ABSTRACT
Some patients suffering from the same neuropsychiatric disorder may have no overlapping symptoms whilst others may share symptoms common to other distinct disorders. Therefore, the Research Domain Criteria initiative recognises the need for better characterisation of the individual symptoms on which to focus symptom‐based treatment strategies. Many of the disorders involve dysfunction within the prefrontal cortex (PFC) and so the marmoset, due to their highly developed PFC and small size, is an ideal species for studying the neurobiological basis of the behavioural dimensions that underlie these symptoms.Here we focus on a battery of tests that address dysfunction spanning the cognitive (cognitive inflexibility and working memory), negative valence (fear generalisation and negative bias) and positive valence (anhedonia) systems pertinent for understanding disorders such as ADHD, Schizophrenia, Anxiety, Depression and OCD. Parsing the separable prefrontal and striatal circuits and identifying the selective neurochemical modulation (serotonin vs dopamine) that underlie cognitive dysfunction have revealed counterparts in the clinical domain. Aspects of the negative valence system have been explored both at individual‐ (trait anxiety and genetic variation in serotonin transporter) and circuit‐based levels enabling the understanding of generalisation processes, negative biases and differential responsiveness to SSRIs. Within the positive valence system, the combination of cardiovascular and behavioural measures provides a framework for understanding motivational, anticipatory and consummatory aspects of anhedonia and their neurobiological mechanisms. Together, the direct comparison of experimental findings in marmosets with clinical studies is proving an excellent translational model to address the behavioural dimensions and neurobiology of neuropsychiatric symptoms. © 2016 The Authors. Developmental Neurobiology Published by Wiley Periodicals, Inc. Develop Neurobiol 77: 328–353, 2016
Keywords: RDoc, marmoset, neuropsychiatric disorders, prefrontal cortex
INTRODUCTION
One in five people will suffer from a neuropsychiatric disorder at some point in their life (Kessler et al., 2009), yet the prognosis for successful treatment is still only about 40%. Currently, a handful of pharmacological and psychological therapies are used to treat multiple psychiatric disorders, with the former mostly targeting the widespread monoamine systems in the forebrain (Arroll et al., 2005; Miyamoto et al., 2005). Why is this? First, disorders such as depression and schizophrenia are broadly defined and two individuals may be diagnosed with the same disorder but have no overlapping symptoms, making it unlikely that they will be treated successfully by the same therapeutic strategy. Second, patients diagnosed with different psychiatric disorders may share the same symptom, which could explain why specific pharmacological therapies can be used successfully across multiple disorders. For example, selective serotonin reuptake blockers can be effective in treating patients with general anxiety disorder and depression (Reinhold et al., 2011; Gorman et al., 2014), probably because they target a shared symptom, such as enhanced negative emotion or negative bias. Third, the clinical symptoms are often poorly characterized so two patients may exhibit similar symptoms that actually have different underlying psychological and neurobiological causes. For instance, anxiety may be the result of poor learning of predictive cues signaling negative consequences, causing uncertainty, a known contributor to anxious behavior. Alternatively, impaired attentional flexibility may promote anxiety by making it more likely that subjects stay focused on salient negative stimuli, unable to switch their attention toward more positive events in the environment (Clarke et al., 2015; Shiba et al., 2016). Fourth, even when treatments are successful there is poor understanding of the underlying psychological and neurobiological mechanisms, making it difficult to match specific treatments to specific symptoms in individual patients.
For all these reasons there is growing emphasis in clinical and preclinical studies of therapeutic strategies to target common symptoms regardless of the disorder with which they are associated. Moreover, improved characterization of these symptoms requires a fundamental understanding of the psychological and neurobiological mechanisms that cause them, as recognized by the Research Domain Criteria [RDoc; National Institute of Mental Health, (Insel et al., 2010)]. Although imaging studies of patients suffering from neuropsychiatric disorders have revealed much, both in terms of the neural circuits that appear dysregulated in untreated patients and reversal of this dysregulation following successful treatment, it cannot be ascertained whether this dysregulation is causal or compensatory. Vital for achieving this understanding is animal‐based research, in which experimental manipulations can establish causal relationships and identify the complex interactions between, and within, neural circuits that underlie adaptive and maladaptive behavior. Such research can also identify the neural circuits upon which current therapies act in order to optimize therapeutic targets, eliminate undesirable side effects, and identify new therapeutic targets.
A key brain structure showing altered activity across the range of neuropsychiatric disorders is the prefrontal cortex (PFC) (Strakowski et al., 2005; Shin et al., 2006; Etkin and Wager, 2007; Milad and Rauch, 2007; Koenigs and Grafman, 2009), a multimodal cortical association region with the most extensive reciprocal connections with the rest of the forebrain of all cortical regions (Carmichael and Price, 1996; Ongur and Price, 2000; Petrides and Pandya, 2002; Petrides, 2005; Petrides and Pandya, 2007; Petrides et al., 2012; Yeterian et al., 2012). It is also the only neocortical region that has regulatory control over the brainstem and forebrain chemically specific arousal pathways (Arnsten and Goldman‐Rakic, 1984) which, along with its direct reciprocal connections, gives this region a pervasive influence on perceptual, motoric, attentional, mnemonic, language and emotional systems of the forebrain, both directly and indirectly. Its anatomical and functional organization is relatively preserved across primate species (Ongur and Price, 2000; Burman et al., 2006; Burman and Rosa, 2009; Petrides et al., 2012) making Old World and New World, non‐human primates particularly valuable for translational studies of the prefrontal circuits that underlie the regulation of behavior.
The marmoset, a New World monkey, is ideal for studying the effects of interventions within prefrontal circuits, including their modulation by the monoamine systems. Their brains are relatively small, compared with the much larger brained Old World monkeys, and their cortex, lissencephalic, making it easier to target localized regions of interest cortically and subcortically, either permanently, via fiber‐sparing excitotoxins (e.g., quinolinic acid) and neurochemically specific toxins (e.g., 5,7 dihydroxytryptamine), or temporarily, by infusions of drugs through indwelling cannulae. This will also prove an advantage when applying state‐of‐the‐art molecular and imaging techniques to neural circuit analysis of cognition and emotion, including optogenetics (MacDougall et al., 2016) and pharmacogenetics. Like humans, vision and audition are dominant senses in monkeys, including the marmoset, (in contrast to rodents in which the dominant sense is olfaction), and the expansion of cortical processing of these senses in humans is also seen in non‐human primates (Orban et al., 2004; Rauschecker and Scott, 2009). In addition, compared with rodents, marmosets show distinct gene expression patterns in the visual and prefrontal cortex despite similarities in genetic markers in many other areas (Mashiko et al., 2012). This makes primates ideal for translational studies of higher‐order cognitive and affective processes using behavioral tests that rely on these dominant senses. An additional advantage of marmosets is that it is possible to maintain purpose bred colonies within spacious accommodation at local institutions as a consequence of their small size and their ease of breeding in captivity. This allows for the necessary large scale studies of neural circuits. Maintaining a large breeding colony allows the investigation of the interaction between genetic and behavioral traits that are known risk factors for neuropsychiatric disorders, for instance, the serotonin transporter polymorphism and high trait anxiety. The onset of many neuropsychiatric disorders occurs during childhood and adolescence (Jones, 2013) and 75% of adults suffering from a mental disorder have an onset before the age of 25 (Kessler et al., 2005). Thus, the short 5 month gestation period of marmosets and the fact that they reach adulthood by 2 years (Abbott and Hearn, 1978; Abbott et al., 2003; Schultz‐Darken et al., 2016) makes them the ideal primate species in which to study the normal and abnormal development of prefrontal circuits related to these genetic and behavioral risk factors.
In this review we will focus on a number of behavioral dimensions common to a variety of neuropsychiatric disorders and describe the range of cognitive and affective tests that have been developed to study their psychological and neurobiological bases in the marmoset. We will include dimensions associated with dysfunction in the cognitive (cognitive inflexibility and working memory), negative valence (fear generalization and negative bias) and positive valence (anhedonia) systems, as defined by RDoC (Fig. 1). Since symptoms are core to neuropsychiatric diagnoses and are often the trigger for individuals to seek help and advice from a clinic, knowledge of the relationship between behavioral dimensions and symptomatology is critical for progress in our understanding of the etiology and treatment of these disorders.
Figure 1.
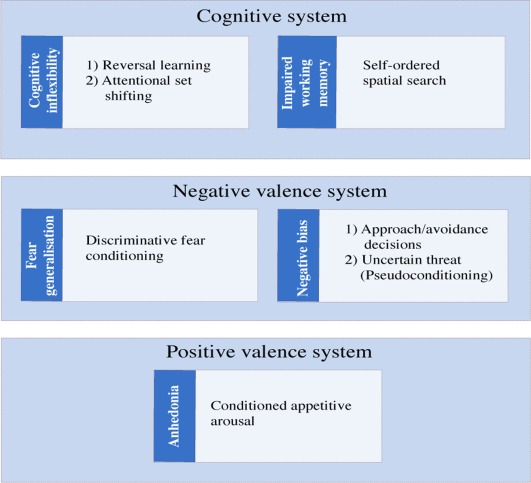
RDoc in the marmoset. Tasks developed in the marmoset are represented in white, linked to the relevant behavioral dimension that is impaired across disorders (blue) and placed within the appropriate system (Cognitive, Negative Valence, and Positive Valence, light blue). [Color figure can be viewed at wileyonlinelibrary.com]
COGNITIVE SYSTEM DYSFUNCTION, COGNITIVE INFLEXIBILITY, AND IMPAIRED WORKING MEMORY
Impairments within the cognitive systems are prominent in disorders such as schizophrenia, obsessive‐compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD). These disorders may share a range of cognitive impairments including deficits in cognitive flexibility and inhibitory response control and aspects of working memory (Morice, 1990; Chamberlain et al., 2005; Castellanos et al., 2006). Such deficits may also be present in affective disorders, including anxiety and mood disorders (Airaksinen et al., 2005; Mantella et al., 2007; Rock et al., 2014). Numerous behavioral tasks have been developed to study cognitive flexibility and working memory abilities in marmosets. Here we focus on those tests that have been successfully translated into clinical and pre‐clinical studies in humans, and back translated into rodents. Specifically, cognitive flexibility has been measured using discrimination reversal‐learning and attentional set shifting tasks, whilst working memory and its underlying mechanisms have been studied using a spatial self‐ordered sequencing task (Fig. 1). The translational success of these tasks for modeling the overlapping deficits in behavioral dimensions and symptoms across disorders has already provided enormous insight into the separable prefrontal circuits and neurochemically specific modulation that underlie such dimensions.
Cognitive flexibility is the ability to adapt mental strategies and actions to the changing contingencies and conditions of the environment (Cañas et al., 2003). It has been suggested that cognitive inflexibility underlies the compulsions present in OCD patients as they shift from flexible, goal directed actions to persistent maladaptive habitual behaviors (Graybiel and Rauch, 2000; Gillan et al., 2011). Although cognitive inflexibility has also been linked to perseverative thinking and the inability to perform mental shifts in schizophrenia (Delahunty et al., 1993; Elliott et al., 1995; Pantelis et al., 1999), it is unclear whether it underpins these symptoms or is simply a by‐product of the prefrontal dysfunction associated with the disorder (Orfei et al., 2013; Weinberger and Berman, 1996). Using the Wisconsin Card Sorting task (WCST, Berg, 1948; Milner, 1963) a commonly used clinical test to evaluate set‐shifting ability in patients with frontal lobe damage, some studies have shown that OCD patients display marked impairments in shifting attentional sets (Okasha et al., 2000; Fontenelle et al., 2001; Veale et al., 2009); but see (Abbruzzese et al., 1995, 1997; Purcell et al., 1998; Simpson et al., 2006). In one such study the level of the impairment correlated with the severity of the symmetry/ordering obsessions (Lawrence et al., 2006) suggesting that difficulties in shifting attention may contribute to the development of these obsessions. Similar difficulties have also been reported not only in patients suffering from schizophrenia (Canavan et al., 1989; Beatty and Monson, 1990) but also Huntington's disease (Malmo, 1974; Lysaker et al., 1995; Everett et al., 2001).
In order to dissect out the underlying cognitive deficits that may contribute to impaired WCST performance, which may include disrupted motivation, attention, learning and memory, a multidimensional discrimination task was developed for use in both humans and marmosets (Roberts et al., 1989). The task was based on intra‐dimensional (ID) and extra‐dimensional (ED) shift studies of animal learning (Mackintosh and Little, 1969). It required subjects to attend to the different aspects of multidimensional stimuli (varying in shape and lines for instance) and either attend to the same dimension across discriminations (intra‐dimensional shift) or shift attention from one dimension to another [extra‐dimensional shifts; Dias et al., 1996a, b; Fig. 2a]; the latter is a direct parallel of the switch from sorting cards according to one category, to sorting them according to another, that is at the core of the WCST. The test also enabled direct comparison with another type of cognitive flexibility that had commonly been studied in primate neuropsychological studies (Jones and Mishkin, 1972), namely reversal learning. Here, having learned to respond to one of two stimuli in order to receive reward, subjects had to reverse their responding to the other, previously unrewarded stimulus in order to gain reward.
