Abstract
Background
Neurodegenerative and cognitive disorders are multifactorial diseases (i.e., involving neurodevelopmental, genetic, age or environmental factors) characterized by an abnormal development that affects neuronal function and integrity. Recently, an increasing number of studies revealed that the dysregulation of microRNAs (miRNAs) may be involved in the etiology of cognitive disorders as Alzheimer, Parkinson, and Huntington‘s diseases, Schizophrenia and Autism spectrum disorders.
Methods
From an extensive search in bibliographic databases of peer-reviewed research literature, we identified relevant published studies related to specific key words such as memory, cognition, neurodegenerative disorders, neurogenesis and miRNA. We then analysed, evaluated and summerized scientific evidences derived from these studies.
Results
We first briefly summarize the basic molecular events involved in memory, a process inherent to cognitive disease, and then describe the role of miRNAs in neurodevelopment, synaptic plasticity and memory. Secondly, we provide an overview of the impact of miRNA dysregulation in the pathogenesis of different neurocognitive disorders, and lastly discuss the feasibility of miRNA-based therapeutics in the treatment of these disorders.
Conclusion
This review highlights the molecular basis of neurodegenerative and cognitive disorders by focusing on the impact of miRNAs dysregulation in these pathological phenotypes. Altogether, the published reports suggest that miRNAs-based therapy could be a viable therapeutic alternative to current treatment options in the future.
Keywords: Cognitive dysfunction, LTP, MicroRNA, neurodegenerative diseases, psychiatric disorders, signaling pathways
1. INTRODUCTION
The term cognition describes the human brain’s ability to think, learn, remember and process information. Dysregulation of learning and memory mechanisms contributes to cognitive disorders in various neuropsychiatric diseases such as schizophrenia, genetic disorders such as ASD (Autism spectrum disorders) and Huntington’s disease or in neuro- degenerative diseases such as Alzheimer and Parkinson diseases. The causes of these disorders are often multiple, arising through a complex interplay between neurodevelop- ment, genetics and environmental factors or through degeneration of the aging brain. Among the molecular mechanisms involved in the regulation of cognitive processes, miRNAs have been recently proposed as interesting biomarkers and/or therapeutic targets for the treatment of these disorders. Since mRNA acts through the regulation of gene expression, a description of the main molecular events involved in memory is required to better understand how their modulation could be proposed as therapeutic approaches to restore cognitive functions. This review first recaps the molecular basis underlying synaptic plasticity and measured as long-term potentiation (LTP) and long-term depression (LTD), together with the molecular events regulating dendritic morphogenesis (number, size and shape of spines). We then review the literature to explore the role of the main miRNAs in the regulation of neurodevelopmental processes, synaptic plasticity and dendritic spine morphology, key processes involved in cognitive functions. Finally, we describe the changes in miRNA expression observed in neurological and neuro- degenerative diseases associated with cognitive deficits such as Alzheimer’s, Parkinson’s, and Huntington’s diseases, Autism spectrum disorders and Schizophrenia. We then finally discuss the perspectives and the feasibility of miRNA-based therapeutics in the treatment of these cognitive disorders.
2. MOLECULAR BASES FOR LEARNING AND MEMORY
2.1. Long Term Potentiation: Properties and Role in Mechanisms of Synaptic Plasticity
Amongst the phenomena involved in learning and memory, long term potentiation (LTP) is considered as one of the main cellular events that underlie synaptic plasticity [1]. Originally discovered in the hippocampus [2], LTP has been observed in many brain areas, such as the cerebral cortex, cerebellum or amygdala [3]. The molecular mechanisms involved in LTP depend on various factors such as the anatomical location where LTP is observed and the age of the organism studied. LTP is divided into a transient early phase (E-LTP) and a late phase (L-LTP) which are independent of and dependent on protein synthesis, respectively. E-LTP is characterized by post-translational events that modulate synaptic function whilst L-LTP requires regulated mRNA translation and new gene transcription [4, 5].
A variety of signaling pathways contribute to LTP. They include the activation of N-methyl-D-aspartate receptor (NMDAR) by glutamate released from presynaptic densities during E-LTP. It has been demonstrated that calcium influx through the NMDA channel activates the calcium/ calmodulin (Ca2+/CaM)-dependent kinase II (CaMKII) which in turn is autophosphorylated [6, 7]. By using pharmacological tools and genetic approaches, it has been demonstrated that CaMKII is responsible for two major mechanisms in early-phase LTP. It modulates synaptic function by phosphorylating AMPA receptors (amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, non-NMDA-type receptors for glutamate) and increases the density of AMPA receptors in the postsynaptic membrane by regulating the reorganization of receptors and their trafficking in the synaptic scaffolding [8, 9]. These events play a crucial role in both LTP induction and maintenance.
The late phase of LTP (L-LTP) is first regulated by the activation of constitutively expressed transcription factors and also by the synthesis of de novo transcription factors. L- LTP is induced by the lasting activation of protein kinases during E-LTP. The extracellular signal-regulated kinase (ERK) is certainly the molecular connection between E-LTP and L-LTP and ERK is activated by many proteins during E-LTP. For example, CaMKII, PKA, and PKC induce ERK activation. Therefore, the phosphorylation of this kinase has been proposed to serve as a molecular memory which underlies the maintenance of L-LTP. Upon activation, ERK phosphorylates various cytoplasmic or nuclear proteins such as the transcription factor CREB which in turn results in the expression of specific proteins such as the protein kinase Mζ (PKMζ), the brain-specific protein kinase C (PKC) isoform. This kinase plays an important role in the maintenance of long-term memory [10]. However, recent studies support a model in which the molecular mechanisms underlying memory maintenance are more complicated than the simple PKMζ-centric model as initially described [11, 12].
Recently some studies have suggested the role of miRNAs in LTP induction and also in the amplification and the diversification of late LTP events [13]. For example, the differential expression of miR-132-3p after LTP induction has been reported and has been associated with the transcriptional modification of key LTP-regulated genes [5]. This observation supports the functional significance of miRNA regulation in response to LTP induction.
2.2. Dendritic Spine Structure and Memory
Changes in dendritic spines underlie complex cognitive processes associated with learning and memory. They are closely related to synaptic function and plasticity, thereby allowing LTP consolidation, and long-term storage of memories. Spines are small, shaped membranous extensions located primarily on the dendrites of neurons [14]. Spines constantly adapt during both development and in adulthood, and are rapidly modulated by experience. Abnormal dendritic spine morphology has been observed in a number of diseases, in Schizophrenia [15], Alzheimer’s disease [16-18] or Parkinson’s disease [19] and in autism spectrum disorders [20].
These changes in the structure and/or the number of dendritic spines involve the impairment of cytoskeleton remodeling which contributes to synaptic dysfunction. Rho GTPases, a subgroup of the Ras superfamily of GTPases that are important for intracellular signaling, are considered as key proteins in membranes to regulate cytoskeletal organization, neuronal development and synaptic functions [21, 22]. These small GTPases bind GTP and hydrolyse it to GDP, and the switching between these two states, the inactive GDP bound form and the active GTP bound form, regulates downstream signaling [22]. RhoA, Rac and Cdc42 GTPases have been reported to play distinct functions in regulating dendritic spine formation and morphology [23]. Indeed, constitutively active Rac1 was shown to increase the formation of the small spines of neurons [24], whereas the expression of the dominant negative Rac1 resulted in a progressive reduction of dendritic spines in rat and mouse hippocampal slices [24, 25]. In contrast, constitutively active RhoA caused a strong simplification of dendritic branch patterns, whilst its inhibition increased the density and the length of dendritic spines [23, 25-27].
