at week 0, 1, 2, 3, 4 then every 4 weeks | 245 | 0 | 12 |
| Secukinumab 300 mg SC at week 0, 1, 2, 3, 4 then every 4 weeks | 245 | 0 | ||
| Placebo at week 0, 1, 2, 3, 4 then every 4 weeks | 247 | 0 | ||
| Blauvelt 2015 (FEATURE)44 | Secukinumab 150 mg SC week 0, 1, 2, 3, 4 and 8 | 59 | 0 | 12 |
| Secukinumab 300 mg SC week 0, 1, 2, 3, 4 and 8 | 59 | 2 | ||
| Placebo SC week 0, 1, 2, 3, 4 and 8 | 59 | 0 | ||
| Paul 2015 (JUNCTURE)45 | Secukinumab 150 mg SC week 0, 1, 2, 3, 4 and 8 | 61 | 0 | 12 |
| Secukinumab 300 mg SC week 0, 1, 2, 3, 4 and 8 | 60 | 0 | ||
| Placebo SC week 0, 1, 2, 3, 4 and 8 | 61 | 0 | ||
| Ustekinumab vs. placebo | ||||
| Tsai 2011 (PEARL)46 | Ustekinumab 45 mg SC at week 0 and 4 | 61 | 0 | 12 |
| Placebo SC at week 0 and 4 | 60 | 0 | ||
| Zhu 2013 (LOTUS)47 | Ustekinumab 45 mg SC at week 0 and 4 | 160 | 0 | 12 |
| Placebo SC at week 0 and 4 | 161 | 0 | ||
| Lebwohl 2015 (AMAGINE 2)48 | Ustekinumab SC (45 mg for patients with a body weight ≤ 100 kg and 90 mg for patients with a body weight > 100 kg) on day 1 and week 4 | 300 | 0 | 12 |
| Placebo | 309 | 0 | ||
| Lebwohl 2015 (AMAGINE 3)48 | Ustekinumab SC (45 mg for patients with a body weight ≤ 100 kg and 90 mg for patients with a body weight > 100 kg) on day 1 and week 4 | 313 | 0 | 12 |
| Placebo | 315 | 0 | ||
| Ustekinumab 45 mg vs. ustekinumab 90 mg vs. placebo | ||||
| Leonardi 2008 (PHOENIX 1)51 | Ustekinumab 45 mg SC at week 0 and 4 | 255 | 1 | 12 |
| Ustekinumab 90 mg SC at week 0 and 4 | 255 | 0 | ||
| Placebo at week 0 and 4 | 255 | 0 | ||
| Papp 2008 (PHOENIX 2)49 | Ustekinumab 45 mg SC at week 0 and 4 | 409 | 0 | 12 |
| Ustekinumab 90 mg SC at week 0 and 4 | 411 | 1 | ||
| Placebo | 410 | 0 | ||
| Igarashi 201250 | Ustekinumab 45 mg SC at week 0 and 4 | 64 | 0 | 12 |
| Ustekinumab 90 mg SC at week 0 and 4 | 62 | 0 | ||
| Placebo SC at week 0 and 4 | 32 | 0 | ||
| Etanercept vs. ustekinumab 45 mg vs. ustekinumab 90 mg | ||||
| Griffiths 2010 (ACCEPT)52 | Etanercept 50 mg SC twice weekly | 347 | 0 | 12 |
| Ustekinumab 45 mg SC at week 0 and 4 | 209 | 0 | ||
| Ustekinumab 90 mg SC at week 0 and 4 | 347 | 0 | ||
| Etanercept vs. ustekinumab vs. no treatment | ||||
| Merck Sharp & Dohme 2015, NCT01276847, clinicaltrials.gov53 | Etanercept 50 mg SC twice weekly for 12 weeks then SC weekly for 4 weeks | 10 | 0 | 16 |
| Ustekinumab 45 mg SC for participants weighing ≤ 100 kg, and ustekinumab 90 mg SC for participants weighing > 100 kg on day 1, and weeks 4 and 16 | 20 | 0 | ||
| No treatment | 10 | 0 | ||
| Etanercept vs. secukinumab 150 mg vs. seckinumab 300 mg vs. placebo | ||||
| Langley 2014 (FIXTURE)43 | Etanercept 50 mg SC twice weekly | 323 | 0 | 12 |
| Secukinumab 150 mg SC weekly week 0, 1, 2, 3, 4 then every 4 weeks | 327 | 0 | ||
| Secukinumab 300 mg SC weekly week 0, 1, 2, 3, 4 then every 4 weeks | 326 | 0 | ||
| Placebo at weeks corresponding to etanercept and secukinumab regimens | 327 | 0 | ||
IV, intravenous; SC, subcutaneous.