Figure 2.

Studying cognitive system dysfunction in the marmoset. (a) Schematic of the attentional set shifting and reversal learning paradigm. In Intra‐dimensional shifts, new exemplars, still varying along the same two perceptual dimensions of shapes and lines are presented and the previously relevant dimension for example, shape, remains relevant and animals must learn which of the two new exemplars from that dimension is rewarded. In extra‐dimensional shifts the relevant dimension is changed (e.g., shape to line) and an exemplar from the new relevant dimension is now rewarded. In contrast, in reversal, the exemplars and dimensions remain the same but the rewarding contingency is reversed (e.g., shape A to shape B). (b) Number of trials required to reach criterion performance during an extra‐dimensional shift (attentional set shifting) and reversal after antOFC or vlPFC excitotoxic lesions. Bars represent the range of scores in each group (Dias et al., 1996a, b). (c) Schematic of the serial reversal learning paradigm, showing an example set of three trials. (d) Perseverative errors (consecutive responses to the unrewarded exemplar, square‐root transformed) made across four reversals after excitotoxic lesions of antOFC or the medial caudate nucleus (Clarke et al., 2008). Error bars represent SEM. (e) Depletions of 5‐HT in the OFC and DA in the medial caudate selectively impair reversal learning. (f) Schematic of the self‐ordered spatial search task with 2‐box and 3‐box examples. The locations of the boxes change across trials. (g) Number of perseverative errors (consecutive responses to the same spatial location) made after antOFC or vlPFC excitotoxic lesions (Walker et al., 2009). Error bars represent SEM. In all figures the location of the excitotoxic lesions are shown on the diagram of the orbital (antOFC, vlPFC) or sagittal (medial caudate) view of the brain. White bars (C) represent control group and stars (*) represent p < 0.05.
By directly comparing the effects of excitotoxic lesions of two distinct PFC regions, namely the orbitofrontal cortex (OFC) and ventrolateral prefrontal cortex (vlPFC), studies in marmosets revealed their differential contribution to these two forms of cognitive flexibility (Dias et al., 1996a, 1996b). While OFC lesions disrupted reversal learning but not attentional set‐shifting, vlPFC lesions disrupted attentional set‐shifting but not reversal learning [Fig. 2(b)], implicating the former in monitoring the changing affective value of stimuli in the environment and the latter in attentional control. This double dissociation was subsequently replicated in human functional neuroimaging studies (Hampshire and Owen, 2006) as well as in rats (Birrell and Brown, 2000) and mice (Bissonette et al., 2008), highlighting the forward and back translatability of these findings. Further studies in the marmoset have implicated the medial caudate nucleus in reversal learning (Clarke et al., 2008) with the pattern of impairment similar to that seen following OFC lesions. In both cases, animals displayed perseverative responding, whereby the previously rewarded option is chosen repeatedly, despite the lack of reward.
Marked differences in the underlying monoaminergic modulation of these circuits have also been revealed. Reductions in dopamine, but not serotonin affect higher‐order attentional selection in the vlPFC (Roberts et al., 1994; Clarke et al., 2005). In contrast, reductions of serotonin, but not dopamine, within the OFC mimic the perseverative effects of excitotoxic OFC lesions on reversal learning (Clarke et al., 2004, 2005, 2007). The converse, however, is the case at the level of the caudate nucleus where reversal learning is impaired following reductions in dopamine but not serotonin (Clarke et al., 2011). Thus, distinct neurochemical systems at the level of the striatum and OFC regulate reversal learning, and although both serotonin and dopamine pathways richly innervate the PFC, their contributions differ with respect to the different forms of cognitive flexibility [Clarke et al., 2008; Fig. 2(e)].
Together these findings provide considerable insight into the specific fronto‐striatal circuitry subserving cognitive flexibility and are beginning to reveal how these different forms of cognitive flexibility contribute to disorders such as schizophrenia and OCD. For example, ED shifting, as assessed in the CANTAB (Cambridge Neuropsychological Test Automated Battery), generally shows a significant impairment in OCD patients (Watkins et al., 2005; Chamberlain et al., 2006). This deficit appears to be pre‐symptomatic as it is also present in their healthy, first degree relatives (Chamberlain et al., 2007). This may implicate altered functioning in vlPFC in OCD patients and a recent report specifically links attentional set‐shifting performance and vlPFC function. Specifically, decreased functional connectivity between vlPFC and caudate at resting state was linked to impairments in attentional set‐shifting performance and predicted greater number of errors during ED shifts (Vaghi et al., 2016). In contrast, OCD patients display mild or no detectable impairment in reversal learning. However, they do show a speed‐for‐accuracy trade off that has been correlated to the severity of compulsions (Chamberlain et al., 2007, 2008; Valerius et al., 2008). Moreover, at the level of underlying brain circuitry, OCD patients and their unaffected first‐degree relatives display decreased recruitment of the OFC during reversal learning (Chamberlain et al., 2007; Remijnse et al., 2013) and symptom provocation (Morgiève et al., 2014) and abnormalities in resting state activity in this region (Menzies et al., 2008). A meta‐analysis of imaging studies highlights not only the OFC but also vlPFC regions as areas with increased likelihood of activation in response to symptom provocation (Rotge et al., 2008). Thus, altered activity in both OFC and vlPFC circuitry involved in response reversal and rule shifting, respectively, may underlie the impairments in cognitive flexibility in OCD and contribute to the compulsions and obsessions, respectively.
Similarly, patients with schizophrenia also show marked deficits in shifting attentional sets (Elliott et al., 1995; Morris et al., 1999; Pantelis et al., 1999; McKirdy et al., 2009) and in reversal learning (Thoma et al., 2007; Waltz and Gold, 2007; Leeson et al., 2009). Performance on the latter is related to the severity of the negative symptoms (Pantelis et al., 1999; Leeson et al., 2009), which if they persisted were linked to greater impairment in set shifting at follow‐up (Leeson et al., 2009). This pattern of impairments also implicates dysfunctional OFC and vlPFC circuits in the cognitive inflexibility associated with schizophrenia, similar to that proposed for OCD. However, the specific neural basis of these deficits across the disorders remains to be determined, given that, for example, similar perseverative responding in reversal learning is associated with disrupted OFC or medial striatal activity (Dias et al., 1996a; Clarke et al., 2008).
In schizophrenia, support for dysfunction within the medial striatum is provided by evidence for disturbances in activation and connectivity within this region (Vink et al., 2006; Rolland et al., 2015) and the finding that reduced functional activity within the ventral striatum is associated specifically with impaired reversal performance in unmedicated patients compared with controls (Schlagenhauf et al., 2014). This can be contrasted with the decreased recruitment of the OFC during reversal learning in OCD patients described above. It can be argued that given vlPFC and OFC form part of a neural network to control attentional set‐shifting and reversal learning respectively, dysregulation at any node within the network will disrupt its overall functioning. However, identifying the underlying cause of that disruption has important implications for the development of therapeutic strategies for the two disorders. For example, studies in the marmoset have revealed that the OFC and striatal mechanisms that underlie reversal learning in the marmoset are differentially modulated by serotonin and dopamine, respectively. Thus, the improvement in OFC function and symptoms in OCD patients following administration of selective serotonin reuptake inhibitors (SSRIs) (Nakao et al., 2005; Saxena et al., 2009) would be predicted by the selective actions of serotonin on reversal learning within the marmoset OFC. It may also explain why medicated OCD patients do not show errors in reversal performance as the boosting of serotonin with SSRI treatment may alleviate their reversal performance deficits. In contrast, pharmacotherapies targeting the dopamine system (rather than SSRIs) would be predicted to alleviate the striatal‐related reversal deficits in schizophrenia. Although both typical (Michara and Goldberg, 2004) and atypical (Keefe et al., 1999) antipsychotics primarily targeting dopaminergic function have been linked to general improvements in cognitive function, whether schizophrenia patients specifically benefit from antipsychotic medication in terms of improvements in striatal based cognitive flexibility remains to be determined.
Within the domain of cognitive symptoms, schizophrenia patients also display impairments in working memory (Green, 1996), which are also a core symptom of attention deficit/hyperactivity disorder (ADHD) (Westerberg et al., 2004; Lee and Park, 2005; Martinussen et al., 2005). Working memory is the ability to hold information “on‐line” in memory, to update that information across time and to use that information to guide responding. A variety of tests has been used to study working memory including delayed response tasks (Pontecorvo et al., 1996), multi‐arm mazes (commonly used in rodents; Dudchenko et al., 2013) and self‐ordered search tasks (Petrides, 1995a, 1995b). An example of the latter developed in the marmoset is conceptually very similar to the CANTAB self‐ordered spatial search task used to study working memory in patients (Owen et al., 1990; Manes et al., 2002; Luciana, 2003; Chase et al., 2008) It requires a combination of working memory, response inhibition and strategy implementation abilities [Fig. 2(f)]. Marmosets are presented with squares simultaneously placed in two, three, or four spatial locations (out of eight possible combinations) on a touch‐sensitive computer screen. On all trials, marmosets are required to respond once only to each square in a self‐ordered sequence in order to receive reward (Collins et al., 1998; Walker et al., 2009). In the marmoset, excitotoxic lesions of the vlPFC, but not OFC, dramatically impair performance [Walker et al., 2009; Fig. 2(g)]. Marmosets are unable to attain pre‐lesion levels of performance and exhibit high numbers of perseverative responses, as they touch the same square repeatedly, failing to move on to the next, previously untouched square. However, performance can be rescued by removing the previously touched square from the touchscreen until another response has been made, thereby preventing perseveration of the immediately preceding response. This suggests that the inability to solve this task stems from a tendency to repeat previous responses.
In the human version of the task, patients with schizophrenia and ADHD patients exhibit similar impairments. Both adults (Dowson et al., 2004) and children (Fried et al., 2015) with ADHD exhibit a profound impairment in this task. Moreover, schizophrenia patients in particular fail to adopt a systematic strategy and make numerous between‐search errors, performing worse than frontal lobe patients (Pantelis et al., 1999; Badcock et al., 2005) and being impaired even at first‐episode psychosis (Joyce, 2002; Joyce et al., 2005). This was evident even when controlling for low visuo‐spatial memory span, suggesting that impaired executive function and strategy implementation are at the core of the working memory problem. Deficits in spatial working memory extend to the healthy monozygotic twins of patients (Pirkola et al., 2005) or first degree relatives (Wood et al., 2003). This would suggest that the working memory impairment is an endophenotype of schizophrenia.
Whilst evidence from the clinical literature supports global prefrontal impairment in ADHD (Lenartowicz et al., 2014; Arai et al., 2015; Mattfeld et al., 2015) and schizophrenia patients (Manoach, 2003; Orfei et al., 2013; Pu et al., 2013; Marumo et al., 2014; Buchy et al., 2015), the identification of the vlPFC as critical for self‐ordered spatial memory in marmosets specifically implicates vlPFC impairment in this working memory deficit in patients. This is supported by the accompanying profound dysfunction in attentional set shifting, which also is associated with vlPFC dysfunction. Similarly, children (Kempton et al., 1999) and adult (Clark et al., 2007) ADHD patients that exhibit marked impairment in the self‐ordered spatial search task also show impaired response inhibition. Such deficits in working memory can be ameliorated by either domain specific cognitive training or psychostimulant medication (Kempton et al., 1999; Bedard et al., 2004; Mehta et al., 2004; Klingberg et al., 2002, 2005; see Del Campo et al., 2011), the latter implicating the dopamine or noradrenaline systems. In support of the dopamine system, administration of a D1 agonist improves a ketamine‐induced deficit on the spatial‐order search task in marmosets (Nakako et al., 2013) suggesting that the working memory processes in this task are under dopaminergic modulation. However, given that striatal dysfunction is also implicated in these disorders (Durston et al., 2003; Cubillo et al., 2012 for a review) it remains to be determined whether the improvements are due to the actions of drugs at the level of the PFC or striatum (see, e.g., Clatworthy et al., 2009).
In summary, the marmoset is proving an excellent model in which to parse the neural circuitry and neurochemical pathways that underlie the cognitive symptomatology associated with a range of neuropsychiatric disorders including OCD, schizophrenia, and ADHD. This is an important first step toward stratification of specific symptoms both within, as well as between, disorders. Moreover, identification of the level within a particular circuit from which a cognitive impairment may arise, for example, PFC or striatum, and the specificity of the neurochemical modulation of that level within the circuit, for example, dopamine or serotonin, has important implications for targeting current therapies more effectively. Future studies employing viral mediated vectors to target specific pathways using optogenetics or pharmacogenetics can further delineate the circuit and the marmoset is an ideal model for such studies with a highly differentiated prefrontal cortex but a relatively small and lissencephalic brain.
