Abstract
This study evaluated the efficacy and safety of 26 weeks of twice‐daily (BID) alogliptin + metformin fixed‐dose combination (FDC) therapy in Asian patients with type 2 diabetes. Patients aged 18 to 75 years with hemoglobin A1c (HbA1c) of 7.5% to 10.0% after ≥2 months of diet and exercise and a 4‐week placebo run‐in were enrolled. Eligible patients were randomized (1:1:1:1) to placebo, alogliptin 12.5 mg BID, metformin 500 mg BID or alogliptin 12.5 mg plus metformin 500 mg FDC BID. The primary endpoint was change in HbA1c from baseline to end of treatment (Week 26). In total, 647 patients were randomized. The least‐squares mean change in HbA1c from baseline to Week 26 was −0.19% with placebo, −0.86% with alogliptin, −1.04% with metformin and −1.53% with alogliptin + metformin FDC. Alogliptin + metformin FDC was significantly more effective (P < .0001) in lowering HbA1c than either alogliptin or metformin alone. The safety profile of alogliptin + metformin FDC was similar to that of the individual components alogliptin and metformin. The study demonstrated that treatment with alogliptin + metformin FDC BID resulted in better glycaemic control than either monotherapy and was well tolerated in Asian patients with type 2 diabetes.
Keywords: alogliptin, DPP‐IV inhibitor, glycaemic control, metformin, type 2 diabetes
1. INTRODUCTION
The prevalence of diabetes mellitus and suboptimal achievement of glycaemic control is increasing in Asian populations.1, 2, 3 Evidence shows that there may be differences in the pathophysiology of type 2 diabetes across ethnic subgroups, with Asian vs non‐Asian patients exhibiting a propensity for lower body mass index, a higher proportion of visceral fat and predominant insulin secretory defects.4, 5 Although pharmacologic agents are available to patients with type 2 diabetes who fail to achieve sufficient glycaemic control through diet and exercise, many of these agents may cause hypoglycaemia or weight gain.6, 7 These adverse events (AEs) often negatively affect treatment adherence and/or long‐term use. Therefore, there is a need for effective, long‐term glycaemic control regimens that can be administered safely.
Alogliptin is a potent, highly selective, orally available dipeptidyl peptidase‐4 (DPP‐4) inhibitor. Since 2013, alogliptin has been available in the USA and the European Union as monotherapy and as a fixed‐dose combination (FDC) with metformin. The FDC formulation was developed to improve convenience and, ultimately, patient adherence. Mechanistically, inhibition of DPP‐4 activity leads to an increase in hormone glucagon‐like peptide 1 levels, which in turn stimulates glucose‐dependent insulin secretion from pancreatic β‐cells.8 This mechanism of achieving glucose homeostasis is distinct from that of metformin, which is a biguanide that suppresses hepatic glucose release and also induces weight loss via reduced calorie intake.9
Given the complementary mechanisms of action of alogliptin and metformin, and the potential for incrementally improving glycaemic control without exacerbating safety events, there has been interest in the use of this FDC in patients with type 2 diabetes. Phase 3 clinical trials in Asian patients have shown that concomitant administration of alogliptin and metformin is effective and has a safety profile similar to that of the individual components.10, 11 The current phase 3 study was designed to evaluate the efficacy and safety of alogliptin 12.5 mg plus metformin 500 mg FDC BID vs alogliptin or metformin alone in Asian patients with type 2 diabetes who are inadequately controlled with diet and exercise.
2. MATERIALS AND METHODS
This was a phase 3, randomized, double‐blind, placebo‐controlled, multicentre study that was conducted at 59 sites in China, Malaysia, Republic of Korea (South Korea) and Taiwan. It was conducted in compliance with the study protocol, the ethical principles originating in the Declaration of Helsinki and the International Conference on Harmonisation of Tripartite Guidelines for Good Clinical Practice. All patients provided written informed consent prior to study procedures.
Patients aged 18 to 75 years were eligible if they had an historical diagnosis of type 2 diabetes for which glycaemic control was inadequate (ie, HbA1c of 7.5%‐10.0% after at least 2 months of diet and exercise prior to the screening period). Key exclusion criteria included a significant history of hepatic and cardiovascular disease.