Meta‐analysis
Patients in 27 RCTs20, 21, 22, 25, 27, 28, 29, 30, 31, 34, 35, 36, 37, 38, 39, 40, 43, 45, 46, 47, 48, 50, 52, 53, 57 did not experience MACEs while exposed to any interventions but 10 MACEs were observed during the randomized controlled phase of nine studies.23, 26, 32, 33, 42, 44, 49, 51 Overall, the pooled analysis of these nine trials found that there was no statistically significant difference in the risk of MACEs when comparing biologic therapies with placebo (pooled OR 1·45, 95% CI 0·34–6·24, P = 0·62), as shown in Figure 2a. We found very low levels of heterogeneity between the included RCTs (χ2 = 7·58; degrees of freedom = 7; P = 0·37; I 2 = 8%).
Figure 2.
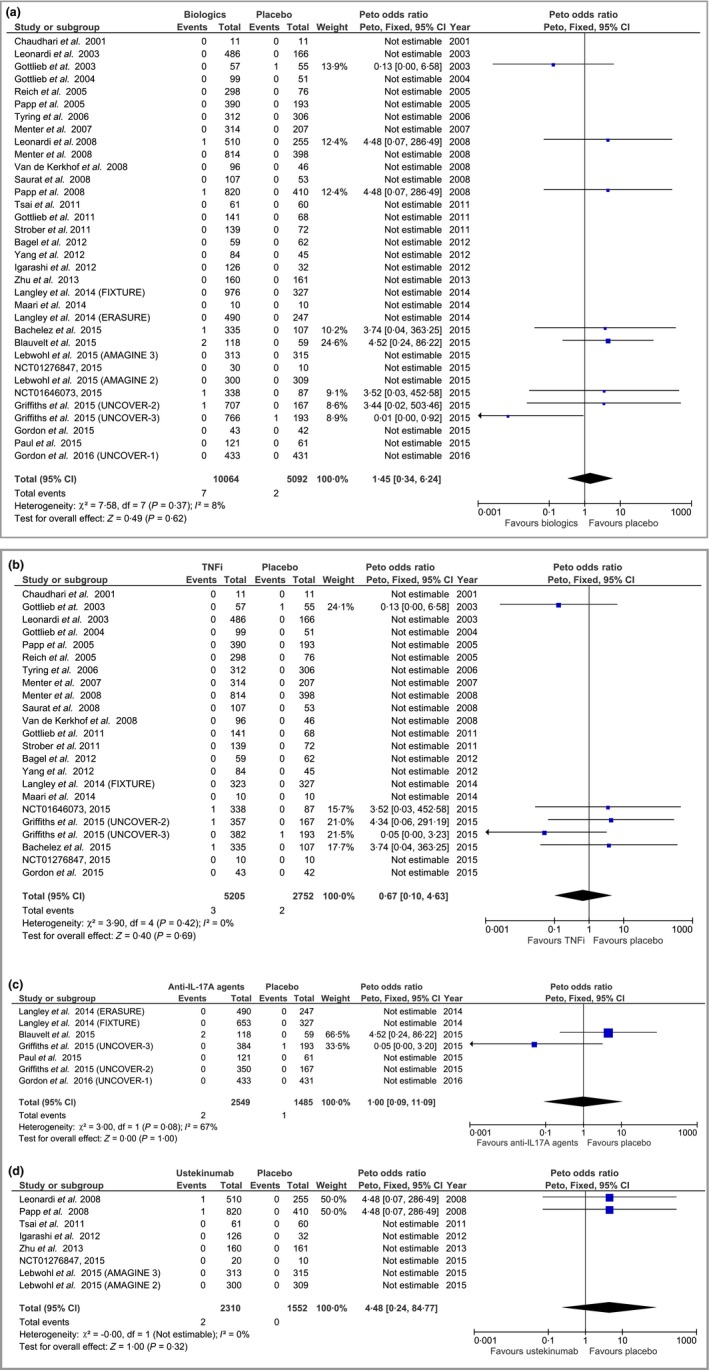
Peto odds ratio (OR) of major adverse cardiovascular events in patients treated with (a) biologic therapies vs. placebo; (b) tumour necrosis factor‐α inhibitors (TNFi) vs. placebo, (c) anti‐interleukin‐(IL)‐17A agents vs. placebo; and (d) ustekinumab vs. placebo. CI, confidence interval; df, degrees of freedom.