DYSREGULATION OF NEGATIVE VALENCE SYSTEMS, ATTENTIONAL BIASES, AND FEAR GENERALIZATION
Although there are evolutionary advantages to fear and anxiety (they can act as a protective defense mechanism in dangerous situations), they can easily become maladaptive and deleterious once left unregulated. Pathological anxiety, affecting more than 28% of the general population (Kessler et al., 2009), specific phobias, PTSD, abnormally low mood in depression and disturbances of emotion in schizophrenia (Staring et al., 2009) are all a result of a dysregulated negative valence system. In generalized anxiety disorder, patients exhibit pathological worry and apprehension over long periods of time about situations that are normally causing no distress to the healthy population (Tyrer and Baldwin, 2006; Craske et al., 2009). In specific phobias and PTSD the worry and distress are focused on, and triggered by, a single event, situation or stimulus (Kessler et al., 2009; Pacella et al., 2013).
A particularly prominent symptom in patients suffering from all types of anxiety and depression is fear over‐generalization, whereby they indiscriminately develop fearful responses to threatening and non‐threatening stimuli alike (Lissek et al., 2008; Craske et al., 2009; Lissek et al., 2014). They also tend to develop negative biases, not only within the affective domain but also extending into the cognitive domain, and influencing attention and memory (Murphy et al., 1999; Gotlib et al., 2004; Mogg and Bradley, 2005; Bar‐Haim et al., 2007). It is still unclear whether fear generalization and negative biases are causal to the development of the disorder, or constitute underlying symptoms that appear in its presence and contribute to its maintenance. Evidence for the former comes from the study of individuals within the healthy population who display quite dramatic differences in anxiety. This variation in anxiety is recognized as a stable personality trait. Individuals at the extreme high end of this trait can be more at risk of developing pathological anxiety and, like individuals suffering from clinical anxiety, have a tendency to over‐generalize in fear provoking situations and display mild negative biases (Sexton et al., 2010; Arnaudova et al., 2013). Such behavioral traits are the product of interactions between genes and early life experiences (Nugent et al., 2011). Life experiences that may trigger the development of these traits include physical and psychological stressors, which impact on the development of brain circuits underlying emotion. Indeed, affective disorders commonly emerge during childhood and adolescence (Jones, 2013), making the study of gene–environmental interactions and brain development important for our understanding of emotion dysregulation.
The common marmoset, like humans, displays marked individual responsivity to anxiety provoking situations that appears stable across time and thus trait‐like in character. This makes the marmoset an ideal species for studying emotion regulation and dysregulation. Consequently, a range of specific tasks have been developed to formally characterize their anxious temperament and to measure fear over‐generalization and negative biases (Fig. 1). An important aim in developing these tasks has been to bridge the gap between existing studies of negative emotion in rodents, monkeys and humans. The neural mechanisms underlying fear and anxiety have been extensively studied in rodents (LeDoux, 2000; Maren, 2001; Davis et al., 2010; Tovote et al., 2015), primarily using Pavlovian fear conditioning paradigms (acute and sustained fear) and tests of innate anxiety, such as the open field and elevated plus maze (Belzung and Griebel, 2001). In contrast, specific tests of innate fear (responsivity to snakes) and anxiety (responsivity to unknown humans) have been more commonly used in non‐human primates (Izquierdo et al., 2005; Rudebeck et al., 2006; Kalin et al., 2007; Machado and Bachevalier, 2008) making cross‐species comparison and translation difficult. Moreover, although fear conditioning has been used to test both humans and rodents alike, the metric of emotion differs between the two species; a behavioral freezing response is commonly measured in rodents contrasted to an autonomic and/or self‐reported state used in humans. Since an emotional state is the product of changes across the range of outputs, both behavioral and physiological, it is important to measure multiple aspects of the response in order to determine the neural circuits that regulate emotional states. This is particularly important since there are strong brain‐body‐brain pathways resulting in patients with anxiety and mood disorders being more likely to suffer cardiovascular disease and vice versa (Khawaja et al., 2009; Stapelberg et al., 2012).
A test commonly used to measure an anxious temperament in macaque monkeys is the human intruder test, whereby the experimental animal encounters an unfamiliar human (Kalin and Shelton, 1989). The unfamiliar intruder could be a potential “friend,” for instance bringing food, or they could be a threat. Hence, the ambiguity of the situation creates a state of anxiety, with subjects varying in the extent to which they approach the intruder. In the marmoset version of the task, the level of anxiety is measured based upon a component score that includes the distance the animal chooses to maintain between themselves and the intruder, their behavioral reactivity in the form of calls made, and other attentive behaviors such as body and head bobbing. This test is relatively quick to perform, as it is based in the home cage, requires no training and thus is ideal for screening large numbers of animals. Another paradigm to assess emotional reactivity in marmosets is based on the response to a snake, which is a stimulus that primates find inherently fearful [Shiba et al., 2014a, b; Fig. 3(a)]. Although the human intruder and snake test measures are somewhat independent of one another [see significant but weak correlation in Fig. 3(b)] and may measure overlapping but also distinct aspects of negative emotion, for example, innate fear versus uncertainty, they both reflect the general anxious temperament of the animal. High scores in both tests on repeated occasions indicate a high‐anxious phenotype in the marmoset. They have been linked to structural alterations in the dorsal Anterior Cingulate Cortex (dACC) [Fig. 3(c)] as measured by MRI and reductions in serotonin release in the amygdala in response to an acute dose of an SSRI, as measured by in vivo microdialysis (Mikheenko et al., 2015). In addition, microPET analysis of the 5‐HTT has also revealed lowered binding in the dACC related to social anxiety in marmosets (Yokoyama et al., 2013).
Figure 3.
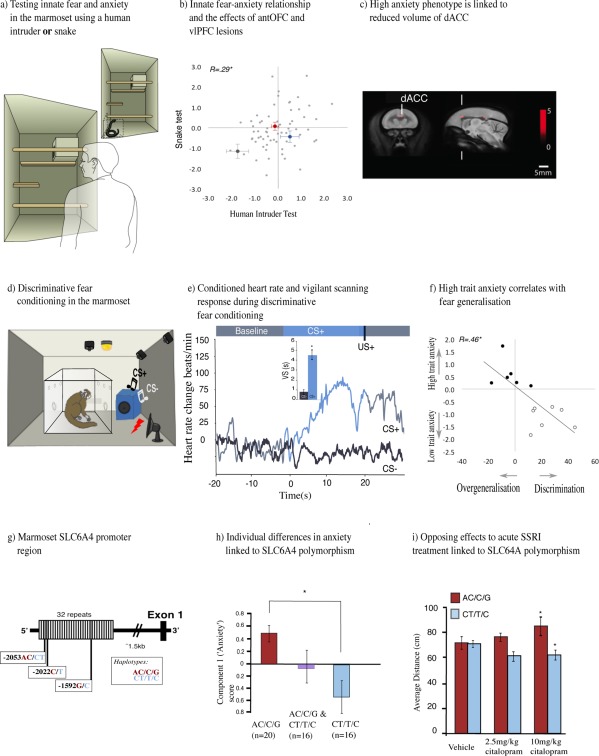
Dysregulation of negative valence systems. (a) Human intruder and snake tests are conducted in the home‐cage, with the animal separated from their cage mate in one quadrant of the cage for a brief period. (b) The negative emotional response to the snake, as measured by the component 1 (“Anxiety”) score in the Principal Component Analysis (PCA), is positively correlated with the negative emotional response to the human intruder. AntOFC (red) and vlPFC (blue) excitotoxic lesions increase negative emotion relative to controls (gray) but the lesioned animals remain within the normal variation of trait anxiety within the colony (Agustín‐Pavón et al., 2012). (c) Two clusters (dACC, labeled, and posterior cingulate cortex) identified by tensor based morphometry analysis were negatively correlated with high anxiety in the human intruder test. (d) Schematic of discriminative fear conditioning apparatus. The CS+ is paired with an aversive loud noise (300–600 ms car siren at 118 dB), whereas the CS− is paired with a neutral event (0.5 s of darkness). (e) Example of conditioned behavioral (vigilant scanning, time in seconds) and cardiovascular (heart rate, beats/min) responses. “Vigilant scanning” includes attentive visual search of surroundings in combination with forward extension of body/head and rearing. A conditioned heart rate rise is observed for the CS+ (blue) but not for the CS− (gray) indicating successful discrimination between the two stimuli. (f) Correlation between component 1 score (“Anxiety”) and performance in discriminative fear conditioning. High scoring in component 1 was associated with failure to achieve discriminative conditioned cardiovascular responses to CS+ and CS−. (g) Schematic representation of the marmoset SLC6A4 promoter region showing 32 repeats. Third, fourth, and 23rd repeats containing the double and the two single‐nucleotide polymorphisms, respectively, are shaded in gray. (h) Comparison of component 1 (“Anxiety”) behavioral scores derived from the PCA of the HIT performance. Error bars represent SEM. (i) The human intruder test was used to assess anxiety levels in response to vehicle and to a single dose of 2.5 mg/kg or 10 mg/kg citalopram, 25 min prior to the intruder phase. Effects on average distance (cm) are shown (Santangelo et al., 2016). Error bars represent SEM, stars (*) represent p < 0.05.
The translational potential of this anxiety phenotype has been determined by studying an individual marmoset's ability to display discriminative fear conditioning, and their responsivity to an uncertain or ambiguous stimulus. The tendency to generalize between threatening and non‐threatening cues and contexts, and the negative appraisal of neutral or ambiguous cues (Hirsch and Mathews, 1997; Constans et al., 1999), is characteristic of high trait‐anxious humans (Craske et al., 2009; Lissek, 2012; Dymond et al., 2015) and rats (Duvarci et al., 2009). Thus, it would be predicted that high anxious marmosets would show a similar phenotype. Consequently, Pavlovian discriminative fear conditioning has been developed for marmosets based on paradigms used effectively in humans (Lau et al., 2008; Lissek et al., 2008) and rodents (Herry et al., 2008). It involves the presentation of two distinct auditory cues, one of which is paired with a mildly aversive loud noise [300–600 ms car siren at 118 dB; the specific parameters are adjusted for each individual animal based on their performance; Fig. 3(d)]. Successful discriminative conditioning is demonstrated by the development of conditioned attentional orienting/vigilant scanning responses and heightened heart rate [Fig. 3(e)] in response to the stimulus paired with the loud noise but not the alternative stimulus paired with a neutral event, (0.5 s period of darkness). Animals that had shown higher anxiety and avoidance of the rubber snake were unable to successfully learn such a discrimination and their conditioned autonomic and behavioral responses generalized to the safety cue or to the context [Shiba et al., 2014b; Fig. 3(f)], much like high trait anxious humans who tend to generalize from threatening, aversive, and negative stimuli to neutral ones. Importantly, marmosets that failed to learn discriminative fear conditioning were perfectly successful in learning an appetitive Pavlovian discrimination paradigm by discriminating the same auditory cues when they were predictive of reward. High anxious marmosets also displayed high levels of behavioral vigilance when presented with a novel, neutral cue in a threatening context (Mikheenko et al., 2015). This is a form of pseudo‐conditioning as the aversive loud noise and a neutral stimulus were presented within the same test session but were not correlated with one another, yet high anxious marmosets still developed conditioned responses to the neutral stimulus.
High anxious marmosets and humans not only display a similar behavioral phenotype but that phenotype may also share common genetic influences. In humans, variation within the serotonin transporter gene (SLC6A4) that results in reduced gene expression has been linked to a high trait anxiety phenotype (Lesch et al., 1996; Canli and Lesch, 2007; Caspi et al., 2010) and marked changes in the activity of emotion circuits in the brain (Brown and Hariri, 2006; Murphy et al., 2013); although meta‐analyses have called into question the gene‐behavior association (Munafò et al., 2009; Risch et al., 2009). Recently, genetic variation in the SLC6A4 upstream repeat region of the marmoset has also been linked to differential SLC6A4 gene expression, with lower gene expression being associated with heightened anxiety on the human intruder test [Santangelo et al., 2016; Fig. 3(g,h)]. This gene–behavioral association in the marmoset highlights the potential advantage to studying such relationships in a purpose‐bred primate colony. The relatively controlled environment dramatically reduces the impact of variation in life experiences on an individual's behavior, allowing gene–behavior relationships to be more easily revealed compared with that of humans.