The study consisted of 4 periods: screening (≤2 weeks), placebo run‐in (4 weeks), treatment (26 weeks) and follow‐up (2 weeks). Eligible patients had HbA1c of 7.5% to 10.0% and fasting plasma glucose (FPG) ≤ 275 mg/dL (15.27 mmol/L) at the Week −1 visit of the placebo run‐in (1 week prior to the treatment phase). After stratifying patients by screening HbA1c (<8.5% vs ≥8.5%) and by country, patients were randomized 1:1:1:1: to receive placebo, alogliptin 12.5 mg twice‐daily (BID), metformin 500 mg BID or FDC of alogliptin 12.5 mg BID plus metformin 500 mg BID.
The primary efficacy variable was change from baseline in HbA1c at Week 26 (or early termination) using last observation carried forward. For this variable, 130 patients per treatment group ensured at least 90% power to demonstrate that alogliptin + metformin FDC BID was statistically superior to constituent doses of alogliptin and metformin. This power calculation assumed a treatment effect of 0.45% between the FDC and constituent doses.
The primary efficacy analysis was conducted using the Full Analysis Set (FAS) and an analysis of covariance (ANCOVA) model, with change from baseline in HbA1c at Week 26 (or early termination) as the response variable. Treatment and country were fixed effects and baseline HbA1c was a continuous covariate (Model 1). Each comparison was performed using contrasts derived from Model 1. A supportive analysis was also conducted using Model 1 and the Per‐Protocol Analysis Set (PPS; ie, patients in the FAS without major protocol deviations).
Changes from baseline in HbA1c and FPG (least‐squares [LS] means) were summarized at each time point using descriptive statistics. The incidences of hyperglycaemic rescue (for FPG ≥ 275 mg/dL [≥15.27 mmol/L] before Week 12 or HbA1c ≥ 8.5% and ≤0.5% reduction in HbA1c between Week 12 and the final visit), marked hyperglycaemia (FPG > 200 mg/dL [11.1 mmol/L]) and clinical response were also summarized (frequency and percentage) by treatment group. Continuous secondary endpoints were analysed using an appropriate statistical model with the Model 1 variables. The analysis was carried out using a Cox proportional hazard model, with treatment as an effect, country as a stratification factor and baseline HbA1c as a covariate. For each efficacy variable, the corresponding baseline value for that variable was modeled as a covariate in place of baseline HbA1c. All secondary variables were analysed at the 2‐sided significance level of 5%.
Treatment‐emergent AEs (including all events, irrespective of treatment relationship and treatment‐related AEs), clinical laboratory changes and hypoglycaemic episodes were summarized.
3. RESULTS
Of 1258 screened patients, 647 were randomized to placebo (n = 163), alogliptin (n = 163), metformin (n = 162) or alogliptin +metformin FDC (n = 159) and were included in the efficacy and safety populations (Figure S1, File S1). Demographic and clinical characteristics for the randomized population were similar between study groups (Table S1, File S1). Of the 647 treated patients, 511 (n = 104, placebo; n = 126, alogliptin; n = 135, metformin; n = 146, alogliptin +metformin FDC) completed the study. The most common reason for study discontinuation was lack of efficacy (n = 86 [64.2%] all groups).
3.1. Efficacy
The LS mean change in HbA1c from baseline to end of treatment (Week 26) was −0.19% with placebo, −0.86% with alogliptin, −1.04% with metformin and −1.53% with alogliptin + metformin BID (Table 1). Changes were significantly greater (P < .0001) for alogliptin + metformin FDC vs metformin alone (LS mean difference −0.49%; 95% confidence interval [CI] −0.700, −0.278) and alogliptin alone (LS mean difference −0.68%; 95% CI −0.889, −0.467). Similar results were observed in a supportive analysis using the PPS: alogliptin + metformin FDC BID led to significantly (P < .0001) greater reductions from baseline in HbA1c at Week 26 compared with metformin BID or alogliptin BID alone (LS mean differences of −0.49% and −0.66%, respectively). Significantly greater decreases in mean HbA1c and FPG were observed with alogliptin + metformin FDC vs either alogliptin or metformin alone, beginning with the first on‐treatment assessment at Week 4 and continuing for all time points throughout the study (P < .02 for HbA1c, for all comparisons; P ≤ .002 for FPG, for all comparisons) (Figure 1).
Table 1.