Considered separately, there was also no statistically significant difference for patients receiving TNFi (adalimumab, etanercept and infliximab) anti‐IL‐17A agents (secukinumab and ixekizumab), or ustekinumab. The corresponding pooled ORs were 0·67, 95% CI 0·10–4·63, P = 0·69 for TNFi (Fig. 2b); 1·00, 95% CI 0·09–11·09, P = 1·00 for anti‐IL‐17A agents (Fig. 2c); and 4·48, 95% CI 0·24–84·77, P = 0·32 for ustekinumab (Fig. 2d). Comparing ustekinumab 45 mg against 90 mg and secukinumab 150 mg against 300 mg, the ORs suggest there were no statistically significant differences in the risk of MACEs (OR 1·00, 95% CI 0·06–16·03, P = 1·00 in four ustekinumab trials and OR 0·13, 95% CI 0·01–1·30, P = 0·08 in five secukinumab trials). The sensitivity analyses using the Mantel–Haenszel risk difference found similar results for all comparisons (Fig. S1; see Supporting Information).
Risk of bias assessment
Our risk of bias assessment found that 28 RCTs (74%; low risk of bias) adequately reported generation of the random sequence, 27 RCTs (71%) adequately concealed allocation; 22 RCTs (58%) and 21 RCTs (55%) blinded patients and personnel, and outcome assessors, respectively. Incomplete outcome data were well balanced in 33 RCTs (87%). Fifteen RCTs (40%) explicitly stated that cardiovascular events were monitored and/or these outcomes were reported. Only 10 RCTs (26%) had a committee for adjudicating suspected MACEs. Among 36 RCTs (95%), patient characteristics in all intervention groups were well balanced (Table S2; see Supporting Information).
Funnel plot analysis using the Peto method was used for assessing potential publication bias; visual inspection of the funnel plot for the outcomes in TNFi studies showed no evidence of publication bias. For the Mantel–Haenszel fixed‐effect method, funnel plot analysis also showed no evidence of publication bias in all comparisons.
Discussion
In this meta‐analysis of RCTs, we found that there was no statistically significant difference in the risk of MACEs in patients with plaque psoriasis exposed to biologic therapies (adalimumab, etanercept, infliximab, ustekinumab, secukinumab and ixekizumab) used at the licensed doses compared with placebo. Moreover, no difference in risk was also found for comparisons between different licensed doses of ustekinumab (45 mg vs. 90 mg) or secukinumab (150 mg vs. 300 mg).