Alternatively, the Cdc42 signaling pathway is activated through the stimulation of GPCRs (G-protein coupled receptors), RTKs (receptor tyrosine kinases) and integrins [28]. Recently, it has been shown that the postnatal disruption of Cdc42 in excitatory neurons impaired synaptic plasticity and induced a reduction in the density of dendritic spines in the hippocampus, together with a pronounced deficit in remote memory performances [29]. In schizophrenia, Cdc42 mRNA levels are low and correlated with layer spine deficits in the prefrontal cortex [30, 31]. Changes in dendritic spines by modifying synaptic morphogenesis and therefore synaptic plasticity can thus dramatically impact brain function and result in cognitive deficits. Understanding how miRNAs contribute to morphological changes in neurons may provide insight into the etiology of cognitive disorders and may reveal new potential therapeutic targets [32].
2.3. The Importance of CREB Signaling in Memory
The nuclear cAMP responsive element binding protein (CREB) signaling appears to play a key role in the molecular mechanisms underlying the conversion of short-term memory to long-term memory. CREB signaling is essential in cognitive dysfunction as it is altered in Huntington’s disease, Rubinstein-Taybi and Coffin-Lowry syndromes and Alzheimer‘s disease [33]. One effector of CREB signaling is the modulation of the transcription of genes bearing cAMP responsive elements in their promoters [33]. Interestingly, by elevating cAMP and/or cGMP levels, phosphodiesterase (PDE) inhibitors have been demonstrated to improve learning and memory in a number of rodent models of impaired cognition [34]. Increases in the concentration of either calcium or cAMP can trigger the phosphorylation and activation of CREB through activation of various kinases such as the CaMKs, PKA, the Ras/ERK-dependent ribosomal S6 kinases (RSKs) and mitogen- and stress-activated protein kinases (MSKs) and ultimately induce changes in gene expression [35]. Once phosphorylated, CREB initiates the transcription of CREB’s target genes including those involved in synaptic plasticity and memory consolidation in the hippocampus such as c-FOS and BDNF [35]. Since a dysregulation of CREB signaling has been involved in the pathogenesis of human cognitive disorders [36], it could be considered as a molecular target for the treatment of these diseases.
Interestingly CREB has been linked to miRNA expression. An upregulation of miRNA-134 related to a downregulation of CREB expression has been associated with a decreased memory performance and impairment of LTP in transgenic mice. Moreover, inhibition of miR-134 restored CREB levels and rescued the plasticity impairments. Altogether, this suggests that the modulation of miRNA-134 might control synaptic plasticity and/or memory, either directly or to a certain extent [37].
2.4. Role of mTOR Signaling in Memory
Among other signaling molecules which contribute to cognitive processes, the mammalian target of rapamycin (mTOR) appears as a crucial component for many forms of synaptic plasticity such as LTP and might also play a role in the process of learning and memory [37]. mTOR is an evolutionarily conserved kinase that has emerged as one of the critical checkpoints of protein synthesis [38]. In neurons, mTOR is implicated in neuronal cytoskeletal organization (neuronal soma size, number and size of dendritic spines), axonal regeneration, and synaptic plasticity [39-41]. mTOR activity is furthermore modulated by a wide variety of signals, ranging from neurotransmitters to changes in energy metabolism status. Alteration of the gene expression of mTOR pathway components (Tsc1, Tsc2, Fkbp1a, S6k1/2, Eif4e-bp2) appears to be a common pathophysiological feature of neurodevelopmental and cognitive disorders. For example, increased levels of mTOR pathway activity have been reported in Tuberous sclerosis [42], in Alzheimer’s disease (AD) patients [43-46] and Fragile X Syndrome [47, 48]. The consequences of mTOR pathway activation include abnormally rapid cell growth and hyperactivation of mRNA translation, thereby impairing synaptic plasticity and long term memory formation. In contrast, loss of mTOR signaling has also been proposed to impair LTP and synaptic plasticity in models of Rett syndrome [49], suggesting that a slight activation of the mTOR pathway may be at least necessary to prevent the progression of these diseases.
Altogether, these data suggest that a fine tuning in the levels of mTOR activity may be crucial to maintain both neuronal homeostasis and normal synaptic plasticity. Again there is some evidence, mainly from studies on the biology of cancer, that the link between the miRNAs and the mTOR pathway may control cell proliferation, migration and invasiveness. MiRNA-99 family members (miRNA-99a, 99b and -100) and miR634 were shown to regulate expression of mTOR and cancer cell proliferation [50-53]. However, considering the importance of mTOR signaling in the processes of learning and memory, it is surprising that, to our knowledge, only one study explored the involvement of miRNAs to regulate the mTOR pathway in the pathogenesis of Rett syndrome, which is caused by mutation of the gene encoding methyl-CpG binding protein 2 (MeCP2) [54]. The authors observed a downregulation of miR-199 in the frontal cortex of RTT patients. This dysregulation was also observed in MeCP2-KO neurons and is associated with an attenuation of mTOR signaling and RTT neuronal phenotype. The expression of the precursor form of miR-199a or inhibition of its downstream targets (Pde4d, sirt1 and Hif1α) completely restored a normal neuronal phenotype. This study demonstrated a positive regulation of MiR-199 on mTOR signaling by targeting inhibitors for mTOR signaling in neurons.
3. MIRNAS IN NEURODEVELOPMENT, SYNAPTIC PLASTICITY AND MEMORY
Cognitive impairment is a frequent clinical feature observed in both neurodegenerative and neuropsychiatric diseases which affect millions of people worldwide. Therefore the management of cognitive disorders has attracted great interest because of their high impact on society. Currently, therapeutic approaches remain focused on the symptomatic treatment of these diseases and include cholinesterase inhibitors to increase cholinergic functions, neuroprotective agents such as N-methyl-D-aspartate receptor antagonist or antioxidants. However these treatments show limited to marginal efficacy with regard to improvements in cognition and memory, highlighting the importance of developing alternative therapeutic options. In this specific context, antisense oligonucleotides show some promise. For instance in Huntington’s disease, the use of antisense oligonucleotide (siRNA) directly against the mutant Huntington protein is currently being evaluated in a clinical setting [55]. However, there are several drawbacks to using an siRNA-based approach in therapeutic applications, including elicitation of the interferon response, off-target effects and interference with the endogenous miRNAs biogenesis [56-58]. In view of the ability of miRNA to tightly regulate several endogenous genes, it might be considered that a miRNA-based pharmacological therapy could open up new and largely unexplored strategies for the treatment of a wide variety of cognitive disorders. This is supported by the observation that miRNAs are highly expressed in the nervous system where they regulate important molecular events. Interestingly, changes in miRNAs have been detected in various cognitive disorders observed in neurodegenerative diseases (e.g. Huntington’s, Parkinson’s and Alzheimer’s diseases) or neurodevelopmental disorders (e.g. Schizophrenia, autism spectrum disorders) and the expression of proteins involved in these diseases (e.g., α-synuclein, sAPP, huntingtin; LRKK2) or involved in the pathways regulating the functions of neurons (neuronal morphology, synaptic plasticity, LTP) is fine-tune regulated by miRNAs [13, 59].
In the next part of this review, after a brief introduction on the biogenesis of miRNAs, we will illustrate the complex regulatory roles of miRNAs during neuronal development by describing their involvement in the control of synaptic functions, from neurotransmission to morphology.