Change from baseline in HbA1c (%) to Week 26
| Placebo (n = 161) | Metformin (n = 161) | Alogliptin (n = 162) | Alogliptin + metformin (n = 158) | |
|---|---|---|---|---|
| Baseline | ||||
| n | 161 | 161 | 162 | 158 |
| Mean (SD) | 8.21 (0.77) | 8.40 (0.77) | 8.48 (0.71) | 8.39 (0.81) |
| Change from baseline to Week 26, Model 1 1 | ||||
| n | 157 | 160 | 160 | 158 |
| LS mean (SE) | −0.19 | −1.04 (0.11) | −0.86 (0.11) | −1.53 |
| LS mean difference 2 | −0.49 | −0.68 | ||
| 95% CI | −0.700, −0.278 | −0.889, −0.467 | ||
| P‐value vs alogliptin + metformin | <0.0001 | <0.0001 | ||
| Change from baseline to Week 26, Model 2 3 | ||||
| N | 157 | 160 | 160 | 158 |
| LS mean (SE) | −0.17 | −1.17 (0.22) | −0.74 (0.22) | −1.53 |
| LS mean difference 2 | −0.36 | −0.78 | ||
| 95% CI | −0.793, 0.073 | −1.213, −0.353 | ||
| P‐value vs alogliptin + metformin | 0.103 | <0.001 |
Abbreviations: CI, confidence interval; HbA1c, haemoglobin A1c; LS, least‐squares; SD, standard deviation; SE, standard error.
Covariates were treatment, country, baseline HbA1c.
Vs alogliptin + metformin.
Covariates were treatment, country, baseline HbA1c, treatment by baseline HbA1c and treatment by country interaction.
Figure 1.
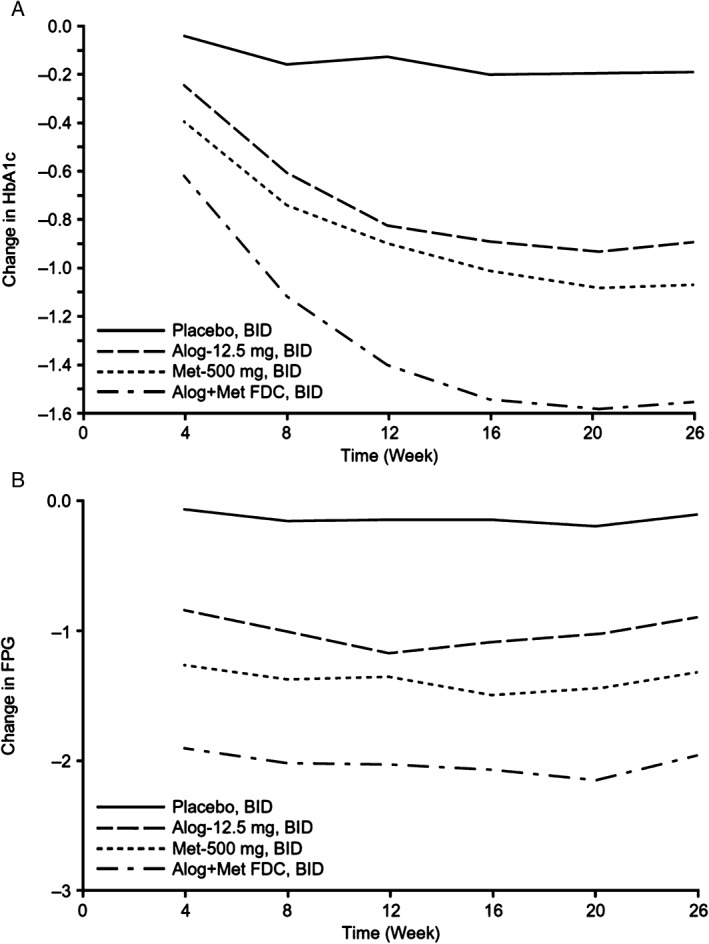
Analysis of adjusted mean change from baseline in (A) haemoglobin A1c (HbA1c; %) and (B) fasting plasma glucose (FPG; mmol/L) to Week 26. P < .02 for HbA1c, for all comparisons; P ≤ .002 for FPG, for all comparisons. BID, twice daily; FDC, fixed‐dose combination.
A lower proportion of patients in the alogliptin + metformin FDC group required hyperglycaemic rescue by Week 26 (4.4%) compared with the proportion of patients in the placebo group (25.5%), alogliptin (14.8%) and metformin (8.7%) groups; this difference was significant as compared with the alogliptin group (P = .002). A similar trend was observed for marked hyperglycaemia (P = .040 vs alogliptin). For time to hyperglycaemic rescue, both placebo and metformin had a significantly lower adjusted risk ratio vs alogliptin + metformin FDC (P < .0001 and P = .002, respectively) (Figure S2, File S1). A higher proportion of patients in the alogliptin + metformin FDC group had HbA1c clinical response at Week 26 as compared with patients in the other 3 treatment groups (Table S2, File S1).