Further exploration of the phenotype has revealed that genetic variation in the SLC6A4 gene is related to opposing effects of an acute dose of a selective serotonin reuptake inhibitor (SSRI) on anxious behavior. The low gene expressing, high trait anxious marmosets displayed an anxiogenic response in contrast to the anxiolytic response of the high gene expressing, low trait anxious marmosets [Santangelo et al., 2016; Fig. 3(i)]. These results bridge the gap between the findings in humans that report reduced responsivity to the therapeutic effects of chronic SSRI treatment in low expressing carriers with anxiety disorders (Perna et al., 2005) and depression (Keers et al., 2011; Porcelli et al., 2012), and the individual differences in sensitivity to the anxiogenic effect of acute SSRIs (Harmer and Cowen, 2013). This finding implicates SCL6A4 genetic variation in the latter (Harmer et al., 2006; Murphy et al., 2009) which may provide insight into the underlying brain mechanisms that account for the later improvement of the clinical symptoms observed in high expressing carriers. The marmoset model therefore has enormous potential for revealing the interactions between the SCL6A4 gene and functional activity in the serotonin system in the control of behavior.
Changes in activity of brain networks that are associated with heightened anxiety and dysregulated negative emotion, upon which serotonin may act, have been reported by functional neuroimaging studies of high trait anxious individuals in the healthy population (Killgore and Yurgelun‐Todd, 2005, Bishop, 2007) as well as in adults (Etkin and Wager, 2007; Milad and Rauch, 2007, Price & Drevets, 2010), children and adolescents (Monk et al., 2006; Guyer et al., 2008; Strawn et al., 2012) with specific anxiety disorders. These implicate a number of prefrontal subregions including the OFC and vlPFC. However, whether those changes are causal to the phenotype or merely compensatory cannot be determined in humans.
Such cause and effect has been established in marmosets, by studying the effects of selective excitotoxic lesions of the anterior OFC (antOFC) and vlPFC on fear and anxiety. At face value, the effects of both lesions seemed almost identical, increasing anxiety as measured by the human intruder test, increasing innate fear in response to a snake stimulus [Fig. 3(b)] and inducing inflexible conditioned cardiovascular and behavioral fear responses following acute alterations in the pairing of a stimulus with aversive loud noise (Agustín‐Pavón et al., 2012; Shiba et al., 2014a). This resembles the robust pattern of conditioned fear found in anxiety patients who display stronger responses to conditioned cues during extinction and are delayed in extinguishing responses compared with controls (Wessa and Flor, 2007; Duits et al., 2015). Whilst the effects of OFC lesions on tests of anxiety and innate fear have been somewhat variable in macaque monkeys (Izquierdo et al., 2005; Rudebeck et al., 2006; Kalin et al., 2007; Machado and Bachevalier, 2008) the reasons for these discrepancies have been attributed mostly to differences in lesion methodology, lesion extent and task sensitivity (for a thorough discussion on the issue see Shiba et al., 2016). The anxiety phenotype induced by antOFC and vlPFC lesions in marmosets altered the entire repertoire of behaviors that contributed to the high anxiety trait, including all aspects of behavior, coping strategy and emotionality. The finding that the lesion‐induced heightened anxiety remained within the “normal” range of such behavior displayed by marmosets within the colony [Fig. 3(b)] highlights the important role that both regions play in determining levels of trait anxiety. Moreover, the apparently similar symptomatology associated with lesions to two distinct regions of PFC illustrates the multivariate nature of anxiety.
The question remains as to what is the distinct contribution of these two prefrontal regions to the anxiety phenotype, the answer of which will help to stratify anxiety disorders and develop targeted treatments. Besides the failure to regulate negative emotions, patients with anxiety and depression often make poor decisions. Because they are particularly sensitive to negative information, they tend to perceive threat with increased intensity and are more risk averse (Murphy et al., 2003; Dickson, 2006; Smoski et al., 2008; Mueller et al., 2010) and thus display negative biases when making decisions (Bradley et al., 1998; Murphy et al., 1999; Mogg et al., 2000; Williams et al., 2007; Peckham et al., 2010). Indeed, reductions in serotonin within the OFC of marmosets have been implicated in such negative biases (Rygula et al., 2015) as identified in a probabilistic discrimination task similar to that used to reveal altered activity in prefronto‐amygdala circuitry related to negative biases in depressed patients (Taylor Tavares et al., 2008).
To compare the impact of antOFC and vlPFC inactivation in marmosets on decision making we developed an approach‐avoidance instrumental decision‐making task. In this task, animals optimize their reward by responding equally to two, equivalently rewarded left and right locations on a touch‐sensitive computer screen. In occasional probe sessions, superimposed over the reward schedule, an aversive loud noise, a form of punishment, is associated with responding at one of the two locations. This results in a conflict at that location between responding to gain reward, and avoiding responding due to the punishment. However, animals normally maintain responding to both stimuli, choosing to optimize reward, despite the occasional punishment. However, transient pharmacological inactivation of the antOFC and vlPFC alters performance in distinct ways. Inactivation of the vlPFC has no impact on overall levels of responding but causes the animal to avoid making the punished response, resulting in many more responses being made to the side on which responding only receives reward. Hence, the cost‐benefit balance of responding for reward in the face of punishment is switched from a positive approach response to a negative avoidance response. In contrast, inactivation of antOFC has no effect on the day of receiving punishment, but it acts to bias responding away from the punished side the following day, presumably as a consequence of its effects on memory consolidation (Clarke et al., 2015).
The vlPFC has been implicated in attentional processing and specifically the shifting of higher‐order attentional sets as described in the cognitive section above. Thus, a parsimonious account of the on‐line negative bias induced by inactivation of the vlPFC is that the marmoset is unable to shift attention away from the intrinsically salient aversive loud noise and toward the rewarding aspects of the context in order to perform a cost‐benefit analysis. Consequently, this prolonged attention to the aversive stimulus leads to the negative bias. This hypothesis is also consistent with the contribution of the vlPFC to cognitive re‐appraisal, whereby individuals explicitly shift their attention from a salient negative interpretation of a context to a more positive interpretation (Buhle et al., 2014), and down‐regulation of emotion during conflict and acceptance of unfair outcomes (Tabibnia et al., 2008; Feng et al., 2015). Consequently, it is proposed that a compromised vlPFC induces anxiety because subjects fail to switch their attention away from negative events.
On the other hand, the antOFC inactivation‐induced negative bias is not linked to the online appraisal and processing of the negative stimulus but rather to its memory. The OFC has been linked to the development of stimulus‐reward associations and the ability to predict outcomes, particularly within probabilistic contexts (Schoenbaum and Roesch, 2005; Schoenbaum et al., 2007; Balleine et al., 2011; Walton et al., 2011; Rudebeck and Murray, 2014). Therefore, it can be argued that a compromised OFC makes the punishment no longer predictable. This learned state of uncertainty leads to establishment of a stronger punishment memory that underlies the negative bias and avoidance behavior upon its retrieval the next day. This is consistent with the role of uncertain environments in the development of anxious responses (Grupe and Nitschke, 2013). The negative bias is abolished by inactivation of either the amygdala or the hippocampus on the day of retrieval, or disconnection of the two, implicating an OFC‐amygdala‐hippocampal network that drives the expression of such negative biases. This account of the symptoms of anxiety and depression induced by compromised OFC function differs from that proposed above for vlPFC dysfunction. Instead of an inability to down‐regulate responses on‐line, a dysfunctional OFC may leave the system unregulated and result in the learning of maladaptive negative associations due to a failure to predict aversive outcomes, supporting the negative cognitions prevalent in anxiety and depression.
In summary, the marmoset model has allowed the investigation of regulation and dysregulation within the negative valence system (Fig. 1) at both the individual‐ (trait anxiety and genetic variation in the serotonin transporter gene) and circuit‐based levels (prefronto‐amygdala‐hippocampal manipulations). The finding of an interaction between the serotonin transporter gene and the effects of acute SSRI treatment on anxious behavior has implications for the individual targeting of treatments. On the other hand, the proposed roles of vlPFC and antOFC, respectively, in attentional processing and in the prediction of negative outcomes, provide an important framework for the stratification of anxiety disorders. Future studies will focus on the contributions of other regions of PFC that have been implicated in the regulation of negative emotion, including primate dorsolateral PFC (Buhle et al., 2014) and peri‐ and sub‐genual anterior cingulate cortex (Drevets et al., 2008; Etkin et al., 2011). While numerous studies have implicated the prelimbic and infralimbic regions of ACC in rodents, respectively, in the expression and extinction of freezing to a conditioned fear stimulus, their putative homology to primate peri‐and subgenual ACC remains unclear (for a critical review see Myers‐Schulz and Koenigs, 2012). This further emphasizes the importance of the marmoset primate model in parsing out the distinct cognitive functions of, and interactions between, these prefrontal brain regions and their downstream targets, in the control of negative emotion. It will also provide the basis by which we can determine the neurobiological and psychological mechanisms underlying the action of current pharmacotherapies that target the serotonin (e.g., SSRIs), noradrenaline (selective noradrenergic reuptake inhibitors) and glutamatergic (e.g., ketamine) systems in the treatment of dysregulated emotions. Only then can these treatments be targeted appropriately to the individual.
IMPAIRED POSITIVE VALENCE: MODELING ANHEDONIA
Positive valence systems, as defined by the RDoC framework, are responsible for responding to positive situations and contexts, including reward seeking, consummatory behaviors and reward/habit learning. Dysfunction within these systems occurs in a number of major psychiatric disorders. One specific example of a clinical symptom involving disturbed positive valence is anhedonia (Fig. 1). Anhedonia is defined according to the DSM (I–V) as “decreased interest or pleasure in most activities, most of each day” and is prevalent in about 37% of depressed patients (Kessler et al., 2009). Indeed, for a patient to be diagnosed with depression they must suffer from one or other form of anhedonia or depressed mood (DSM V). Besides depression, anhedonia constitutes one of the primary negative symptoms of schizophrenia, being present in half of the patients (Pelizza and Ferrari, 2009), is present in eating disorders (Davis and Woodside, 2002) and is associated with withdrawal in drug abuse (Markou and Koob, 1991). It has also been reported in a number of neurodegenerative disorders, including Parkinson's Disease (Isella et al., 2003) and Alzheimer's disease (Starkstein et al., 2005).
Clinically, it has been identified by self‐report measures of hedonic experiences using a variety of questionnaires, such as the Snaith Hamilton (Snaith et al., 1995) and Fawcett–Clark Pleasure scales (Fawcett et al., 1983). The majority of the questions on these scales focus on the consummatory aspects of positive experiences such as “one food tastes as good as another to me.” As a consequence, pre‐clinical models of depression have tended to use the sucrose consumption test as the primary measure of anhedonia (Slattery et al., 2007) when investigating novel pharmacotherapies. However, preclinical neurobiological studies of positive valence systems have recognized motivational, reinforcing, decision making and consummatory components [see Treadway and Zald (2011) for a comprehensive review], all of which can be parsed at the level of neural circuits and neurochemistry. Thus, anhedonia could arise from a lack of the ability not only to experience pleasure (consummatory anhedonia) but also to anticipate pleasure (anticipatory anhedonia). Indeed, a failure to anticipate pleasure has a major impact on an individual's motivation to seek out pleasure (motivational anhedonia). Treadway and Zald (2011) further proposed the concept of decisional anhedonia to include the effects of anhedonia on decision making in the context of reward.
Recently, distinctions between these different types of anhedonia have begun to emerge in clinical research. For example, patients with depression show anticipatory (McFarland and Klein, 2009) as opposed to consummatory anhedonia (Dichter et al., 2010). On the other hand, schizophrenia patients show intact anticipation of reward but deficits in motivational/effortful responding for reward, a form of apathy (Gard et al., 2009, 2014). Another distinction to have arisen is that between self‐report measures of pleasurable experiences retrospectively and experiencing pleasure per se. In particular, patients with schizophrenia and depression do not show reductions in their hedonic response to different sucrose solutions compared with controls, despite scoring high on the Chapman and Fawcett anhedonia questionnaires (Amsterdam et al., 1987; Berlin et al., 1998). Moreover, self‐reported affective flattening and anhedonia in schizophrenia, as measured by questionnaires, is actually associated with an almost normal experience of emotion as measured by hedonic ratings during the experience of pleasant stimuli, including pictures, films, words, or faces (Burbridge and Barch, 2007; Heerey and Gold, 2007; Trémeau et al., 2010). This inconsistency between retrospective self‐reports of anhedonia and the actual on‐line experience may well be a consequence of the reliance of clinical questionnaires on the ability of patients to “represent” hedonic experience, as opposed to the hedonic experience per se. This ability to “represent” consummatory pleasure is also a major component of anticipatory and motivational hedonic processes and thus illustrates the difficulty in interpreting self‐report questionnaires in the clinical population.
Considerable insight into the distinct consummatory, anticipatory and motivational components of reward processing has been gained from neurobiological and neurochemical studies at the level of the striatum and amygdala of both rodents (Cardinal et al., 2002; Wassum and Izquierdo, 2015) and monkeys (Schultz et al., 2000; Schultz, 2004; Murray, 2007; Bermudez and Schultz, 2010). The anhedonia associated with depression, however, is linked to altered activity within the ventromedial PFC (Keedwell et al., 2005). This is a complex region in humans composed of a number of discrete cytoarchitectonic areas, including Brodmann's areas 10, 14, 25, and 32, and their contribution to the regulation of reward processing and their interaction with striatal and amygdala reward systems is unknown. This again highlights the need for studies in a primate in which the organization of ventromedial PFC appears far similar to humans than that of rodents (Myers‐Schulz and Koenigs, 2012).