3.1. MiRNAs: Biogenesis and Target Recognition
Mature miRNAs are a family of short, single-strand 21-22 nucleotides-long non-coding RNAs. Transcription of miRNAs is performed by either RNA polymerase II or RNA polymerase III to produce a long primary transcript miRNA (pri-miRNA). Then, the pri-miRNAs are recognized and processed by the Drosha/DGCR8 microprocessor machinery to generate a small precursor miRNA transcript (pre-miRNA) about 70 nucleotides long [60, 61]. These pre-miRNAs are exported to the nucleus by the exportin-5 where they are cleaved by Dicer, a type-III ribonuclease, to generate 20-22 nucleotides-long miRNA duplexes. At this stage, the Argonaute 2 protein (Ago2) unwinds the miRNA duplex, freeing the guide-strand (mature miRNA) which can then be incorporated into the miRNA-induced Silencing Complex (miRISC) while the passenger strand is degraded (Fig. 1). In the miRISC two mechanisms for miRNA silencing are currently known: translational inhibition and/or target mRNA degradation. Both processes are dependent on the degree of complementary sequence between the miRNA and often with the 5-UTR part of mRNA. If there is a perfect sequence match (100% homology), Ago2 can cleave the mRNA, leading to mRNA degradation whilst if complementation is not complete (less than 100% homology), the silencing is achieved by preventing translation and inducing later mRNA degradation by deadenylation and decapping [61].
Fig. (1).
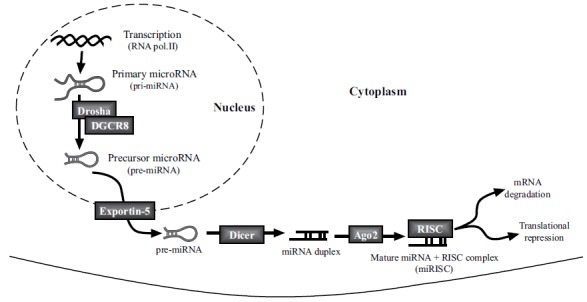
Schematic overview of microRNA biogenesis. RNA polymerase II transcribes the primary microRNA transcript in the nucleus which is processed by Drosha and the microprocessor complex into the pre-miRNA (70 nucleotide stem loop structure) and exported to the cytoplasm by Exportin-5. This pre-miRNA is cleaved by Dicer, leaving a 20-23 nucleotides long, imperfectly base-paired RNA duplex. One of these strands is the mature miRNA, which is integrated into the miRNA-Induced Silencing Complex (miRISC), a complex of proteins that silences gene expression by translational inhibition or target RNA degradation.
3.2. MiRNAs in Neurodevelopment
Although the etiology of cognitive dysfunctions in schizophrenia and autism spectrum disorders is complex and largely unclear, the neurodevelopment hypothesis is one of the most widely acknowledged and is based on neuro- anatomical and neuroimaging experiments. The development of the human brain includes an initial period of neurogenesis, followed by the migration of immature neurons, neuronal differentiation and generation of the synapses to finally form a neuronal network [62]. These neurodevelopmental processes are very malleable and are believed to be dependent on environmental and experiential events. As a result, any deviation from this program early in life can result in aberrations in normal processes of neurogenesis and may lead to cognitive dysfunction later in life. In the next part of this review, we will describe recent progress on understanding the roles of miRNAs in the regulation of normal brain development and neurogenesis and may lead to cognitive dysfunction later in life. Then we will describe recent progress on understanding the roles of miRNAs in the regulation of normal brain development and neurogenesis.
In order to identify possible functions of miRNAs in the brain, initial studies focused on targeting critical components of miRNA biogenesis such as DGCR8, Drosha or Dicer. The first evidence of the importance of miRNAs in neuronal development came from Dicer loss in zebrafish which resulted in abnormal brain morphogenesis and neural differentiation [63]. Dicer plays an important role during mammalian development as shown by the lethality of a global genetic depletion of Dicer in mice [64]. In the mouse central nervous system, conditional deletion of Dicer results in the apoptosis of neural progenitors and abnormal differentiation in the cortex and striatum [65]. Dicer function is crucial for the proper development of neurons and glia during early and late embryonic stages of CNS development [65]. In addition, conditional inactivation of Dicer in postmitotic Purkinje neurons in the cerebellum using Purkinje cell–specific Pcp2 promotor–driven Cre recombinase resulted in the rapid disappearance of miRNA expression and a slow neurodegeneration of Purkinje cells [66]. In mice, Dicer loss in postmitotic midbrain dopaminergic neurons induced a progressive loss of dopaminergic neurons due to apoptosis and induction of Parkinson-like symptoms as measured by a decrease in locomotion [67, 68]. Davis et al. demonstrated that loss of Dicer function in the cortex and hippocampus of mice resulted in dramatic effects on cell number and function, increased apoptosis and led to changes in dendritic branch patterns, underlying the role of Dicer in neuronal development [69]. Altogether, these studies illustrate the role of Dicer on neuronal differentiation and survival and support the hypothesis that dysregulation of Dicer and miRNA synthesis may contribute to neurodevelopmental disorders and neurodegenerative diseases.
Many miRNAs are enriched in mouse and human brain and significant progress has been made in identifying and characterizing their specific functions in brain development and homeostasis. Three of the best-studied miRNAs with developmental roles are miRNA-9, miRNA-124 and miRNA-137.
miRNA-9 is one of the most abundant miRNAs in the brain and has been extensively studied in neurogenesis. In the embryo, miRNA-9 is preferentially expressed by neural progenitor cells located in active neurogenesis areas [70-73]. In double-mutant mice in which two of the miRNA-9 genomic loci (miRNA-9-2/3) were mutated, Shibata et al. demonstrated that miRNA-9 controls both neural progenitor proliferation and differentiation by targeting multiple transcription factors [74]. Interestingly, miRNA-9 targets the transcriptional repressor RE1-silencing transcription factor (REST, also known as neuron restrictive silencer factor, NRSF) whereas miRNA-9* targets its partner CoREST (corepressor protein). The authors also demonstrated that there is a regulatory loop between miRNA-9 and REST during neural differentiation [75]. REST and coREST are essential during brain development. They orchestrate different epigenetic mechanisms to inactivate neuronal genes in non-neuronal cells [76]. Loss of REST is critical to the acquisition of neuronal phenotype, thereby underlining the importance of miRNA-9 which controls its expression.
Unlike miRNA-9 which is mainly expressed by neural progenitor cells, miRNA-124 increases during neuronal differentiation [77]. This miRNA regulates neuronal develop-ment by controlling key factors of neuronal differentiation such as REST and the Polypyrimidine Tract-binding Protein (PTBP1) which encodes a global repressor of alternative pre-mRNA splicing in non-neuronal cells [78, 79]. Thus, miRNA-124 promotes neuronal progenitor differentiation but also maturation by targeting the expression of cAMP response element-binding protein (CREB), Rho-associated coiled-coil-containing protein kinase 1 (ROCK1) and the small GTPase Ras homolog growth-related (RhoG) [78, 79]. In addition, miRNA‐124, by targeting the expression of the transcription factor Sox9, increases adult neurogenesis in the subventricular zone stem cell niche [80]. In another study, miRNA-124 was shown to regulate hippocampal neuro- genesis in juvenile rats, by increasing cell proliferation and survival [81]. An upregulation of miRNA-124 was observed in rats exposed to environmental enrichment and was associated to an improvement in learning and memory, as assessed by habituation learning and place object recognition [81], highlighting the importance of miRNA-124 on brain plasticity.