3.2. Safety
The overall frequency of treatment‐emergent AEs was similar for the 4 treatment groups: 48.4% for placebo, 46.3% for alogliptin, 47.2% for metformin and 45.6% for alogliptin + metformin FDC (Table S3, File S1). Across all treatment groups, upper respiratory tract infection was the most common AE, and the only AE with ≥5% incidence (range 4.9%‐6.8%). The overall frequency of serious AEs (SAEs) ranged from 2.5% to 3.1%. Two SAEs were considered to be related to treatment: moderate gastroenteritis (1 event; placebo) that resolved in 8 days, and moderate unstable angina (1 event; alogliptin + metformin FDC) that resolved in 9 days. Overall, 19 patients (3.0%) experienced 23 hypoglycaemic events during the study, 6.2% in the metformin group, 3.8% in the alogliptin + metformin FDC group, 1.2% in the alogliptin group and 0.6% in the placebo group.
4. DISCUSSION
This phase 3 study demonstrated significantly greater reductions in HbA1c over 26 weeks with alogliptin + metformin FDC vs alogliptin or metformin. The advantages of the FDC regimen in reducing HbA1c (primary endpoint) and FPG (secondary endpoint) were evident at Week 4, with sustained effects throughout the study. By Week 26, the rate of achieving clinical response measures was higher and the rate of hyperglycaemic rescue or marked hyperglycaemia was lower with alogliptin + metformin FDC.
Overall, alogliptin + metformin FDC demonstrated a favourable safety profile. The incidence of hypoglycaemia (6.2%) was highest in the metformin‐alone group, which is difficult to explain, as the incidence of hypoglycaemia in the alogliptin + metformin FDC group was lower (3.8%). The overall incidence of AEs and SAEs did not differ with alogliptin + metformin FDC vs each monotherapy. This finding is consistent with data from prior phase 3 clinical trials that tested concomitant alogliptin + metformin in Asian patients.10, 11, 12 In addition, a meta‐analysis of various DDP‐4 inhibitors plus metformin as initial therapy revealed no increase in the risk of hypoglycaemia or prolonged gastrointestinal complaints relative to metformin monotherapy.13
The efficacy and safety of alogliptin + metformin FDC demonstrated in this study is of particular importance because of potential differences in the pathophysiology of type 2 diabetes in Asians compared with other populations. Furthermore, there are multiple reasons why alogliptin + metformin FDC represents an important addition to the glycaemic‐lowering armamentarium in Asia. First, patients are predisposed to multiple comorbidities and polypharmacy, and improving medication adherence is associated with better glycaemic control.14 Second, the FDC incorporates 2 commonly prescribed classes of a type 2 diabetes agent with different mechanisms of action. Metformin is recommended in international diabetes guidelines as first‐line treatment of patients with type 2 diabetes,15 while DPP‐4 inhibitors are recommended in the second‐line setting. Similar recommendations have been made in Asian diabetes guidelines, such as those published by the Chinese Diabetes Society.16 Last, the ENDURE study, where alogliptin was added to stable doses of metformin, demonstrated the sustainability of glycaemic control.17
One limitation of the study is its relatively short duration, which provided no insight into the durability of HbA1c lowering, long‐term tolerability or adherence. However, the durability of the treatment effect was addressed in the ENDURE study.17 Additionally, our study was conducted in eastern Asian countries and employed extensive eligibility criteria, which precludes the extrapolation of results to the entire type 2 diabetes population.
In conclusion, alogliptin + metformin FDC BID resulted in better glycaemic control as compared with alogliptin or metformin alone in Asian patients with type 2 diabetes who were inadequately controlled with diet and exercise. The safety profile of the combination was comparable to that of the individual components.
Supporting information
Appendix S1. Eligibility criteria, patient demographic and clinical characteristics, incidence of HbA1c clinical response at week 26, adverse events, patient disposition, and time to hyperglycemic rescue
ACKNOWLEDGMENTS
Medical writing support was provided by BlueMomentum, a division of Ashfield Healthcare Communications (a UDG Healthcare plc company), and was funded by Takeda Pharmaceutical Company Limited.
Conflict of interest
L.J. has received research grants and consulting fees from Takeda. L.L. has no relevant conflicts of interest to disclose. J.K. has received research grants and consulting fees from Takeda. T.Y. has no relevant conflicts of interest to disclose. D.K. has received research grants and consulting fees from Takeda. A.A.K. has received research grants and consulting fees from Takeda. C.H. has received research grants and consulting fees from Takeda. D.L. reports employment with Takeda.