We have developed a paradigm in the marmoset that distinguishes between the consummatory and anticipatory aspects of pleasure and uses independent measures of the behavioral and autonomic arousal (cardiovascular) components. The latter is seldom measured in studies of reward processing in animals (but see Rudebeck et al., 2014) but can be an important component of positive emotional responses, as it is for negative emotions, and is often the metric used in humans, alongside self‐report questionnaires. Using a Pavlovian procedure [Fig. 4(a)], one version of the paradigm used the “sight of the food” viewed through a transparent door of a food box as the conditioned stimulus. This constitutes the anticipatory period and was followed by the consummatory period when the door opened, resulting in access to the reward. With training, a conditioned rise in systolic blood pressure accompanied by approach behavior toward the food box developed during the anticipatory period, as the animals learned that the presentation of the food box was predictive of subsequent reward [Fig. 4(b)]. In contrast, the rise in blood pressure that accompanied the consummatory period was present from the beginning. The consummatory arousal was, nevertheless, motivational in nature since it was only seen in the context of the marmosets gaining access to their preferred, high incentive reward and not the food pellets that they received daily (Braesicke et al., 2005). Consistent with the role of the amygdala in appetitive conditioning, excitotoxic lesions of the amygdala in the marmoset reduced anticipatory behavioral responses directed toward the conditioned stimulus, as shown previously in rodents (Holland, 1999) and also reduced the conditioned cardiovascular arousal. In contrast there was no effect on the food box approach response or the cardiovascular arousal during the consummatory period. Thus, damage to the amygdala induced anticipatory but not consummatory anhedonia [Fig. 4(c)].
Figure 4.
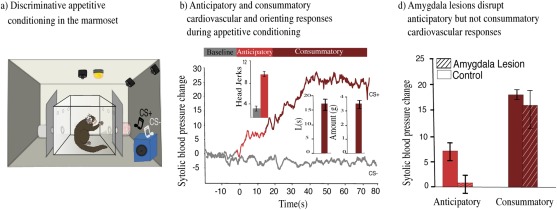
Parsing consummatory and anticipatory aspects of positive valence in the marmoset. (a) Schematic of discriminative appetitive conditioning apparatus. CS+ resulted in opening of the door revealing food box with reward (e.g., right), whereas CS− resulted in presentation of empty food box (e.g., left). The anticipatory period entails viewing the full food box and is followed by the consummatory period, when marmosets are allowed access to the reward. (b) Conditioned behavioral response during the anticipatory phase is measured by the number of orienting head movements (head jerks) toward the food box. Consummatory behavior is measured by the latency to eat the reward (“L”, seconds) and the amount of reward eaten (grams). A conditioned systolic blood pressure rise is observed for the CS+ (Light red for anticipatory period, dark red for consummatory period) but not for the CS− (gray). (c) Systolic blood pressure response during the CS+ after excitotoxic lesions of the amygdala (lined bars) presented for the anticipatory (Light red) and the consummatory (Dark red) periods compared with controls (empty bars) (Braesicke et al., 2005). Error bars represent SEM, stars (*) represent p < 0.05.
A revised version of the Pavlovian paradigm has since been developed to identify the brain mechanisms underlying the ability of marmosets to adapt their anticipatory hedonic responses to changes in environmental contingencies. Animals were conditioned to discriminate between two auditory stimuli, either predicting reward (CS+) or an empty food box (CS−). Changes in behavioral and cardiovascular arousal during the CSs were assessed both after a single extinction session, in which the decline in conditioned cardiovascular arousal was measured following omission of the expected reward and after reversal of the reinforcing contingencies, in which the previous CS− became the CS+, and vice versa. So far this revised version has been used to study the contribution of the antOFC to the regulation of appetitive Pavlovian conditioning. Marmosets with excitotoxic lesions of the antOFC failed to show the normal decline in the conditioned systolic blood pressure response after reward omission, instead showing a prolonged arousal response. Moreover, upon reversal, antOFC lesioned animals were much slower to reverse their blood pressure response. Significantly, even when the cardiovascular response was successfully reversed, conditioned behavior was not (Reekie et al., 2008). This was in marked contrast to controls in which there was strong coupling between the rate of reversal learning of the conditioned behavioral and cardiovascular responses. Thus, antOFC lesions caused uncoupling of cardiovascular and behavioral responses. This is particularly relevant for the motivational‐anticipatory distinction in anhedonia, as a patient may display cardiovascular anticipatory arousal, but due to uncoupling this may fail to result in motivated behavior.
Future studies in marmosets are ideally placed to focus on the contribution of the distinct regions of the ventromedial PFC in the regulation of striatal and amygdala reward processing, including their modulation of the monoamine systems. Downregulation of striatal activity (Heller et al., 2009; Robinson et al., 2012) and of striatal dopamine receptors in particular (Cannon et al., 2009), has been reported in depressed subjects, along with reductions in amygdala responsivity to happy faces (Beesdo et al., 2009), the latter being correlated with symptoms of anhedonia (Stuhrmann et al., 2013). An understanding of the relationship between these cortical and subcortical changes and their causal role in the anhedonic symptoms will inform treatment strategies since the symptom of anhedonia appears particularly resistant to current pharmacotherapies. It is largely unresponsive to SSRI based treatments of depression (Shelton and Tomarken, 2001; Dunlop and Nemeroff, 2007) and similarly fails to improve when SSRI administration is used as an add‐on therapy in schizophrenia (Sepehry et al., 2007). Whilst drugs that target the dopamine system are successful in alleviating many symptoms of depression and schizophrenia, reports of the direct effects of such drugs on anhedonic symptoms are mixed (Nutt et al., 2007). This may not be surprising since evidence from psychopharmacological experiments in animals implicates dopamine primarily in motivational and reward prediction processes rather than pleasure per se (Robbins and Everitt, 1992; Salamone, 1997; Berridge and Robinson, 1998; Denk et al., 2005; Schultz et al., 2015). Thus, depending upon what type of anhedonia a patient is displaying, for example, consummatory, anticipatory, or motivational, the neurochemical target for alleviating anhedonia may differ.
UTILITY OF THE MARMOSET IN FUTURE INVESTIGATIONS OF NEURAL AND COGNITIVE DEVELOPMENT RELEVANT TO MENTAL HEALTH
Seventy five per cent of patients with a mental disorder will have shown neuropsychiatric symptoms by age 25 (Kessler et al., 2005), making the first years of life extremely important for understanding the development of neuropsychiatric disorders and their behavioral dimensions. The marmoset has distinct but, relatively, short duration developmental stages, compared with old world monkeys, lasting just a few months each, until they reach adulthood and sexual maturity at approximately 18‐20 months (Abbott et al., 2003; see Schultz‐Darken et al., 2016 for a comprehensive review). These developmental stages have been well characterized in terms of typical behaviors observed, including social interactions (Chalmers and Locke‐Haydon, 1984), vocalizations (Pistorio et al., 2006; Braun et al., 2015), and sensorimotor abilities (Piper et al., 1992; Kaplan and Rogers, 2006; Izumi et al., 2012). Moreover, the impact of early life experiences, such as maternal deprivation and infant isolation, on some of these behavioral outcomes, as well as on levels of cortisol, adrenaline and noradrenaline are already known (Dettling et al., 2002; Pryce et al., 2004; Dettling et al., 2007). This research provides the community with a useful toolbox to study gene‐environment interactions and the effects of early life experiences on cognitive and affective development and pinpoints the different milestones of physical ability according to age. As Schultz‐Darken et al. (2016) highlight, marmosets pose the great advantage of primarily breeding twins, which allows the direct comparison of subjects with the same genetic background after being placed in different experimental conditions. What remains to be studied is how cognitive functions are acquired in the marmoset in relation to brain development in order to inform our understanding of the emergence of neuropsychiatric disorders.
In humans, the development of cognitive functions, including reversal learning and attentional set‐shifting occur at different ages (Luciana, 2003; Davidson et al., 2006) and are affected by early life experiences and the home environment (Sarsour et al., 2011). Unraveling the genetic and environmental contribution to this development is difficult in humans because of the complexity of, and the lack of experimental control over, their environment. In the marmoset, the relationship between the development of these cognitive processes and their underlying neural circuit can be determined in a highly controlled environment. Taking advantage of their quick progression from infancy to adulthood, the common problem of high attrition rates in human studies of development (Barnett, 1995) and the impossibility of following subjects over a period of twenty years can be overcome. Young marmosets can be assessed with CANTAB tasks using a home‐cage apparatus that allows testing in a relaxed environment (Crofts et al., 1999; Takemoto et al., 2011) while neuroimaging will be used to determine brain alterations across the different developmental stages. These aspects of development can also be studied as predictors of anxious phenotypes in adulthood in conjunction with genetic variations, which would provide a rich dataset for studying the development of neuropsychiatric disorders.
CONCLUSIONS
In conclusion, the marmoset is proving a valuable species in which to study many of the symptoms of neuropsychiatric disorders associated with dysfunction within cognitive, negative and positive valence systems (Fig. 1). In the marmoset, a range of tests have been developed which are designed to dissect out the behavioral dimensions that underlie these symptoms. Some of these dimensions are already being successfully mapped on to specific prefrontal circuits and neurochemical pathways within the marmoset using a range of neurobiological techniques including temporary or permanent brain manipulations, microdialysis, microPET, and structural MRI. The new generation of viral mediated tools for targeting chemically specific neural pathways, including opto‐ and pharmacogenetics, in combination with fluorodeoxyglucose and receptor ligand based microPET and high field MRI for measuring functional and resting state activity will reveal interactions between and within cognitive and emotional circuits. While marmosets may not replace Old World macaques for studying certain aspects of higher‐order executive functioning, their compact, but highly developed primate brains, small body size and thus ease of keeping large groups of marmosets in spacious accommodation make them the ideal primate for large scale research programs investigating cortical–subcortical circuit interactions. Moreover, their relatively short 5 month gestation and 2 year period of development provides a major opportunity to determine the effects of genetic and behavioral risk factors for neuropsychiatric disorders on the development of these neurocognitive circuits and neurochemical modulatory pathways across childhood and adolescence, in order to understand their impact on complex cognitive and emotional behaviors.
This work was supported by long term funding from the MRC (ACR) and the Wellcome Trust (TWR) and was performed within the Behavioral and Clinical Neuroscience Institute, University of Cambridge, funded jointly by the Wellcome Trust and MRC. LO is supported by an MRC studentship. We thank Stacey Jackson, Ian Bolton and Adrian Newman for advice on illustration and figure formatting.
Contract grant sponsors: MRC (ACR) and the Wellcome Trust (TWR).