MiRNA-137 is another extensively studied neuronal microRNA which is highly expressed in the dentate gyrus, an area that is highly active in adult neurogenesis [82]. Overexpression of miRNA-137 decreases neural stem cell proliferation and promotes neuronal differentiation by inhibiting the expression of lysine-specific histone demethylase 1 (LSD1), and by forming a regulatory loop with TLX, an essential regulator of adult neural stem cell self-renewal [83]. By targeting the mindbomb homolog 1 (Mib1), a ubiquitin ligase that is important for neurogenesis and neurodevelopment, miRNA-137 also exerts a specific role in neuronal maturation [82]. In addition, Szulwach et al. demonstrated the role of miRNA-137 in adult hippocampal neurogenesis by post-transcriptionally repressing the expression of the enhancer of zeste homolog 2 (Ezh2), a histone methyltransferase that regulates neuroprogenitor cell maintenance and differentiation [84]. Given the important role that neurogenesis plays in learning and memory, together with the role of miRNA-137 in neurogenesis, it is not surprising that several links between this miRNA and neurodevelopmental disorders have been reported. The largest Genome-Wide Association Study (GWAS), a genetic study of a large cohort of schizophrenic subjects, allowed the identification of a single nucleotide polymorphism (SNP) rs1625579 close to miR137 thereby supporting a strong association of this polymorphism with Schizophrenia [85]. Further, it has been suggested that the SNP identified was associated with miRNA-137 expression levels in post-mortem patients’ samples [86]. Two other studies established a significant association of the risk allele with cognitive deficits in patients with schizophrenia [87, 88].
3.3. MiRNAs in Synaptic Plasticity and Memory
Recently, specific miRNAs involved in the regulation of dendritic arborization and synaptogenesis in the adult brain have also been identified as key players of synaptic plasticity and therefore learning and memory.
MiRNA-132 is enriched in neurons and was identified as a target of CREB [89], a key regulator of developmental plasticity which stabilizes the structural and functional changes in synapses necessary for memory processes [90]. The effect of miRNA-132 on spine plasticity is mediated in part by targeting, the Rho GTPase-activating protein 32, p250GAP, leading to an activation of the RAC1-PAK actin-cytoskeleton pathway [91, 92]. In addition, miRNA-132 was reported to target methyl CpG-binding protein 2 (MeCP2), a known regulator of synaptic formation and neuronal morphogenesis [93-95]. Numakawa et al. found an induction of miRNA-132 by basic Fibroblast Growth Factor which is mediated through activation of the ERK/MAPK cascade, a key signaling pathway in synaptic plasticity and memory [96]. Transfection of exogenous miRNA-132 induced a strong increase in the expression of glutamate receptors (NR2A, NR2B, and GluR1), providing additional evidence that miRNA-132 has a positive effect by enhancing postsynaptic protein levels [97]. The positive role of miRNA-132 in neuronal morphogenesis and connectivity was further confirmed in vivo in mice. The deletion of the miRNA-132 locus in mice decreased dendritic elongation and branching of newborn neurons in the adult hippocampus [98]. Accordingly, in vivo inhibition of miRNA-132 affected the maturation of dendritic spines and disrupted ocular dominance plasticity [99]. Conversely, while transgenic overexpression of miRNA-132 in mice induced an increase in spine density, it impaired the ability of mice to perform in novel object recognition [95]. Altogether these data demonstrate the critical role of miRNA-132 expression in neuronal development and plasticity.
As highlighted above, MiRNA-134 is a brain-specific microRNA which is expressed in neuronal dendrites. It was shown to negatively regulate the size of dendritic spines of rat hippocampal neurons by inhibiting the expression of Lim-domain-containing protein kinase 1 (LIMK1) which reorganizes the actin cytoskeleton dynamics through phosphorylation and thereby inactivation of the actin depolymerizing factor cofilin [100]. Furthermore, it was shown that inhibition of miRNA-134 with antisense oligonucleotide restores CREB and BDNF levels, two proteins involved in synaptic plasticity, and rescues the impairment of long-term potentiation and plasticity observed in a knockout mice model [37].
It was also shown that the brain-enriched miRNA-138, which is located in the dendritic compartment on rat hippocampal neurons, negatively regulates the size of dendritic spines. Indeed, miRNA-138 targets the expression of acyl protein thioesterase 1 (APT1), an enzyme known to depalmitoylate a number of substrates implicated in synaptic plasticity, including the Gα13 subunits of G proteins [101]. More specifically, miRNA-138 increases their membrane-bound state by reducing depalmitoylation of Gα13 subunits. This results in a prolonged activation of the downstream RhoA pathway and subsequent spine growth inhibition through the actin cytoskeleton reorganization.
Along the same lines, recent studies have shown that miRNA-128 [102], another brain-enriched miRNA was implicated in the control of synaptic plasticity and memory. Its upregulation in the infralimbic prefrontal cortex is required to regulate plasticity in adult post-mitotic neurons and is involved in the formation of fear-extinction memory. This effect of miRNA128b on memory is mediated through negative regulation of the expression of plasticity-related genes such as the regulator of calmodulin signaling, Rcs and CREB1 [102]. More recently, it has been shown that its expression reduces dendritic growth and arborization of neurons, and changes their intrinsic excitability by targeting PHF6, a gene mutated in the cognitive disorder Börjeson-Forssman-Lehmann syndrome [103].
Thanks to genetic approaches performed in Drosophila, other studies have provided direct evidence that miRNA pathways regulate learning and memory. A genetic screen identified four microRNAs, miRNA-9c, miRNA-31a, miRNA-974, mirNA-305 that reduced olfactory learning and memory formation and one microRNA, MiRNA-980 that, when inhibited, enhances memory formation [104]. More recently, it was shown that miR-980 overexpression impaired olfactory memory in Drosophila by targeting expression of the autism-susceptibility gene, A2bp1, a known RNA binding protein involved in alternative splicing [105].
Given the important role of miRNAs in the development and functions of the brain (Table 1) as detailed here, it is not surprising that there is increasing evidence suggesting that the dysregulation or altered expression of these miRNAs may be associated to cognitive disorders. Some of these examples will be discussed in the following section.
Table 1. Experimental validated miRNA-target interaction for miRNA involved in synaptic plasticity and function.