Author contributions
L.J. contributed to the study design, study conduct/data collection, and writing of the manuscript. L.L. contributed to study conduct/data collection and the writing of the manuscript. J.K. contributed to study conduct/data collection and the writing of the manuscript. T.Y. contributed to study conduct/data collection and the writing of the manuscript. D.K. contributed to study design, study conduct/data collection, and the writing of the manuscript. A.A.K. contributed to study conduct/data collection and the writing of the manuscript. C.H. contributed to study design, study conduct/data collection, and the writing of the manuscript. D.L. contributed to study conduct/data collection, data analysis, and the writing of the manuscript.
Ji L, Li L, Kuang J, Yang T, Kim D‐J, Kadir AA, Huang C‐N and Lee D. Efficacy and safety of fixed‐dose combination therapy, alogliptin plus metformin, in Asian patients with type 2 diabetes: A phase 3 trial. Diabetes Obes Metab. 2017;19:754–758. https://doi.org/10.1111/dom.12875
Funding information This study was sponsored by Takeda Pharmaceutical Company Limited.
REFERENCES
- 1. Xu Y, Wang L, He J, et al; 2010 China Noncommunicable Disease Surveillance Group . Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948‐959. [DOI] [PubMed] [Google Scholar]
- 2. Fung CS, Wan EY, Jiao F, Lam CL. Five‐year change of clinical and complications profile of diabetic patients under primary care: a population‐based longitudinal study on 127,977 diabetic patients. Diabetol Metab Syndr. 2015;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu NC, Su HY, Chiou ST, et al. Trends of ABC control 2006‐2011: a National Survey of Diabetes Health Promotion Institutes in Taiwan. Diabetes Res Clin Pract. 2013;99:112‐119. [DOI] [PubMed] [Google Scholar]
- 4. Eastwood SV, Tillin T, Dehbi HM, et al. Ethnic differences in associations between fat deposition and incident diabetes and underlying mechanisms: the SABRE study. Obesity (Silver Spring). 2015;23:699‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mearns ES, Sobieraj DM, White CM, et al. Comparative efficacy and safety of antidiabetic drug regimens added to metformin monotherapy in patients with type 2 diabetes: a network meta‐analysis. PLoS One. 2015;10:e0125879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case‐control analysis. Diabetes Care. 2008;31:2086‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1:8‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non‐insulin‐dependent diabetes. Obes Res. 1998;6:47‐53. [DOI] [PubMed] [Google Scholar]
- 10. Pan C, Li W, Zeng J, et al. Efficacy and safety of alogliptin in treatment of type 2 diabetes mellitus: a multicenter, randomized, double‐blind, placebo‐controlled phase III clinical trial in mainland China. Zhonghua Nei Ke Za Zhi. 2015;54:949‐953. [PubMed] [Google Scholar]
- 11. Seino Y, Miyata Y, Hiroi S, Hirayama M, Kaku K. Efficacy and safety of alogliptin added to metformin in Japanese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial with an open‐label, long‐term extension study. Diabetes Obes Metab. 2012;14:927‐936. [DOI] [PubMed] [Google Scholar]
- 12. Pan C, Han P, Ji Q, et al. Efficacy and safety of alogliptin in patients with type 2 diabetes mellitus: a multicentre, randomized, double‐blind, placebo‐controlled, phase 3 study in mainland China, Taiwan, and Hong Kong. J Diabetes. 2016. doi:10.1111/1753‐0407.12425. [DOI] [PubMed] [Google Scholar]
- 13. Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase‐4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta‐analysis. Diabetes Obes Metab. 2014;16:30‐37. [DOI] [PubMed] [Google Scholar]
- 14. Rozenfeld Y, Hunt JS, Plauschinat C, Wong KS. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care. 2008;14:71‐75. [PubMed] [Google Scholar]
- 15. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. [DOI] [PubMed] [Google Scholar]
- 16. Weng J, Ji L, Jia W, et al; Chinese Diabetes Society . Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32:442‐458.27464265 [Google Scholar]
- 17. Del Prato S, Camisasca R, Wilson C, Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2‐year study. Diabetes Obes Metab. 2014;16:1239‐1246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Eligibility criteria, patient demographic and clinical characteristics, incidence of HbA1c clinical response at week 26, adverse events, patient disposition, and time to hyperglycemic rescue