REFERENCES
- Abbott DH, Hearn JP. 1978. Physical, hormonal and behavioural aspects of sexual development in the marmoset monkey, Callithrix jacchus. Reproduction 53:155–166. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz‐Darken NJ. 2003. Aspects of common marmoset basic biology and life history important for biomedical research. Comp Med 53:339–350. [PubMed] [Google Scholar]
- Abbruzzese M, Ferri S, Scarone S. 1995. Wisconsin Card Sorting Test performance in obsessive‐compulsive disorder: No evidence for involvement of dorsolateral prefrontal cortex. Psychiatry Res 58:37–43. [DOI] [PubMed] [Google Scholar]
- Abbruzzese M, Ferri S, Scarone S. 1997. The selective breakdown of frontal functions in patients with obsessive–compulsive disorder and in patients with schizophrenia: A double dissociation experimental finding. Neuropsychologia 35:907–912. [DOI] [PubMed] [Google Scholar]
- Agustín‐Pavón C, Braesicke K, Shiba Y, Santangelo AM, Mikheenko Y, Cockroft G, Asma F, Clarke H, Man M‐S, Roberts AC. 2012. Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol Psychiatry 72:266–272. [DOI] [PubMed] [Google Scholar]
- Airaksinen E, Larsson M, Forsell Y. 2005. Neuropsychological functions in anxiety disorders in population‐based samples: Evidence of episodic memory dysfunction. J Psychiatr Res 39:207–214. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. 1987. Taste and smell perception in depression. Biol Psychiatry 22:1481–1485. [DOI] [PubMed] [Google Scholar]
- Arai S, Okamoto Y, Fujioka T, Inohara K, Ishitobi M, Matsumura Y, Jung M, Kawamura K, Takiguchi S, Tomoda A, et al. 2015. Altered frontal pole development affects self‐generated spatial working memory in ADHD. Brain Dev 38:471–480. [DOI] [PubMed] [Google Scholar]
- Arnaudova I, Krypotos A‐M, Effting M, Boddez Y, Kindt M, Beckers T. 2013. Individual differences in discriminatory fear learning under conditions of ambiguity: A vulnerability factor for anxiety disorders?. Front Psychol 4:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman‐Rakic PS. 1984. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res 306:9–18. [DOI] [PubMed] [Google Scholar]
- Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, Crombie I. 2005. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: A meta‐analysis. Ann Fam Med 3:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock JC, Michie PT, Rock D. 2005. Spatial working memoryand planning ability: Contrasts between schizophreniaand bipolar i disorder. Cortex 41:753–763. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Leung BK, Ostlund SB. 2011. The orbitofrontal cortex, predicted value, and choice. Ann N Y Acad Sci 1239:43–50. [DOI] [PubMed] [Google Scholar]
- Bar‐Haim Y, Lamy D, Pergamin L, Bakermans‐Kranenburg MJ, van IJzendoorn MH. 2007. Threat‐related attentional bias in anxious and nonanxious individuals: A meta‐analytic study. Psychol Bull 133:1–24. [DOI] [PubMed] [Google Scholar]
- Barnett WS. 1995. Long‐term effects of early childhood programs on cognitive and school outcomes. Futur Child 5:25. [PubMed] [Google Scholar]
- Beatty WW, Monson N. 1990. Problem solving in parkinson's disease: Comparison of performance on the wisconsin and california card sorting tests. J Geriatr Psychiatry Neurol 3:163–171. [DOI] [PubMed] [Google Scholar]
- Bedard A‐C, Martinussen R, Ickowicz A, Tannock R. 2004. Methylphenidate improves visual‐spatial memory in children with attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 43:260–268. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JYF, Guyer AE, McClure‐Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen H‐U, Leibenluft E, et al. 2009. Common and distinct amygdala‐function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry 66:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. 2001. Measuring normal and pathological anxiety‐like behaviour in mice: A review. Behav Brain Res 125:141–149. [DOI] [PubMed] [Google Scholar]
- Berg EA. 1948. A simple objective technique for measuring flexibility in thinking. J Gen Psychol 39:15–22. [DOI] [PubMed] [Google Scholar]
- Berlin I, Givry‐Steiner L, Lecrubier Y, Puech AJ. 1998. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry 13:303–309. [DOI] [PubMed] [Google Scholar]
- Bermudez MA, Schultz W. 2010. Reward magnitude coding in primate amygdala neurons. J Neurophysiol 104:3424–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. 1998. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience?. Brain Res Rev 28:309–369. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. 2000. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20:4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. 2007. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci 11:307–316. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. 2008. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci 28:11124–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Falla SJ, Hamilton LR. 1998. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cogn Emot 12:737–753. [Google Scholar]
- Braesicke K, Parkinson JA, Reekie Y, Man M‐S, Hopewell L, Pears A, Crofts H, Schnell CR, Roberts AC. 2005. Autonomic arousal in an appetitive context in primates: A behavioural and neural analysis. Eur J Neurosci 21:1733–1740. [DOI] [PubMed] [Google Scholar]
- Braun K, Schultz‐Darken N, Schneider M, Moore CF, Emborg ME. 2015. Development of a novel postnatal neurobehavioral scale for evaluation of common marmoset monkeys. Am J Primatol 77:401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Hariri AR. 2006. Neuroimaging studies of serotonin gene polymorphisms: Exploring the interplay of genes, brain, and behavior. Cogn Affect Behav Neurosci 6:44–52. [DOI] [PubMed] [Google Scholar]
- Buchy L, Hawco C, Joober R, Malla A, Lepage M. 2015. Cognitive insight in first‐episode schizophrenia: Further evidence for a role of the ventrolateral prefrontal cortex. Schizophr Res 166:65–68. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. 2014. Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cereb Cortex 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbridge JA, Barch DM. 2007. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol 116:30–42. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Rosa MGP. 2009. Architectural subdivisions of medial and orbital frontal cortices in the marmoset monkey (Callithrix jacchus). J Comp Neurol 514:11–29. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Palmer SM, Gamberini M, Rosa MGP. 2006. Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix jacchus), and their projections to dorsal visual areas. J Comp Neurol 495:149–172. [DOI] [PubMed] [Google Scholar]
- Cañas J, Quesada JF, Antolí A, Fajardo I. 2003. Cognitive flexibility and adaptability to environmental changes in dynamic complex problem‐solving tasks. Ergonomics 46:482–501. [DOI] [PubMed] [Google Scholar]
- Canavan AGM, Passingham RE, Marsden CD, Quinn N, Wyke M, Polkey CE. 1989. The performance on learning tasks of patients in the early stages of Parkinson's disease. Neuropsychologia 27:141–156. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch K‐P. 2007. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat Neurosci 10:1103–1109. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Klaver JM, Peck SA, Rallis‐Voak D, Erickson K, Drevets WC. 2009. Dopamine type‐1 receptor binding in major depressive disorder assessed using positron emission tomography and [11C]NNC‐112. Neuropsychopharmacology 34:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. 2002. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. 1996. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371:179–207. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. 2010. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Focus (Madison) 8:398–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga‐Barke EJS, Milham MP, Tannock R. 2006. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci 10:117–123. [DOI] [PubMed] [Google Scholar]
- Chalmers NR, Locke‐Haydon J. 1984. Correlations among measures of playfulness and skillfulness in captive common marmosets (Callithrix jacchus jacchus). Dev Psychobiol 17:191–208. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. 2005. The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev 29:399–419. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. 2006. Motor inhibition and cognitive flexibility in obsessive‐compulsive disorder and trichotillomania. Am J Psychiatry 163:1282–1284. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. 2007. A neuropsychological comparison of obsessive‐compulsive disorder and trichotillomania. Neuropsychologia 45:654–662. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, et al. 2008. Orbitofrontal dysfunction in patients with obsessive‐compulsive disorder and their unaffected relatives. Science 321:421–422. [DOI] [PubMed] [Google Scholar]
- Chase HW, Clark L, Sahakian BJ, Bullmore ET, Robbins TW. 2008. Dissociable roles of prefrontal subregions in self‐ordered working memory performance. Neuropsychologia 46:2650–2661. [DOI] [PubMed] [Google Scholar]
- Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, Sahakian BJ. 2007. Association between response inhibition and working memory in adult ADHD: A link to right frontal cortex pathology?. Biol Psychiatry 61:1395–1401. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. 2004. Cognitive inflexibility after prefrontal serotonin depletion. Science 304:878–880. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. 2005. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci 25:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. 2007. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 17:18–27. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. 2008. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci 28:10972–10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. 2011. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci 31:4290–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Horst NK, Roberts AC. 2015. Regional inactivations of primate ventral prefrontal cortex reveal two distinct mechanisms underlying negative bias in decision making. Proc Natl Acad Sci U S A 112:4176–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJG, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron J‐C, Fryer TD, et al. 2009. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci 29:4690–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P, Roberts AC, Dias R, Everitt BJ, Robbins TW. 1998. Perseveration and strategy in a novel spatial self‐ordered sequencing task for nonhuman primates: Effects of excitotoxic lesions and dopamine depletions of the prefrontal cortex. J Cogn Neurosci 10:332–354. [DOI] [PubMed] [Google Scholar]
- Constans JI, Penn DL, Ihen GH, Hope DA. 1999. Interpretive biases for ambiguous stimuli in social anxiety. Behav Res Ther 37:643–651. [DOI] [PubMed] [Google Scholar]
- Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. 2009. What is an anxiety disorder?. Depress Anxiety 26:1066–1085. [DOI] [PubMed] [Google Scholar]
- Crofts HS, Muggleton NG, Bowditch AP, Pearce PC, Nutt DJ, Scott EA. 1999. Home cage presentation of complex discrimination tasks to marmosets and rhesus monkeys. Lab Anim 33:207–214. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. 2012. A review of fronto‐striatal and fronto‐cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex 48:194–215. [DOI] [PubMed] [Google Scholar]
- MC Davidson, D Amso, LC Anderson, A Diamond. 2006. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 44:2037–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Woodside DB. 2002. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Compr Psychiatry 43:189–194. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. 2010. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35:105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. 2011. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention‐deficit/hyperactivity disorder. Biol Psychiatry 69:e145–e157. [DOI] [PubMed] [Google Scholar]
- Delahunty A, Morice R, Frost B. 1993. Specific cognitive flexibility rehabilitation in schizophrenia. Psychol Med 23:221–227. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MFS, Bannerman DM. 2005. Differential involvement of serotonin and dopamine systems in cost‐benefit decisions about delay or effort. Psychopharmacology (Berl) 179:587–596. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. 2002. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, primates) and analysis of its effects on early development. Biol Psychiatry 52:1037–1046. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Schnell CR, Maier C, Feldon J, Pryce CR. 2007. Behavioral and physiological effects of an infant‐neglect manipulation in a bi‐parental, twinning primate: Impact is dependent on familial factors. Psychoneuroendocrinology 32:331–349. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. 1996a. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110:872–886. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. 1996b. Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380:69–72. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov‐Polevoy AB, Gallop R, Garbutt JC. 2010. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety 27:859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson JM. 2006. Perceived consequences underlying approach goals and avoidance goals in relation to anxiety. Pers Individ Dif 41:1527–1538. [Google Scholar]
- Dowson JH, McLean A, Bazanis E, Toone B, Young S, Robbins TW, Sahakian BJ. 2004. Impaired spatial working memory in adults with attention‐deficit/hyperactivity disorder: Comparisons with performance in adults with borderline personality disorder and in control subjects. Acta Psychiatr Scand 110:45–54. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. 2008. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. 2013. Animal models of working memory: A review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev 37:2111–2124. [DOI] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, van den Hout MA, Baas JMP. 2015. Updated meta‐analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety 32:239–253. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. 2007. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti I‐M, Yang Y, Ulug AM, Casey B. 2003. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 53:871–878. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Paré D. 2009. The bed nucleus of the stria terminalis mediates inter‐individual variations in anxiety and fear. J Neurosci 29:10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D. 2015. Fear generalization in humans: Systematic review and implications for anxiety disorder research. Behav Ther 46:561–582. [DOI] [PubMed] [Google Scholar]
- Elliott R, McKenna PJ, Robbins TW, Sahakian BI. 1995. Specific neuropsychological deficits in schizophrenic patients with preserved intellectual function. Cogn Neuropsychiatry 25:619–630. [Google Scholar]
- Etkin A, Wager TD. 2007. Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J, Lavoie K, Gagnon JF, Gosselin N. 2001. Performance of patients with schizophrenia on the Wisconsin Card Sorting Test (WCST). J Psychiatry Neurosci 26:123–130. [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. 1983. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry 40:79–84. [DOI] [PubMed] [Google Scholar]
- Feng C, Luo Y‐J, Krueger F. 2015. Neural signatures of fairness‐related normative decision making in the ultimatum game: A coordinate‐based meta‐analysis. Hum Brain Mapp 36:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenelle L, Marques C, Engelhardt E, Versiani M. 2001. Impaired set‐shifting ability and therapeutic response in obsessive‐compulsive disorder. J Neuropsychiatry Clin Neurosci 13:508–510. [DOI] [PubMed] [Google Scholar]
- Fried R, Hirshfeld‐Becker D, Petty C, Batchelder H, Biederman J. 2015. How informative is the cantab to assess executive functioning in children with ADHD? A controlled study. J Atten Disord 19:468–475. [DOI] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. 2009. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophr Res 115:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. 2014. Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both?. J Abnorm Psychol 123:771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Papmeyer M, Morein‐Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, de Wit S. 2011. Disruption in the balance between goal‐directed behavior and habit learning in obsessive‐compulsive disorder. Am J Psychiatry 168:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Korotzer A, Su G. 2014. Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: Pooled analysis of placebo‐controlled trials. CNS Spectr 7:40–44. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. 2004. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol 113:121–135. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. 2000. Toward a neurobiology of obsessive‐compulsive disorder. Neuron 28:343–347. [DOI] [PubMed] [Google Scholar]