| miRNA | Identified Target Symbol | Identified Target Gene Name | Functions | Refs. |
|---|---|---|---|---|
| miRNA-9 | Foxg1 REST |
forkhead box protein G1 RE-1 silencing transcription factor |
Cell differentiation Neurogenesis |
[73, 74] [75] |
| miRNA-29 | BACE1 | beta-site amyloid precursor protein cleaving enzyme 1 | Neurodevelopment | [109] |
| miRNA-124 | CREB Sox9 RhoG ROCK-1 PTBP1 REST |
cAMP response element-binding protein SRY-box transcription factor Sox9 Ras homology Growth-related GTP-binding protein Serine/threonine Rho kinase 1 polypyrimidine tract binding protein RE-1-silencing transcription factor |
Synaptic plasticity Adult neurogenesis Axonal and dendritic branching Neurite outgrowth Neuronal differentiation Neurodevelopment |
[123] [80] [124] [125] [78] [79] |
| miRNA-128 | PHF6 NMD |
plant homeodomain finger 6 Nonsense-mediated decay |
Neurogenesis, synaptogenesis Neuronal differentiation |
[103] [126] |
| miRNA-132 | p250GAP MeCP2 CREB BDNF NR2A NR3B GluR1 |
Rho GTPase activating protein 32 Methyl CpG binding protein 2 (Rett syndrome) cAMP response element-binding protein brain-derived neurotrophic factor Subunit 2A NMDA receptor Subunit 2A NMDA receptor Glutamate receptor AMPA1 |
Synaptic plasticity Synaptogenesis Synaptic plasticity Synaptic plasticity Synaptic plasticity Synaptic plasticity Synaptic plasticity |
[92] [93-95] [93] [93] [97] [97] [97] |
| miRNA-134 | LIMK1 | LIM domain kinase 1 | Dendritic spine morphogenesis | [37, 100] |
| miRNA-137 | Mib1 Ezh2 LSD1 |
ubiquitin ligase mind bomb-1 the enhancer of zeste homolog 2 lysine-specific demethylase 1 |
Neurogenesis neurogenesis Neuronal differentiation |
[82] [84] [83] |
| miRNA-138 | APT1 | Depalmitoylation enzyme acyl-protein-thioesterase 1 | Dendritic spine morphogenesis | [101] |
4. MIRNA DYSREGULATION IN NEURO-COGNITIVE DISORDERS
4.1. Alzheimer’s Disease
Alzheimer’s disease (AD), the most common form of dementia, is a progressive degenerative brain disease that leads to neuron death, particularly in the hippocampus, restricting the patient’s ability to form new memories [106]. From a histopathologic perspective, AD is characterized by the production and deposition of amyloid-β peptide (Aβ) plaques and the accumulation of tau-containing intraneuronal neurofibrillary tangles [107, 108]. Recently, expression profiles of miRNAs in AD brain revealed alterations in many miRNAs, suggesting that a dysregulation could contribute to AD. Hébert et al. identified the miRNA-29a/b-1 cluster as a potential major suppressor of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1), which is involved in the production of Aβ [109]. They found a significant reduction of miRNA-29a/b-1 expression associated with an increase of BACE1 levels in the subgroup of sporadic AD patients when compared to controls. This loss of miRNA-29 may also contribute to the increase of Aβ generation by decreasing suppression of BACE1 expression in the aging neurons.
Using integrative genetics, Zovoilis et al. found an increase in miRNA-34c expression in the hippocampus of both AD patients and AD mouse models [110]. In contrast, miRNA-132 is downregulated in the human hippocampus of late onset AD which contributes to the disease progression by targeting the transcription factor Forkhead box protein O1 (FOXO1) in neurons displaying tau hyper-phosphorylation [111]. Moreover, miRNA-132 directly targets the neuronal splicing factor polypyrimidine tract-binding protein 2 (PTBP2) which could lead to the abnormal splicing of tau exon 10 in the brain [112]. miRNA-132 and miRNA-212 were also reported to directly regulate Phosphatase and tensin homolog (PTEN), FOXO3a and E1A binding protein p300, which are known to be involved in the AKT signaling pathway, and to contribute to AD neurodegeneration [113].
Over the last few years, a growing number of studies reported dysregulation of miRNA expression in biofluids, such as blood, plasma, serum or cerebrospinal fluid (CSF) [114]. Recently, the analysis of the expression profile of 1178 miRNAs in the cerebrospinal fluid (CSF) samples of AD patients identified three miRNAs, miRNA-100, miRNA-1274a and miRNA-146a, as the best candidates for potential AD biomarkers [115]. Interestingly, beside its biomarker potential miRNA-100 could also be an interesting target for therapeutic interventions as its predicted targets include mTOR [116].
4.2. Parkinson’s Disease
Parkinson’s disease (PD) is a progressive disorder of the nervous system characterized by degeneration of the dopamine neurons (DN) in the substantia nigra pars compacta of the midbrain and the presence of α-synuclein intraneuronal inclusions aggregates [117]. A progressive loss of midbrain DN has been shown in Dicer conditional knockout mice, suggesting that miRNAs may be involved in PD pathogenesis [68].
Amongst a panel of 224 miRNA precursors, miRNA-133b is specifically enriched in midbrain DNs and is downregulated in the midbrain of Parkinson’s disease patients. Overexpression of miRNA-133b in primary midbrain cultures suppresses DN maturation and reduces the depolarization-induced dopamine release by targeting the paired-like homeodomain transcription factor Pitx3 [68]. Two studies described miRNA-7 as a regulator of α-synuclein expression, a major component of Lewy bodies, and a pathologic hallmark of PD [118, 119]. Junn et al. also found that miRNA-7, mainly expressed in neurons, represses α-synuclein expression and protects cells against oxidative stress. It was also shown that in a MPTP-induced neurotoxin model of PD, miRNA-7 was down-regulated, which may contribute to an increased expression level of α-synuclein [119]. Unlike miRNA-7 and miRNA-153, miRNA-16 promotes the aberrant α-synuclein accumulation in PD via targeting heat shock protein 70 in a human neuroblastoma cell line [120]. Given that the expression level of α-synuclein is elevated in PD patients and that α-synuclein expression may contribute to DA neuron degeneration, miRNA-based strategies may be an alternative exploitable therapeutic approach to modulate this abnormal upregulation in PD.
Besides α-synuclein, the leucine-rich repeat kinase 2 (LRRK2), an enzyme involved in the early development of neuronal processes [121], is another key protein involved in the etiology of PD. Mutations in LRKK2 are the most common genetic lesions associated with familial and sporadic PD cases [122]. Interestingly, it was shown that miRNA-205, which is highly expressed in wild type mouse midbrain DN, directly inhibits the expression of LRRK2 protein. Furthermore, miRNA-205 expression is decreased in the frontal cortex of sporadic PD patients, who exhibited enhanced LRKK2 protein levels compared to age-matched non pathological controls. In vitro experiments on neurons from transgenic mutant mice for LRRK2 showed that the overexpression of miRNA-205 rescues the impaired neurite outgrowth in these neurons [127]. Altogether, these data suggest that miRNA-205 may be considered as another interesting therapeutic target to restore normal levels of LRKK2 protein in PD.
Finally, in another recent study, Alvarez-Erviti et al. demonstrated that in two brain regions (substantia nigra pars compacta and amygdala) associated with PD, there were significant increases in specific miRNAs (miRNA-106a, miRNA-224) that target Lysosome-associated membrane protein 2 and heat shock cognate protein 70, two autophagic mediators [128].
4.3. Schizophrenia
Schizophrenia (SCZ) is a mental disorder characterized by chronic psychotic symptoms and abnormal social behavior. It has a multifactorial mode of inheritance arising through a complex interplay between several genetic and non-genetic (epigenetic, environmental) factors acting in combination to produce the disorder [129, 130]. Anomalies in the size, number and density of neurons have been reported in the hippocampus of SCZ patients [131-134]. SCZ is also associated with deficits in cognitive functions including processing speed, episodic memory, working memory, and executive functions [135].
Alterations in the expression of many miRNAs have been reported in the postmortem brains of SCZ patients [136-138]. For instance, miRNA-26b, miRNA-29b, miRNA-30b, miRNA-106b and miRNA-132/132* are downregulated [139-141] whereas up-regulation of the miRNA-15 family and miRNA-181b is associated with SCZ pathogenesis [142, 143]. Furthermore, two single-nucleotide polymorphisms in miRNA-198 and miRNA-206 showed a significant allelic association to SCZ in the Danish and Norwegian samples respectively [144]. In silico analysis revealed that these two miRNAs have a large number of targets in common, eight of which are also connected by the same transcription factors such as c-jun which is known to be upregulated in the post-mortem brains of SCZ patients [145]. Future studies will help to characterize the molecular mechanisms involved in the neuronal functions of miRNA-198 and miRNA-206.