Two earlier meta‐analyses of RCTs have examined the risk of MACEs and biologic therapies for the treatment of psoriasis. The first included 22 trials and reported that TNFi (adalimumab, etanercept and infliximab) and anti‐IL‐12/23 agents (ustekinumab and briakinumab) were not associated with an increased risk of MACEs.58 This meta‐analysis used a Mantel–Haenszel fixed effect model to examine absolute risk difference, which is generally considered a less appropriate method for detecting rare events (lower than 1%).19 The second meta‐analysis included nine trials to examine the association between MACEs and anti‐IL‐12/23 agents (ustekinumab and briakinumab).59 The results of this analysis suggested that anti‐IL‐12/23 agents were significantly associated with an increased risk of MACEs. In our meta‐analysis, we did not include briakinumab as this has not been licensed for use by the regulatory agencies. However, we did include newer licensed biologic therapies (secukinumab and ixekizumab) in our analysis. One important limitation of the earlier meta‐analyses is that they included patients treated with both nonlicensed and licensed doses of biologic therapies, while our meta‐analysis has focused only on those patients receiving biologic therapies at licensed dose regimens.
Nonetheless, we were faced with several important limitations that should be considered when interpreting the findings of our meta‐analysis. Firstly, the primary aim of all the included trials was to examine efficacy and only 10 trials explicitly provided a definition of MACEs and established a committee for adjudicating suspected cases. Most of the included trials had a relatively small sample size and a short duration of the randomized controlled phase of treatment (ranging from 10 to 30 weeks). These factors would impact on the power of the included studies to detect a change in risk of MACEs and this uncertainty was reflected by the wide CIs surrounding some of our risk estimates. For instance, ustekinumab has been suggested to increase the risk of MACEs during the initial stage of therapy because of temporary increases in inflammatory mediators.60 A phase 2 study showed that serum levels of IL‐12/23 p40, which is proatherogenic, in patients receiving ustekinumab dramatically increased at week 12 and decreased to a little above baseline levels by week 32.61 Thus, assessment of the potential association requires continued surveillance of emerging trial data. In cardiovascular research, it is also well established to use composite outcomes including MACE to detect rare events; this will increase the power to detect clinically important differences in event rates.62 Ideally, the recent calls to facilitate the sharing of clinical trial data will also provide new opportunities to examine individual patient‐level data from RCTs thereby enabling more robust time‐to‐event meta‐analysis to be performed.63
The majority of the included studies were phase 3 trials, which tend to enrol patients with fewer comorbidities than those seen in routine clinical practice and also exclude elderly patients, who are at increased risk of MACEs. Thus, the background risk for both the exposed and nonexposed groups is likely to be lower, which may limit the generalizability of the findings.
Two cohort studies have recently reported that biologic therapies were not associated with the risk of MACEs in patients with psoriasis.64, 65 The first study, using a Danish nationwide cohort, analysed the association with biologic therapies; the results suggested that TNFi were associated with a significantly decreased risk of MACEs but there was no difference in risk associated with ustekinumab. This study did not examine the TNFi separately, which is likely to be due to the relatively small numbers of patients receiving TNFi (n = 959) and ustekinumab (n = 178). In addition, the study did not adjust for important confounders such as body mass index or smoking status in the analysis. The second study recruited 12 095 participants with psoriasis receiving biologic therapies and nonbiologic systemic therapies (such as MTX, or ciclosporin). In this study, 96·5%, 85·6% and 85·8% of patients in the infliximab, ustekinumab, and other biologic therapies groups, respectively, received at least one biologic therapy before study entry. Thus, the MACEs that occurred during the study period might not be solely related to their treatment group and a ‘new user’ study design would have been preferable to avoid this risk of bias.66 In addition, a number of important cardiovascular risk factors such as diabetes mellitus, hypertension and dyslipidaemia were not adjusted for when assessing the association between MACEs and biologic therapies.