- Green MF. 1996. What are the functional consequences of neurocognitive deficits in schizophrenia?. Am J Psychiatry 153:321–330. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. 2013. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure‐Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, et al. 2008. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 65:1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. 2006. Fractionating attentional control using event‐related fMRI. Cereb Cortex 16:1679–1689. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Cowen PJ. 2013. "It"s the way that you look at it’–a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc Lond B Biol Sci 368:20120407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. 2006. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59:816–820. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. 2007. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol 116:268–278. [DOI] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. 2009. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto‐striatal brain activation. Proc Natl Acad Sci U S A 106:22445–22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. 2008. Switching on and off fear by distinct neuronal circuits. Nature 454:600–606. [DOI] [PubMed] [Google Scholar]
- Hirsch C, Mathews A. 1997. Interpretative inferences when reading about emotional events. Behav Res Ther 35:1123–1132. [PubMed] [Google Scholar]
- Holland G. 1999. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci 3:65–73. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. 2010. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry 167:748–751. [DOI] [PubMed] [Google Scholar]
- Isella V, Iurlaro S, Piolti R, Ferrarese C, Frattola L, Appollonio I, Melzi P, Grimaldi M. 2003. Physical anhedonia in Parkinson's disease. J Neurol Neurosurg Psychiatry 74:1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. 2005. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci 25:8534–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi A, Tsuchida J, Yamaguchi C. 2012. Effects of rearing conditions on early visual development in common marmosets. Dev Psychobiol 54:700–705. [DOI] [PubMed] [Google Scholar]
- Jones PB. 2013. Adult mental health disorders and their age at onset. Br J Psychiatry Suppl 54:s5–s10. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. 1972. Limbic lesions and the problem of stimulus—Reinforcement associations. Exp Neurol 36:362–377. [DOI] [PubMed] [Google Scholar]
- Joyce E. 2002. Executive dysfunction in first‐episode schizophrenia and relationship to duration of untreated psychosis: The West London Study. Br J Psychiatry 181:38s–344. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Hutton SB, Mutsatsa SH, Barnes TRE. 2005. Cognitive heterogeneity in first‐episode schizophrenia. Br J Psychiatry 187:516–522. [DOI] [PubMed] [Google Scholar]
- Kalin N, Shelton S. 1989. Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science 243:1718–1721. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. 2007. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry 62:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G, Rogers LJ. 2006. Head‐cocking as a form of exploration in the common marmoset and its development. Dev Psychobiol 48:551–560. [DOI] [PubMed] [Google Scholar]
- RS Keefe, SG Silva, DO Perkins, JA Lieberman. 1999. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull 25:201–222. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. 2005. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 58:843–853. [DOI] [PubMed] [Google Scholar]
- Keers R, Uher R, Huezo‐Diaz P, Smith R, Jaffee S, Rietschel M, Henigsberg N, Kozel D, Mors O, Maier W, et al. 2011. Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. Pharmacogenomics J 11:138–145. [DOI] [PubMed] [Google Scholar]
- Kempton S, Vance A, Maruff P, Luk E, Costin J, Pantelis C. 1999. Executive function and attention deficit hyperactivity disorder: Stimulant medication and better executive function performance in children. Psychol Med 29:527–538. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar‐Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Üstün TB, Wang PS. 2009. The global burden of mental disorders: An update from the WHO World Mental Health (WMH) Surveys. Epidemiol Psichiatr Soc 18:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja IS, Westermeyer JJ, Gajwani P, Feinstein RE. 2009. Depression and coronary artery disease: The association, mechanisms, and therapeutic implications. Psychiatry (Edgmont) 6:38–51. [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun‐Todd DA. 2005. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport 16:1671–1675. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. 2002. Training of working memory in children with ADHD. J Clin Exp Neuropsychol 24:781–791. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, Gillberg CG, Forssberg H, Westerberg H. 2005. Computerized training of working memory in children with ADHD–a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 44:177–186. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. 2009. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 201:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JYF, Lissek S, Nelson EE, Lee Y, Roberson‐Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. 2008. Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. J Am Acad Child Adolesc Psychiatry 47:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Wooderson S, Mataix‐Cols D, David R, Speckens A, Phillips ML. 2006. Decision making and set shifting impairments are associated with distinct symptom dimensions in obsessive‐compulsive disorder. Neuropsychology 20:409–419. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. 2000. Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. 2005. Working memory impairments in schizophrenia: A meta‐analysis. J Abnorm Psychol 114:599–611. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TRE, Joyce EM. 2009. Discrimination learning, reversal, and set‐shifting in first‐episode schizophrenia: Stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry 66:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Delorme A, Walshaw PD, Cho AL, Bilder RM, McGough JJ, McCracken JT, Makeig S, Loo SK. 2014. Electroencephalography correlates of spatial working memory deficits in attention‐deficit/hyperactivity disorder: Vigilance, encoding, and maintenance. J Neurosci 34:1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K‐P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. 1996. Association of anxiety‐related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531. [DOI] [PubMed] [Google Scholar]
- Lissek S. 2012. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear‐learning: The case for conditioned overgeneralization. Depress Anxiety 29:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, Grillon C. 2008. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry 165:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. 2014. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry 75:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. 2003. Practitioner Review: Computerized assessment of neuropsychological function in children: Clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). J Child Psychol Psychiatry 44:649–663. [DOI] [PubMed] [Google Scholar]
- Lysaker P, Bell M, Beam‐Goulet J. 1995. Wisconsin card sorting test and work performance in schizophrenia. Psychiatry Res 56:45–51. [DOI] [PubMed] [Google Scholar]
- MacDougall M, Nummela SU, Coop S, Disney A, Mitchell JF, Miller CT. 2016. Optogenetic manipulation of neural circuits in awake marmosets. J Neurophysiol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. 2008. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology 33:926–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ, Little L. 1969. Intradimensional and extradimensional shift learning by pigeons. Psychon Sci 14:5–6. [Google Scholar]
- Malmo HP. 1974. On frontal lobe functions: Psychiatric patient controls. Cortex 10:231–237. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. 2002. Decision‐making processes following damage to the prefrontal cortex. Brain 125:624–639. [DOI] [PubMed] [Google Scholar]
- Manoach DS. 2003. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophr Res 60:285–298. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Dew MA, Mulsant BH, Begley AE, Tracey B, Shear MK, Reynolds CF, Lenze EJ. 2007. Cognitive impairment in late‐life generalized anxiety disorder. Am J Geriatr Psychiatry 15:673–679. [DOI] [PubMed] [Google Scholar]
- Maren S. 2001. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. 1991. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4:17–26. [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg‐Johnson S, Tannock R. 2005. A meta‐analysis of working memory impairments in children with attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 44:377–384. [DOI] [PubMed] [Google Scholar]
- Marumo K, Takizawa R, Kinou M, Kawasaki S, Kawakubo Y, Fukuda M, Kasai K. 2014. Functional abnormalities in the left ventrolateral prefrontal cortex during a semantic fluency task, and their association with thought disorder in patients with schizophrenia. Neuroimage 85 Pt 1:518–526. [DOI] [PubMed] [Google Scholar]
- Mashiko H, Yoshida AC, Kikuchi SS, Niimi K, Takahashi E, Aruga J, Okano H, Shimogori T. 2012. Comparative anatomy of marmoset and mouse cortex from genomic expression. J Neurosci 32:5039–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld AT, Whitfield‐Gabrieli S, Biederman J, Spencer T, Brown A, Fried R, Gabrieli JDE. 2015. Dissociation of working memory impairments and attention‐deficit/hyperactivity disorder in the brain. NeuroImage Clin 10:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland BR, Klein DN. 2009. Emotional reactivity in depression: Diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety 26:117–122. [DOI] [PubMed] [Google Scholar]
- McKirdy J, Sussmann JED, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. 2009. Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med 39:1289–1293. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ. 2004. Methylphenidate improves working memory and set‐shifting in AD/HD: Relationships to baseline memory capacity. J Child Psychol Psychiatry 45:293–305. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. 2008. Integrating evidence from neuroimaging and neuropsychological studies of obsessive‐compulsive disorder: The orbitofronto‐striatal model revisited. Neurosci Biobehav Rev 32:525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheenko Y, Shiba Y, Sawiak S, Braesicke K, Cockcroft G, Clarke H, Roberts AC. 2015. Serotonergic, brain volume and attentional correlates of trait anxiety in primates. Neuropsychopharmacology 40:1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. 2007. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci 1121:546–561. [DOI] [PubMed] [Google Scholar]
- Milner B. 1963. Effects of different brain lesions on card sorting. Arch Neurol 9:90. [Google Scholar]
- AL Mishara, TE Goldberg. 2004. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biol Psychiatry 55:1013–1022. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. 2005. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10:79–104. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. 2005. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognit Ther Res 29:29–45. [Google Scholar]
- Mogg K, Bradley BP, Dixon C, Fisher S, Twelftree H, McWilliams A. 2000. Trait anxiety, defensiveness and selective processing of threat: An investigation using two measures of attentional bias. Pers Individ Dif 28:1063–1077. [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJR, Chen G, Charney DS, Ernst M, et al. 2006. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry 163:1091–1097. [DOI] [PubMed] [Google Scholar]
- Morgiève M, N'Diaye K, Haynes WIA, Granger B, Clair A‐H, Pelissolo A, Mallet L. 2014. Dynamics of psychotherapy‐related cerebral haemodynamic changes in obsessive compulsive disorder using a personalized exposure task in functional magnetic resonance imaging. Psychol Med 44:1461–1473. [DOI] [PubMed] [Google Scholar]
- Morice R. 1990. Cognitive inflexibility and pre‐frontal dysfunction in schizophrenia and mania. Br J Psychiatry 157:50–54. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. 1999. A subcortical pathway to the right amygdala mediating "unseen" fear. Proc Natl Acad Sci 96:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller EM, Nguyen J, Ray WJ, Borkovec TD. 2010. Future‐oriented decision‐making in Generalized Anxiety Disorder is evident across different versions of the Iowa Gambling Task. J Behav Ther Exp Psychiatry 41:165–171. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, Järvelin M‐R, Taanila A, Flint J. 2009. 5‐HTTLPR genotype and anxiety‐related personality traits: A meta‐analysis and new data. Am J Med Genet B Neuropsychiatr Genet 150B:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. 1999. Emotional bias and inhibitory control processes in mania and depression. Psychol Med 29:1307–1321. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo‐Smith I, Lawrence AD. 2003. Functional neuroanatomy of emotions: A meta‐analysis. Cogn Affect Behav Neurosci 3:207–233. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. 2009. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ, Munafò MR. 2013. The effect of the serotonin transporter polymorphism (5‐HTTLPR) on amygdala function: A meta‐analysis. Mol Psychiatry 18:512–520. [DOI] [PubMed] [Google Scholar]
- Murray EA. 2007. The amygdala, reward and emotion. Trends Cogn Sci 11:489–497. [DOI] [PubMed] [Google Scholar]
- Myers‐Schulz B, Koenigs M. 2012. Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry 17:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, et al. 2005. Brain activation of patients with obsessive‐compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biol Psychiatry 57:901–910. [DOI] [PubMed] [Google Scholar]
- Nakako T, Murai T, Ikejiri M, Ishiyama T, Taiji M, Ikeda K. 2013. Effects of a dopamine D1 agonist on ketamine‐induced spatial working memory dysfunction in common marmosets. Behav Brain Res 249:109–115. [DOI] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH. 2011. Gene–environment interactions: Early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berl) 214:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S. 2007. The other face of depression, reduced positive affect: The role of catecholamines in causation and cure. J Psychopharmacol 21:461–471. [DOI] [PubMed] [Google Scholar]
- Okasha A, Rafaat M, Mahallawy N, Nahas J, Seif El Dawla A, Sayed M, El Kholi S. 2000. Cognitive dysfunction in obsessive?compulsive disorder. Acta Psychiatr Scand 101:281–285. [PubMed] [Google Scholar]
- Ongur D, Price JL. 2000. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. 2004. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci 8:315–324. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Piras F, Macci E, Caltagirone C, Spalletta G. 2013. The neuroanatomical correlates of cognitive insight in schizophrenia. Soc Cogn Affect Neurosci 8:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. 1990. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28:1021–1034. [DOI] [PubMed] [Google Scholar]
- Pacella ML, Hruska B, Delahanty DL. 2013. The physical health consequences of PTSD and PTSD symptoms: A meta‐analytic review. J Anxiety Disord 27:33–46. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. 1999. Comparison of set‐shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res 37:251–270. [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. 2010. A meta‐analysis of the magnitude of biased attention in depression. Depress Anxiety 27:1135–1142. [DOI] [PubMed] [Google Scholar]