More recently, the brain-enriched miRNA-137 has been identified as a novel candidate gene for SCZ. Carriers of the miRNA-137 risk allele are more likely to represent a subgroup of psychotic patients with lower scores for psychotic symptoms and more cognitive deficits [87]. Supporting the role of this miRNA in the etiology of the disease, several schizophrenia-associated genes, such as transcription factor 4 (TCF4), calcium channel, voltage-dependent, L type, alpha 1C subunit (CACNA1C), cub and sushi multiple domains 1(CSMD1) and chromosome 10 open reading frame 26 (C10orf26) were also validated as miRNA-137 target genes [146, 147].
Other miRNAs such as miRNA-9, miRNA-124, and miRNA-219 were also shown to be associated with SCZ pathogenesis by regulating respectively the dopamine receptor D2 (DRD2) [148], the regulator of G protein signaling 4 (RGS4) [149] and the N-methyl-D-aspartate (NMDA) receptor signaling [150] respectively.
Taken together all these studies suggest that miRNAs, by targeting genes known to be associated with schizophrenia, may contribute to this complex neuropsychiatric disorder.
4.4. Austism Spectrum Disorder (ASD)
Autism, also known as autism spectrum disorder (ASD), is a neurodevelopmental disorder characterized by social deficits, impaired communication and stereotyped or repetitive patterns of behavior, activities and interests [151]. The genetic component has a key role in the etiology of autism, in conjunction with developmentally early environmental factors [152]. ASD is a disorder of the neuro-cortical organization characterized by alterations in the organization of cortical circuits and connectivity and abnormal dendritic spine morphogenesis and plasticity [153]. As in schizophrenia, the problem of multiple susceptibility genes of small effect has led to an increased interest in defining the roles of miRNAs in ASD.
A recent investigation of patients with 22q11.2 deletion syndrome found a reduced expression in DGCR8, one of the components of the nuclear miRNA processing complex [154]. The localization of DGCR8 within an ASD susceptibility locus strongly suggests a link between dysregulated miRNA expression and ASD pathogenesis. Using a series of computational tools, Vaishnavi et al. pointed out that certain miRNAs present in autism-associated copy number variants (CNVs) loci could contribute to the genetic heterogeneity and phenotypic variability of ASD [155]. Among the 71 CNVs associated with miRNAs, miRNA-7, miRNA-195, miRNA-211, miRNA-484 and miRNA-598 were previously reported to be associated with autism [156-158]. Another study conducted by Willemsen et al. revealed reduced levels of precursor and mature miRNA-137 forms [159], which are known to be involved in neurogenesis as described previously. Additionally, they found an upregulation of downstream targets of miRNA-137 (MITF, EZH2, Klf4) in patients with 1p21.3 microdeletions and with intellectual disability, compared to controls. Molecular analysis of gene expression linked to ASD has also implicated RORA (retinoic acid-related orphan receptor alpha), a transcriptional gene regulator, as a novel autism candidate gene [160]. It was shown that RORA levels were reduced in the cerebellum and frontal cortex of autistic brain [160]. Given the role of miRNA-137 in the regulation of RORA expression [161], it can be speculated that dysregulation of miRNA-137 and subsequent inappropriate RORA expression may contribute to ASD.
In another study involving a global miRNA expression profiling in lymphoblastoid cells from severe autistic patients, Ghahramani Seno et al. identified twelve dysregulated miRNAs in ASD compared to controls [158], one of which, miRNA-199b-5p, was the most deregulated. This miRNA has several potential targets with interesting neuronal functions. Among them, the Hairy and enhancer of split (HES1), one of the experimentally validated targets, is a protein involved in CNS development. Undoubtedly, future studies aiming at investigating whether the upregulation of miRNA-199b-5p has an impact on the expression of genes associated with ASD will increase our knowledge of the complex molecular mechanisms involved in the etiology of these cognitive disorders.
4.5. Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant progressive neurodegenerative disorder affecting the central nervous system [162]. It is characterized by uncontrolled movements, behavioral and psychiatric disorders, cognitive decline and dementia [163, 164]. HD results from an expanded CAG repeat (36 repeats or more) in the gene encoding the huntingtin protein. The major neuropathological hallmark is extensive loss of neurons within the striatum and cerebral cortex [165-168]. Pharmacological treatments are available to help relieve the symptoms and maintain the quality of HD patients' lives [169-171] but there is currently no cure.
The recent discovery of miRNAs and their involvement in HD pathogenesis could lead to novel approaches for HD therapy. The first evidence for the involvement of miRNAs in HD came from a study by Johnson et al. [172] in which they demonstrated that the expression of a number of brain-enriched microRNAs is dysregulated in HD patients, probably as a result of increased repression by REST [172]. In addition to the well described role of REST as a transcriptional repressor in neuronal function [76], it appeared that REST can regulate the expression of multiple neuron-specific miRNAs involved in neuronal functions.
This dysregulation in the biogenesis of miRNAs in the pathogenesis of HD was also confirmed in murine models of the disease. In YAC128 and R6/2 mice transgenic models of HD, an abnormal miRNAs biogenesis was reported. The expressions of Dicer, Drosha-DGCR8, Exportin-5, and Dcp1, key molecules involved in miRNA biogenesis and functions, were dysregulated [173]. In addition nine miRNAs (miRNA-22, miRNA-29c, miRNA-128, miRNA-132, miRNA-138, miRNA-218, miRNA-222, miRNA-344, and miRNA-674*) were commonly down-regulated in mouse models of HD. Among them, miRNA-132, described above, is again also more weakly expressed in the cortex of human HD patients [172].
Using the N171-82Q HD animal model of HD, Jin et al. observed an abnormal expression of miRNA-200 family members, miRNA-200a, and miRNA-200c in the cerebral cortex and the striatum, at the presymptomatic stage of the disease process. Interestingly, the predicted target genes of miRNA-200a and miRNA-200c are involved in the regulation of important neuronal functions such as synaptic plasticity, neurogenesis, and neuronal survival [174]. In transgenic mice, and human induced pluripotent stem cells derived from HD patients, Chen et al. showed that miRNA-196a ameliorates the phenotype of HD [175], underlining the putative interest of this miRNA as a therapeutic target. Furthermore, in postmortem brain tissue studies, Hoss et al. identified 75 miRNAs differentially expressed in HD brain and among them five miRNAs including miRNA-196a as having a significant relationship to CAG length-adjusted age of onset [176].
Recently, it was discovered that extracellular miRNAs circulate in the bloodstream in a highly stable form [177, 178]. In the plasma from HD patients, prior to symptom onset, miRNA-34b was shown to be significantly elevated [179]. Interestingly, downregulation of miRNA-34b is associated with a decrease in the expression of DJ1 and Parkin, two proteins associated to familial forms of PD [180]. These data suggest that miRNAs may be used as molecular biomarkers to provide an early indication of symptom onset and progression of HD. However, how these biomarker miRNAs contribute to HD phenotypes remains to be elucidated.
5. DISCUSSION
Over the last fifteen years or so, numerous studies have pointed out the importance of miRNAs in the regulation of gene expression involved in important physiological processes such as cellular proliferation or differentiation. miRNAs are found in high abundance within the central nervous system. Interestingly, some of them display brain-specific expression patterns and are usually found to be co-expressed with their targets.