An earlier retrospective cohort study involving 8845 patients with psoriasis examined the association between TNFi (etanercept, infliximab or adalimumab) and risk of MI.67 The study reported that TNFi were associated with a significantly reduced risk of MI when compared with topical therapies but this association was not found when comparisons were made with oral systemic agents (ciclosporin, acitretin and MTX) or phototherapy. Comparing TNFi with topical therapies would not be an appropriate comparison because patients treated with topical therapies are at a much earlier stage in the treatment pathway, and those patients with psoriasis who are most likely to experience MI (including fatal events) may do so before being exposed to biologic therapies, which could bias results.68
In conclusion, the existing evidence suggests that biologic therapies including TNFi, an anti‐IL‐12/23 agent (ustekiumab) and anti‐IL‐17A agents (secukinumab and ixekizumab) had no significant impact on the risk of MACEs in adult patients with plaque psoriasis over the short term. Moreover, nor did the different licensed dosages of ustekinumab and secukinumab impact on the risk of MACEs. However, these results should be interpreted with caution given the short duration of follow‐up and the characteristics of patients participating in RCTs. Well‐designed observational studies that involve larger numbers of patients and longer durations of treatment exposure reflecting routine clinical practice are required in order to better examine the impact of biologic therapies on the risk of MACEs in patients with psoriasis.
Supporting information
Appendix S1. Search strategy.
Table S1. Characteristics of included randomized controlled trials.
Table S2. Risk of bias assessment for randomized controlled trials.
Fig S1. Mantel–Haenszel risk difference of major adverse cardiovascular events between therapies.
Video S1. Author video.
Funding sources
W.R. is funded by the Royal Thai Government to undertake her PhD at the University of Manchester. This study forms part of the PhD programme. The funding organization played no part in the interpretation of the findings or decision to publish the work. Z.Z.N.Y. is funded by a National Institute for Health Research (NIHR) doctoral research fellowship (ref. no. DRF‐2015‐08‐089). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health. C.E.M.G. is an NIHR senior investigator. C.E.M.G. and R.B.W. are funded in part by the Medical Research Council (MR/L011808/1).
Conflicts of interest
R.B.W. has acted as a consultant and/or speaker and/or received research grants for AbbVie, Amgen, Celgene, Eli Lilly, Pfizer, Novartis and Janssen, all of whom manufacture biologic therapies. C.E.M.G. has received honoraria and/or research grants from AbbVie, Actelion, Amgen, Celgene, LEO Pharma, Eli Lilly, GSK‐Stiefel, Janssen, MSD, Novartis, Pfizer, Sandoz and UCB Pharma. D.M.A. has received grant funding from AbbVie and served on advisory boards for Pfizer and GSK.
References
- 1. Gelfand JM, Neimann AL, Shin DB et al Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296:1735–41. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong AW, Harskamp CT, Ledo L et al Coronary artery disease in patients with psoriasis referred for coronary angiography. Am J Cardiol 2012; 109:976–80. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong AW, Lin SW, Chambers CJ et al Psoriasis and hypertension severity: Results from a case‐control study. PLOS One 2011; 6:e18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samarasekera EJ, Neilson JM, Warren RB et al Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta‐analysis. J Invest Dermatol 2013; 133:2340–6. [DOI] [PubMed] [Google Scholar]
- 5. Parisi R, Rutter MK, Lunt M et al Psoriasis and the risk of major cardiovascular events: cohort study using the Clinical Practice Research Datalink. J Invest Dermatol 2015; 135:2189–97. [DOI] [PubMed] [Google Scholar]
- 6. Stern RS, Huibregtse A. Very severe psoriasis is associated with increased noncardiovascular mortality but not with increased cardiovascular risk. J Invest Dermatol 2011; 131:1159–66. [DOI] [PubMed] [Google Scholar]
- 7. Wakkee M, Herings RM, Nijsten T. Psoriasis may not be an independent risk factor for acute ischemic heart disease hospitalizations: results of a large population‐based Dutch cohort. J Invest Dermatol 2010; 130:962–7. [DOI] [PubMed] [Google Scholar]
- 8. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–95. [DOI] [PubMed] [Google Scholar]
- 9. Serruys PW, García‐García HM, Buszman P et al Effects of the direct lipoprotein‐associated phospholipase A2 inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008; 118:1172–82. [DOI] [PubMed] [Google Scholar]
- 10. Libby P, Ridker P, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009; 54:2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost 2009; 7 (Suppl. 1):332–9. [DOI] [PubMed] [Google Scholar]
- 12. Menter A, Griffiths CEM. Current and future management of psoriasis. Lancet 2007; 370:272–84. [DOI] [PubMed] [Google Scholar]
- 13. Gordon KB, Langley RG, Gottlieb AB et al A phase III, randomized, controlled trial of the fully human IL‐12/23 mAb briakinumab in moderate‐to‐severe psoriasis. J Invest Dermatol 2012; 132:304–14. [DOI] [PubMed] [Google Scholar]
- 14. Krueger GG, Langley RG, Leonardi C et al A human interleukin‐12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007; 356:580–92. [DOI] [PubMed] [Google Scholar]
- 15. Petrou I. Systemic medications for psoriasis therapy must be prescribed with caution. Available at: http://dermatologytimes.modernmedicine.com/dermatology-times/news/clinical/clinical-pharmacology/systemic-medications-psoriasis-therapy-must-be (last accessed 17 January 2017).