- Pelizza L, Ferrari A. 2009. Anhedonia in schizophrenia and major depression: State or trait?. Ann Gen Psychiatry 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna G, Favaron E, Di Bella D, Bussi R, Bellodi L. 2005. Antipanic efficacy of paroxetine and polymorphism within the promoter of the serotonin transporter gene. Neuropsychopharmacology 30:2230–2235. [DOI] [PubMed] [Google Scholar]
- Petrides M. 1995a. Impairments on nonspatial self‐ordered and externally ordered working memory tasks after lesions of the mid‐dorsal part of the lateral frontal cortex in the monkey. J Neurosci 15:359–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. 1995b. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci 769:85–96. [DOI] [PubMed] [Google Scholar]
- Petrides M. 2005. Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 360:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2002. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16:291–310. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2007. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci 27:11573–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. 2012. The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex 48:46–57. [DOI] [PubMed] [Google Scholar]
- Piper MC, Pinnell LE, Darrah J, Maguire T, Byrne PJ. 1992. Construction and validation of the Alberta Infant Motor Scale (AIMS). Can J Public Health 83 Suppl 2:S46–S50. [PubMed] [Google Scholar]
- Pirkola T, Tuulio‐Henriksson A, Glahn D, Kieseppä T, Haukka J, Kaprio J, Lönnqvist J, Cannon TD. 2005. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biol Psychiatry 58:930–936. [DOI] [PubMed] [Google Scholar]
- Pistorio AL, Vintch B, Wang X. 2006. Acoustic analysis of vocal development in a New World primate, the common marmoset (Callithrix jacchus). J Acoust Soc Am 120:1655–1670. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Sahgal A, Steckler T. 1996. Further developments in the measurement of working memory in rodents. Cogn Brain Res 3:205–213. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Fabbri C, Serretti A. 2012. Meta‐analysis of serotonin transporter gene promoter polymorphism (5‐HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol 22:239–258. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. 2010. Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. 2004. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry 56:72–79. [DOI] [PubMed] [Google Scholar]
- Pu S, Nakagome K, Yamada T, Itakura M, Satake T, Ishida H, Nagata I, Kaneko K. 2013. Association between cognitive insight and prefrontal function during a cognitive task in schizophrenia: A multichannel near‐infrared spectroscopy study. Schizophr Res 150:81–87. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, Pantelis C. 1998. Cognitive deficits in obsessive–compulsive disorder on tests of frontal–striatal function. Biol Psychiatry 43:348–357. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. 2009. Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nat Neurosci 12:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reekie YL, Braesicke K, Man MS, Roberts AC. 2008. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc Natl Acad Sci U S A 105:9787–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold JA, Mandos LA, Rickels K, Lohoff FW. 2011. Pharmacological treatment of generalized anxiety disorder. Expert Opin Pharmacother 12:2457–2467. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, van den Heuvel OA, Nielen MMA, Vriend C, Hendriks G‐J, Hoogendijk WJG, Uylings HBM, Veltman DJ. 2013. Cognitive inflexibility in obsessive‐compulsive disorder and major depression is associated with distinct neural correlates. PLoS One 8:e59600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K‐Y, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. 2009. Interaction between the serotonin transporter gene (5‐HTTLPR), stressful life events, and risk of depression: A meta‐analysis. JAMA 301:2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. 1992. Functions of dopamine in the dorsal and ventral striatum. Semin Neurosci 4:119–127. [Google Scholar]
- Roberts AC, Robbins TW, Everitt BJ. 1989. The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non‐human primates. Q J Exp Psychol 40:321–341. [PubMed] [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 1994. 6‐Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. J Neurosci 14:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. 2012. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry 169:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD. 2014. Cognitive impairment in depression: A systematic review and meta‐analysis. Psychol Med 44:2029–2040. [DOI] [PubMed] [Google Scholar]
- Rolland B, Amad A, Poulet E, Bordet R, Vignaud A, Bation R, Delmaire C, Thomas P, Cottencin O, Jardri R. 2015. Resting‐state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr Bull 41:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge J‐Y, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. 2008. Provocation of obsessive‐compulsive symptoms: A quantitative voxel‐based meta‐analysis of functional neuroimaging studies. J Psychiatry Neurosci 33:405–412. [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. 2014. The orbitofrontal oracle: Cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron 84:1143–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. 2006. Separate neural pathways process different decision costs. Nat Neurosci 9:1161–1168. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SEV, Murray EA. 2014. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc Natl Acad Sci 111:5391–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Clarke HF, Cardinal RN, Cockcroft GJ, Xia J, Dalley JW, Robbins TW, Roberts AC. 2015. Role of central serotonin in anticipation of rewarding and punishing outcomes: Effects of selective amygdala or orbitofrontal 5‐ht depletion. Cereb Cortex 25:3064–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J. 1997. Behavioral functions of nucleus accumbens dopamine: Empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev 21:341–359. [DOI] [PubMed] [Google Scholar]
- Santangelo AM, Ito M, Shiba Y, Clarke HF, Schut EH, Cockcroft G, Ferguson‐Smith AC, Roberts AC. 2016. Novel primate model of serotonin transporter genetic polymorphisms associated with gene expression, anxiety and sensitivity to antidepressants. Neuropsychopharmacology 41:2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru‐Jeter A, Hinshaw S, Boyce WT. 2011. Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. J Int Neuropsychol Soc 17:120–132. [DOI] [PubMed] [Google Scholar]
- Saxena S, Gorbis E, O'Neill J, Baker SK, Mandelkern MA, Maidment KM, Chang S, Salamon N, Brody AL, Schwartz JM, et al. 2009. Rapid effects of brief intensive cognitive‐behavioral therapy on brain glucose metabolism in obsessive‐compulsive disorder. Mol Psychiatry 14:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Huys QJM, Deserno L, Rapp MA, Beck A, Heinze H‐J, Dolan R, Heinz A. 2014. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage 89:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. 2005. Orbitofrontal cortex, associative learning, and expectancies. Neuron 47:633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Stalnaker TA. 2007. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Ann N Y Acad Sci 1121:320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. 2004. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol 14:139–147. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. 2000. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex 10:272–283. [DOI] [PubMed] [Google Scholar]
- Schultz W, Abe H, Lee D, Adamantidis A, Tsai H, Boutrel B, Zhang F, Stuber G, Budygin E, Touriño C, et al. 2015. Neuronal reward and decision signals: From theories to data. Physiol Rev 95:853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz‐Darken N, Braun KM, Emborg ME. 2016. Neurobehavioral development of common marmoset monkeys. Dev Psychobiol 58:141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehry AA, Potvin S, Élie R, Stip E. 2007. Selective serotonin reuptake inhibitor (ssri) add‐on therapy for the negative symptoms of schizophrenia. J Clin Psychiatry 68:604–610. [DOI] [PubMed] [Google Scholar]
- Sexton KA, Norton PJ, Walker JR, Norton GR. 2010. Hierarchical model of generalized and specific vulnerabilities in anxiety. Cogn Behav Ther 32:82–94. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tomarken AJ. 2001. Can recovery from depression be achieved?. Psychiatr Serv 52:1469–1478. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Kim C, Santangelo AM, Roberts AC. 2014a. Lesions of either anterior orbitofrontal cortex or ventrolateral prefrontal cortex in marmoset monkeys heighten innate fear and attenuate active coping behaviors to predator threat. Front Syst Neurosci 8:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Santangelo AM, Braesicke K, Agustín‐Pavón C, Cockcroft G, Haggard M, Roberts AC. 2014b. Individual differences in behavioral and cardiovascular reactivity to emotive stimuli and their relationship to cognitive flexibility in a primate model of trait anxiety. Front Behav Neurosci 8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Santangelo AM, Roberts AC. 2016. Beyond the medial regions of prefrontal cortex in the regulation of fear and anxiety. Front Syst Neurosci 10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. 2006. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071:67–79. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Rosen W, Huppert JD, Lin S‐H, Foa EB, Liebowitz MR. 2006. Are there reliable neuropsychological deficits in obsessive‐compulsive disorder?. J Psychiatr Res 40:247–257. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Cryan JF. 2007. Evaluation of reward processes in an animal model of depression. Psychopharmacology (Berl) 190:555–568. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, Krishnan RR. 2008. Decision‐making and risk aversion among depressive adults. J Behav Ther Exp Psychiatry 39:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. 1995. A scale for the assessment of hedonic tone the Snaith‐Hamilton Pleasure Scale. Br J Psychiatry 167:99–103. [DOI] [PubMed] [Google Scholar]
- Stapelberg NJ, Hamilton‐Craig I, Neumann DL, Shum DH, McConnell H. 2012. Mind and heart: Heart rate variability in major depressive disorder and coronary heart disease ‐ a review and recommendations. Aust N Z J Psychiatry 46:946–957. [DOI] [PubMed] [Google Scholar]
- Staring ABP, Van der Gaag M, Van den Berge M, Duivenvoorden HJ, Mulder CL. 2009. Stigma moderates the associations of insight with depressed mood, low self‐esteem, and low quality of life in patients with schizophrenia spectrum disorders. Schizophr Res 115:363–369. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Mizrahi R, Garau L. 2005. Specificity of symptoms of depression in alzheimer disease: A longitudinal analysis. Am J Geriatr Psychiatry 13:802–807. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, Adler CM. 2005. The functional neuroanatomy of bipolar disorder: A review of neuroimaging findings. Mol Psychiatry 10:105–116. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Bitter SM, Weber WA, Chu W‐J, Whitsel RM, Adler C, Cerullo MA, Eliassen J, Strakowski SM, DelBello MP. 2012. Neurocircuitry of generalized anxiety disorder in adolescents: A pilot functional neuroimaging and functional connectivity study. Depress Anxiety 29:939–947. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Dohm K, Kugel H, Zwanzger P, Redlich R, Grotegerd D, Rauch AV, Arolt V, Heindel W, Suslow T, et al. 2013. Mood‐congruent amygdala responses to subliminally presented facial expressions in major depression: Associations with anhedonia. J Psychiatry Neurosci 38:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. 2008. The sunny side of fairness: Preference for fairness activates reward circuitry (and disregarding unfairness activates self‐control circuitry). Psychol Sci 19:339–347. [DOI] [PubMed] [Google Scholar]
- Takemoto A, Izumi A, Miwa M, Nakamura K. 2011. Development of a compact and general‐purpose experimental apparatus with a touch‐sensitive screen for use in evaluating cognitive functions in common marmosets. J Neurosci Methods 199:82–86. [DOI] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. 2008. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage 42:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Wiebel B, Daum I. 2007. Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophr Res 92:168–180. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. 2015. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. 2011. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev 35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trémeau F, Antonius D, Cacioppo JT, Ziwich R, Butler P, Malaspina D, Javitt DC. 2010. Anticipated, on‐line and remembered positive experience in schizophrenia. Schizophr Res 122:199–205. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Baldwin D. 2006. Generalised anxiety disorder. Lancet (London, England) 368:2156–2166. [DOI] [PubMed] [Google Scholar]
- Vaghi MM, Vértes PE, Kitzbichler MG, Apergis‐Schoute AM, van der Flier FE, Fineberg NA, Sule A, Zaman R, Voon V, Kundu P, et al. 2016. Specific fronto‐striatal circuits for impaired cognitive flexibility and goal‐directed planning in Obsessive‐Compulsive Disorder: Evidence from resting‐state functional connectivity. Biol Psychiatry 0:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerius G, Lumpp A, Kuelz A‐K, Freyer T, Voderholzer U. 2008. Reversal learning as a neuropsychological indicator for the neuropathology of obsessive compulsive disorder? A behavioral study. J Neuropsychiatry Clin Neurosci 20:210–218. [DOI] [PubMed] [Google Scholar]
- Veale DM, Sahakian BJ, Owen AM, Marks IM. 2009. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive–compulsive disorder. Psychol Med 26:1261. [DOI] [PubMed] [Google Scholar]
- Vink M, Ramsey NF, Raemaekers M, Kahn RS. 2006. Striatal dysfunction in schizophrenia and unaffected relatives. Biol Psychiatry 60:32–39. [DOI] [PubMed] [Google Scholar]
- Walker SC, Robbins TW, Roberts AC. 2009. Response disengagement on a spatial self‐ordered sequencing task: Effects of regionally selective excitotoxic lesions and serotonin depletion within the prefrontal cortex. J Neurosci 29:6033–6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TEJ, Noonan MP, Rushworth MFS. 2011. Giving credit where credit is due: Orbitofrontal cortex and valuation in an uncertain world. Ann N Y Acad Sci 1239:14–24. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. 2007. Probabilistic reversal learning impairments in schizophrenia: Further evidence of orbitofrontal dysfunction. Schizophr Res 93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. 2015. The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev 57:271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LH, Sahakian BJ, Robertson MM, Veale DM, Rogers RD, Pickard KM, Aitken MRF, Robbins TW. 2005. Executive function in Tourette's syndrome and obsessive–compulsive disorder. Psychol Med 35:571–582. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. 1996. Prefrontal function in schizophrenia: Confounds and controversies. Philos Trans R Soc Lond B Biol Sci 351:1495–1503. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H. 2007. Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second‐order conditioning. Am J Psychiatry 164:1684–1692. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Hirvikoski T, Forssberg H, Klingberg T. 2004. Visuo‐spatial working memory span: A sensitive measure of cognitive deficits in children with ADHD. Child Neuropsychol 10:155–161. [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Liddell BJ, Palmer DM, Bryant RA. 2007. Neural biases to covert and overt signals of fear: Dissociation by trait anxiety and depression. J Cogn Neurosci 19:1595–1608. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Pantelis C, Proffitt T, Phillips LJ, Stuart GW, Buchanan JA, Mahony K, Brewer W, Smith DJ, McGorry PD. 2003. Spatial working memory ability is a marker of risk‐for‐psychosis. Psychol Med 33:1239–1247. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. 2012. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex 48:58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Kawasaki A, Hayashi T, Onoe H. 2013. Linkage between the midline cortical serotonergic system and social behavior traits: Positron emission tomography studies of common marmosets. Cereb Cortex 23:2136–2145. [DOI] [PubMed] [Google Scholar]