We have presented here converging lines of evidence suggesting that miRNAs modulate memory formation including synaptic plasticity and neurogenesis. The hypothesis that miRNAs regulate cognitive functions is emphasized by the studies presented in this paper. Table 1 summarizes some of these studies and highlights the influence of individual miRNAs on neuronal function and communication through their action on multiple gene targets. For example, miRNA-124 has been shown to suppress the translation of RhoG, ROCK-1 or CREB which are involved in dendritic morphogenesis and known also to regulate the expression of transcription factors (Sox9, REST, PTBP1) involved in neurogenesis. Taking into consideration the fact that each miRNA can control hundreds of target genes, the identification of the accurate miRNA targets for cognitive functions remains a huge challenge. One of the most important key steps in the future will be to confirm interactions of miRNAs with their mRNA targets often described in vitro but less so in vivo, in physiological conditions. For this purpose, two issues may be considered as research strategies. The first one will be to improve target prediction algorithms in order to facilitate the identification of relevant targets of these miRNAs. This approach would provide great insight into the functional significance of miRNA expression in physiological conditions. The second one will be to improve strategies to modulate miRNA expression in vivo by using mimics or antagomirs which will make it possible to confirm the importance of a specific miRNA to control the expression of specific genes involved in cognitive processes. For this purpose, the design and synthesis of pharmacological miRNA modulators will be necessary. Strategies developed to obtain such phar- macological tools are extensively described in the following review. Thus, recent studies demonstrated that the manipulation of miRNA brain expression in animal models by silencing or mimicking them may be a useful strategy to confirm their importance in brain function. Further, the importance of miRNA expression levels for the correct functioning of the nervous system is reflected by an increasing number of studies demonstrating a link between miRNA dysregulation and the etiology and/or patho- physiology of cognitive dysfunction observed in several neurologic and neuropsychiatric disorders.
In this review we have described the main miRNAs that are dysregulated in various forms of cognitive dysfunction including Parkinson’s disease, Alzheimer’s disease, Schizophrenia, autism spectrum disorders and Huntington's disease and discussed their possible application as biomarkers of such diseases. Although the contribution of some individual miRNAs in regulating neural morphology and function has been extensively explored in animal models, available studies in patients have only provided snapshots of the disease process and have not shown how miRNAs change with disease progression and contribute to specific clinical features. It is now necessary to obtain consistent knowledge about the role of miRNAs in brain for the maintenance of cognitive functions or the appearance of cognitive deficits. Frequently, a large range of miRNAs are found dysregulated in pathological conditions. Increasing the complexity, individual miRNAs are known to target hundreds of different mRNAs. Altogether, it means that a variety of targets and combinatorial effects are likely to be involved in the etiology of complex neurological disorders. The obvious challenge will be to choose the most promising miRNAs for further investigations. For this purpose, one strategy will be to focus on the most differentially expressed miRNAs across samples in combination with statistical testing. However, whether altered expression of miRNAs plays a part in the etiology of the disease or conversely is the consequence of the disease to compensate brain dysfunctions should be further explored before any diagnostic or therapeutic utilization can be accomplished. In any event, it appears that miRNA-based pharmacological therapies could open a new and largely unexplored strategy for the treatment of a wide variety of cognitive disorders.
ACKNOWLEDGEMENT
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Malenka R.C., Nicoll R.A. Long-term potentiationa decade of progress? Science. 1999;285(5435):1870–1874. doi: 10.1126/science.285.5435.1870. [http://dx.doi.org/ 10.1126/science.285.5435.1870]. [PMID: 10489359]. [DOI] [PubMed] [Google Scholar]
- 2.Lømo T. The discovery of long-term potentiation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358(1432):617–620. doi: 10.1098/rstb.2002.1226. [http://dx. doi.org/10.1098/rstb.2002.1226]. [PMID: 12740104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clugnet M.C., LeDoux J.E. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J. Neurosci. 1990;10(8):2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [PMID: 2388089]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham W.C., Williams J.M. Properties and mechanisms of LTP maintenance. Neuroscientist. 2003;9(6):463–474. doi: 10.1177/1073858403259119. [http://dx.doi. org/10.1177/1073858403259119]. [PMID: 14678579]. [DOI] [PubMed] [Google Scholar]
- 5.Joilin G., Guévremont D., Ryan B., Claudianos C., Cristino A.S., Abraham W.C., Williams J.M. Rapid regulation of microRNA following induction of long-term potentiation in vivo. Front. Mol. Neurosci. 2014;7:98. doi: 10.3389/fnmol.2014.00098. [http://dx.doi.org/10.3389/fnmol.2014.00098]. [PMID: 25538559]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi Y., Shi S.H., Esteban J.A., Piccini A., Poncer J.C., Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287(5461):2262–2267. doi: 10.1126/science.287.5461.2262. [http://dx.doi.org/10.1126/ science.287.5461.2262]. [PMID: 10731148]. [DOI] [PubMed] [Google Scholar]
- 7.Shi S.H., Hayashi Y., Petralia R.S., Zaman S.H., Wenthold R.J., Svoboda K., Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284(5421):1811–1816. doi: 10.1126/science.284.5421.1811. [http://dx.doi.org/10.1126/ science.284.5421.1811]. [PMID: 10364548]. [DOI] [PubMed] [Google Scholar]
- 8.Malenka R.C., Bear M.F. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [http://dx.doi.org/10.1016/ j.neuron.2004.09.012]. [PMID: 15450156]. [DOI] [PubMed] [Google Scholar]
- 9.Whitlock J.R., Heynen A.J., Shuler M.G., Bear M.F. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [http://dx.doi.org/10.1126/science.1128134]. [PMID: 16931756]. [DOI] [PubMed] [Google Scholar]
- 10.Kwapis J.L., Helmstetter F.J. Does PKM(zeta) maintain memory? Brain Res. Bull. 2014;105:36–45. doi: 10.1016/j.brainresbull.2013.09.005. [http://dx.doi.org/10.1016/ j.brainresbull.2013.09.005]. [PMID: 24076105]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volk L.J., Bachman J.L., Johnson R., Yu Y., Huganir R.L. PKM-ζ is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493(7432):420–423. doi: 10.1038/nature11802. [http://dx.doi.org/ 10.1038/nature11802]. [PMID: 23283174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee A.M., Kanter B.R., Wang D., Lim J.P., Zou M.E., Qiu C., McMahon T., Dadgar J., Fischbach-Weiss S.C., Messing R.O. Prkcz null mice show normal learning and memory. Nature. 2013;493(7432):416–419. doi: 10.1038/nature11803. [http://dx.doi.org/10.1038/nature11803]. [PMID: 23283171]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan B., Joilin G., Williams J.M. Plasticity-related microRNA and their potential contribution to the maintenance of long-term potentiation. Front. Mol. Neurosci. 2015;8:4. doi: 10.3389/fnmol.2015.00004. [http://dx.doi.org/ 10.3389/fnmol.2015.00004]. [PMID: 25755632]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris K.M., Kater S.B. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [http://dx.doi.org/10.1146/ annurev.ne.17.030194.002013]. [PMID: 8210179]. [DOI] [PubMed] [Google Scholar]