- 16. Moher D, Liberati A, Tetzlaff J et al Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annu Intern Med 2009; 151:264–9. [DOI] [PubMed] [Google Scholar]
- 17. Lefebvre C, Manheimer E, Glanville J. Searching for studies In: Cochrane Handbook for Systematic Reviews of Interventions. (Higgins JPT, Green S, eds). Chichester: John Wiley & Sons Ltd, 2008; 95–150. [Google Scholar]
- 18. Higgins JP, Altman DG. Assessing risk of bias in included studies In: Cochrane Handbook for Systematic Reviews of Interventions (Higgins JPT, Green S, eds). Chichester: John Wiley & Sons Ltd, 2008; 187–242. [Google Scholar]
- 19. Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Stat Med 2007; 26:53–77. [DOI] [PubMed] [Google Scholar]
- 20. Menter A, Tyring SK, Gordon K et al Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58:106–15. [DOI] [PubMed] [Google Scholar]
- 21. Maari C, Bolduc C, Nigen S et al Effect of adalimumab on sleep parameters in patients with psoriasis and obstructive sleep apnea: a randomized controlled trial. J Dermatolog Treat 2014; 25:57–60. [DOI] [PubMed] [Google Scholar]
- 22. Gordon KB, Duffin KC, Bissonnette R et al A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015; 373:136–44. [DOI] [PubMed] [Google Scholar]
- 23. AbbVie . Safety and efficacy study of adalimumab in the treatment of plaque psoriasis. Available at: https://clinicaltrials.gov/show/NCT01646073 (last accessed 17 January 2017).
- 24. AbbVie . Adalimumab M13‐606 clinical study report R&D/13/997. Available at: https://www.abbvie.com/wp-content/uploads/2016/10/adalimumab-M13-606.pdf (last accessed 17 January 2017).
- 25. Saurat JH, Stingl G, Dubertret L et al Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008; 158:558–66. [DOI] [PubMed] [Google Scholar]
- 26. Gottlieb AB, Matheson RT, Lowe N et al A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003; 139:1627–32. [DOI] [PubMed] [Google Scholar]
- 27. Tyring S, Gottlieb A, Papp K et al Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double‐blind placebo‐controlled randomised phase III trial. Lancet 2006; 367:29–35. [DOI] [PubMed] [Google Scholar]
- 28. van de Kerkhof PCM, Segaert S, Lahfa M et al Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate‐to‐severe plaque psoriasis: a randomized controlled trial with open‐label extension. Br J Dermatol 2008; 159:1177–85. [DOI] [PubMed] [Google Scholar]
- 29. Gottlieb A, Leonardi C, Kerdel F et al Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 2011; 165:652–60. [DOI] [PubMed] [Google Scholar]
- 30. Strober BE, Crowley JJ, Yamauchi PS et al Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 2011; 165:661–8. [DOI] [PubMed] [Google Scholar]
- 31. Bagel J, Lynde C, Tyring S et al Moderate to severe plaque psoriasis with scalp involvement: a randomized, double‐blind, placebo‐controlled study of etanercept. J Am Acad Dermatol 2012; 67:86–92. [DOI] [PubMed] [Google Scholar]
- 32. Bachelez H, van de Kerkhof PCM, Strohal R et al Tofacitinib versus etanercept or placebo in moderate‐to‐severe chronic plaque psoriasis: A phase 3 randomised non‐inferiority trial. Lancet 2015; 386:552–61. [DOI] [PubMed] [Google Scholar]
- 33. Griffiths CEM, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386:541–51. [DOI] [PubMed] [Google Scholar]
- 34. Leonardi CL, Powers JL, Matheson RT et al Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349:2014–22. [DOI] [PubMed] [Google Scholar]
- 35. Papp KA, Tyring S, Lahfa M et al A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005; 152:1304–12. [DOI] [PubMed] [Google Scholar]
- 36. Chaudhari U, Romano P, Mulcahy LD et al Efficacy and safety of infliximab monotherapy for plaque‐type psoriasis: a randomised trial. Lancet 2001; 357:1842–7. [DOI] [PubMed] [Google Scholar]
- 37. Gottlieb AB, Evans R, Li S et al Infliximab induction therapy for patients with severe plaque‐type psoriasis: a randomized, double‐blind, placebo‐controlled trial. J Am Acad Dermatol 2004; 51:534–42. [DOI] [PubMed] [Google Scholar]
- 38. Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366:1367–74. [DOI] [PubMed] [Google Scholar]
- 39. Menter A, Feldman SR, Weinstein GD et al A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate‐to‐severe plaque psoriasis. J Am Acad Dermatol 2007; 56:31.e1–15. [DOI] [PubMed] [Google Scholar]
- 40. Yang HZ, Wang K, Jin HZ et al Infliximab monotherapy for Chinese patients with moderate to severe plaque psoriasis: a randomized, double‐blind, placebo‐controlled multicenter trial. Chin Med J (Engl) 2012; 125:1845–51. [PubMed] [Google Scholar]
- 41. Barker J, Hoffmann M, Wozel G et al Efficacy and safety of infliximab vs. methotrexate in patients with moderate‐to‐severe plaque psoriasis: results of an open‐label, active‐controlled, randomized trial (RESTORE1). Br J Dermatol 2011; 165:1109–17. [DOI] [PubMed] [Google Scholar]
- 42. Mrowietz U, Leonardi CL, Girolomoni G et al Secukinumab retreatment‐as‐needed versus fixed‐interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double‐blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol 2015; 73:27–36. [DOI] [PubMed] [Google Scholar]
- 43. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med 2014; 371:326–38. [DOI] [PubMed] [Google Scholar]
- 44. Blauvelt A, Prinz JC, Gottlieb AB et al Secukinumab administration by pre‐filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol 2015; 172:484–93. [DOI] [PubMed] [Google Scholar]
- 45. Paul C, Lacour J‐P, Tedremets L et al Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol 2015; 29:1082–90. [DOI] [PubMed] [Google Scholar]
- 46. Tsai T‐F, Ho J‐C, Song M et al Efficacy and safety of ustekinumab for the treatment of moderate‐to‐severe psoriasis: a phase III, randomized, placebo‐controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci 2011; 63:154–63. [DOI] [PubMed] [Google Scholar]
- 47. Zhu X, Zheng M, Song M et al Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque‐type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol 2013; 12:166–74. [PubMed] [Google Scholar]
- 48. Lebwohl M, Strober B, Menter A et al Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015; 373:1318–28. [DOI] [PubMed] [Google Scholar]
- 49. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371:1675–84. [DOI] [PubMed] [Google Scholar]
- 50. Igarashi A, Kato T, Kato M et al Efficacy and safety of ustekinumab in Japanese patients with moderate‐to‐severe plaque‐type psoriasis: long‐term results from a phase 2/3 clinical trial. J Dermatol 2012; 39:242–52. [DOI] [PubMed] [Google Scholar]
- 51. Leonardi CL, Kimball AB, Papp KA et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371:1665–74. [DOI] [PubMed] [Google Scholar]
- 52. Griffiths CEM, Strober BE, van de Kerkhof P et al Comparison of ustekinumab and etanercept for moderate‐to‐severe psoriasis. N Engl J Med 2010; 362:118–28. [DOI] [PubMed] [Google Scholar]
- 53. Merck Sharp & Dohme Corp . A study to assess the effect of ustekinumab (Stelara®) and etanercept (Enbrel®) in participants with moderate to severe psoriasis. Available at: https://clinicaltrials.gov/ct2/show/NCT01276847 (last accessed 17 January 2017).
- 54. Goldminz AM, Suárez‐Fariñas M, Wang AC et al CCL20 and IL22 Messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol 2015; 151:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Janssen Pharmaceutical K.K. An efficacy and safety study of ustekinumab (CNTO 1275) in participants with plaque psoriasis. Available at: https://clinicaltrials.gov/ct2/show/results/NCT00723528 (last accessed 17 January 2017).
- 56. Leonardi C, Blauvelt A, Langley RG et al Maintenance of efficacy among patients who achieve sPGA (0, 1): 60‐week results from UNCOVER‐1, a phase 3 trial of ixekizumab for moderate‐to‐severe plaque psoriasis. Presented at the 74th American Academy of Dermatology Annual Meeting, Washington, D.C., U.S.A., 4–8 March 2016.
- 57. Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med 2016; 375:345–56. [DOI] [PubMed] [Google Scholar]
- 58. Ryan C, Leonardi CL, Krueger JG et al Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta‐analysis of randomized controlled trials. JAMA 2011; 306:864–71. [DOI] [PubMed] [Google Scholar]
- 59. Tzellos T, Kyrgidis A, Zouboulis CC. Re‐evaluation of the risk for major adverse cardiovascular events in patients treated with anti‐IL‐12/23 biological agents for chronic plaque psoriasis: a meta‐analysis of randomized controlled trials. J Eur Acad Dermatol Venereol 2013; 27:622–7. [DOI] [PubMed] [Google Scholar]
- 60. Callen JP. Are major adverse cardiovascular events associated with anti–IL‐12/23 therapies? Available at: http://www.jwatch.org/jd201109160000001/2011/09/16/are-major-adverse-cardiovascular-events (last accessed 17 January 2017).
- 61. Reddy M, Torres G, McCormick T et al Positive treatment effects of ustekinumab in psoriasis: analysis of lesional and systemic parameters. J Dermatol 2010; 37:413–25. [DOI] [PubMed] [Google Scholar]
- 62. Rauch G, Rauch B, Schüler S, Kieser M. Opportunities and challenges of clinical trials in cardiology using composite primary endpoints. World J Cardiol 2015; 7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taichman DB, Backus J, Baethge C et al Sharing clinical trial data – a proposal from the International Committee of Medical Journal Editors. N Engl J Med 2016; 374:384–6. [DOI] [PubMed] [Google Scholar]
- 64. Ahlehoff O, Skov L, Gislason G et al Cardiovascular outcomes and systemic anti‐inflammatory drugs in patients with severe psoriasis: 5‐year follow‐up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol 2015; 29:1128–34. [DOI] [PubMed] [Google Scholar]
- 65. Gottlieb AB, Kalb RE, Langley RG et al Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol 2014; 13:1441–8. [PubMed] [Google Scholar]
- 66. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol 2003; 158:915–20. [DOI] [PubMed] [Google Scholar]
- 67. Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol 2012; 148:1244–50. [DOI] [PubMed] [Google Scholar]
- 68. Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non‐experimental pharmacoepidemiologic research. J Clin Epidemiol 1994; 47:731–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.
Table S1. Characteristics of included randomized controlled trials.
Table S2. Risk of bias assessment for randomized controlled trials.
Fig S1. Mantel–Haenszel risk difference of major adverse cardiovascular events between therapies.
Video S1. Author video.