- 15.Glausier J.R., Lewis D.A. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [http://dx.doi.org/ 10.1016/j.neuroscience.2012.04.044]. [PMID: 22546337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendoza-Naranjo A., Contreras-Vallejos E., Henriquez D.R., Otth C., Bamburg J.R., Maccioni R.B., Gonzalez-Billault C. Fibrillar amyloid-β142 modifies actin organization affecting the cofilin phosphorylation state: a role for Rac1/cdc42 effector proteins and the slingshot phosphatase. J. Alzheimers Dis. 2012;29(1):63–77. doi: 10.3233/JAD-2012-101575. [PMID: 22204905]. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza-Naranjo A., Gonzalez-Billault C., Maccioni R.B. Abeta142 stimulates actin polymerization in hippocampal neurons through Rac1 and Cdc42 Rho GTPases. J. Cell Sci. 2007;120(Pt 2):279–288. doi: 10.1242/jcs.03323. [http://dx.doi.org/10.1242/jcs.03323]. [PMID: 17200137]. [DOI] [PubMed] [Google Scholar]
- 18.Pozueta J., Lefort R., Shelanski M.L. Synaptic changes in Alzheimers disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [http://dx. doi.org/10.1016/j.neuroscience.2012.05.050]. [PMID: 22687952]. [DOI] [PubMed] [Google Scholar]
- 19.Chan D., Citro A., Cordy J.M., Shen G.C., Wolozin B. Rac1 protein rescues neurite retraction caused by G2019S leucine-rich repeat kinase 2 (LRRK2). J. Biol. Chem. 2011;286(18):16140–16149. doi: 10.1074/jbc.M111.234005. [http://dx.doi.org/10.1074/jbc.M111.234005]. [PMID: 21454543]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelleher R.J., III, Bear M.F. The autistic neuron: troubled translation? Cell. 2008;135(3):401–406. doi: 10.1016/j.cell.2008.10.017. [http://dx.doi.org/10.1016/ j.cell.2008.10.017]. [PMID: 18984149]. [DOI] [PubMed] [Google Scholar]
- 21.Negishi M., Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist. 2005;11(3):187–191. doi: 10.1177/1073858404268768. [http://dx.doi.org/10.1177/ 1073858404268768]. [PMID: 15911868]. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese B., Wilson M.S., Halpain S. Development and regulation of dendritic spine synapses. Physiology (Bethesda) 2006;21:38–47. doi: 10.1152/physiol.00042.2005. [http://dx.doi.org/10.1152/physiol.00042.2005]. [PMID: 16443821]. [DOI] [PubMed] [Google Scholar]
- 23.Newey S.E., Velamoor V., Govek E.E., Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J. Neurobiol. 2005;64(1):58–74. doi: 10.1002/neu.20153. [http://dx.doi.org/10.1002/neu.20153]. [PMID: 15884002]. [DOI] [PubMed] [Google Scholar]
- 24.Tashiro A., Minden A., Yuste R. Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb. Cortex. 2000;10(10):927–938. doi: 10.1093/cercor/10.10.927. [http://dx. doi.org/10.1093/cercor/10.10.927]. [PMID: 11007543]. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama A.Y., Harms M.B., Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 2000;20(14):5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [PMID: 10884317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott E.K., Reuter J.E., Luo L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J. Neurosci. 2003;23(8):3118–3123. doi: 10.1523/JNEUROSCI.23-08-03118.2003. [PMID: 12716918]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahnert-Hilger G., Höltje M., Grosse G., Pickert G., Mucke C., Nixdorf-Bergweiler B., Boquet P., Hofmann F., Just I. Differential effects of Rho GTPases on axonal and dendritic development in hippocampal neurones. J. Neurochem. 2004;90(1):9–18. doi: 10.1111/j.1471-4159.2004.02475.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02475.x]. [PMID: 15198662]. [DOI] [PubMed] [Google Scholar]
- 28.Chen C., Wirth A., Ponimaskin E. Cdc42: an important regulator of neuronal morphology. Int. J. Biochem. Cell Biol. 2012;44(3):447–451. doi: 10.1016/j.biocel.2011.11.022. [http://dx.doi.org/10.1016/j.biocel.2011.11.022]. [PMID: 22172377]. [DOI] [PubMed] [Google Scholar]
- 29.Kim I.H., Wang H., Soderling S.H., Yasuda R. Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall. eLife. 2014;3:3. doi: 10.7554/eLife.02839. [http://dx.doi.org/10.7554/eLife.02839]. [PMID: 25006034]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill J.J., Hashimoto T., Lewis D.A. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2006;11(6):557–566. doi: 10.1038/sj.mp.4001792. [http://dx.doi.org/10.1038/sj.mp.4001792]. [PMID: 16402129]. [DOI] [PubMed] [Google Scholar]
- 31.Datta D., Arion D., Corradi J.P., Lewis D.A. Altered expression of CDC42 signaling pathway components in cortical layer 3 pyramidal cells in schizophrenia. Biol. Psychiatry. 2015;78(11):775–785. doi: 10.1016/j.biopsych.2015.03.030. [http://dx.doi.org/10.1016/j.biopsych.2015.03.030]. [PMID: 25981171]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu B., Hsu P.K., Karayiorgou M., Gogos J.A. MicroRNA dysregulation in neuropsychiatric disorders and cognitive dysfunction. Neurobiol. Dis. 2012;46(2):291–301. doi: 10.1016/j.nbd.2012.02.016. [http://dx.doi. org/10.1016/j.nbd.2012.02.016]. [PMID: 22406400]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saura C.A., Valero J. The role of CREB signaling in Alzheimers disease and other cognitive disorders. Rev. Neurosci. 2011;22(2):153–169. doi: 10.1515/RNS.2011.018. [http://dx.doi.org/10.1515/rns.2011.018]. [PMID: 21476939]. [DOI] [PubMed] [Google Scholar]
- 34.García-Barroso C., Ugarte A., Martínez M., Rico A.J., Lanciego J.L., Franco R., Oyarzabal J., Cuadrado-Tejedor M., García-Osta A. Phosphodiesterase inhibition in cognitive decline. J. Alzheimers Dis. 2014;42(Suppl. 4):S561–S573. doi: 10.3233/JAD-141341. [PMID: 25125473]. [DOI] [PubMed] [Google Scholar]
- 35.Kida S., Serita T. Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res. Bull. 2014;105:17–24. doi: 10.1016/j.brainresbull.2014.04.011. [http://dx.doi.org/10.1016/j.brainresbull. 2014.04.011]. [PMID: 24811207]. [DOI] [PubMed] [Google Scholar]
- 36.Paramanik V., Thakur M.K. Role of CREB signaling in aging brain. Arch. Ital. Biol. 2013;151(1):33–42. doi: 10.4449/aib.v151i1.1461. [PMID: 23807618]. [DOI] [PubMed] [Google Scholar]
- 37.Gao J., Wang W.Y., Mao Y.W., Gräff J., Guan J.S., Pan L., Mak G., Kim D., Su S.C., Tsai L.H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–1109. doi: 10.1038/nature09271. [http://dx.doi.org/10.1038/nature09271]. [PMID: 20622856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmelzle T., Hall M.N. TOR, a central controller of cell growth. Cell. 2000;103(2):253–262. doi: 10.1016/s0092-8674(00)00117-3. [http://dx.doi.org/10.1016/S0092-8674(00)00117-3]. [PMID: 11057898]. [DOI] [PubMed] [Google Scholar]
- 39.Campbell D.S., Holt C.E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32(6):1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [http://dx.doi.org/10.1016/S0896-6273(01)00551-7]. [PMID: 11754834]. [DOI] [PubMed] [Google Scholar]
- 40.Kumar V., Zhang M.X., Swank M.W., Kunz J., Wu G.Y. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005;25(49):11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.2284-05.2005]. [PMID: 16339024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaworski J., Spangler S., Seeburg D.P., Hoogenraad C.C., Sheng M. Control of dendritic arborization by the phosphoinositide-3-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci. 2005;25(49):11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI. 2270-05.2005]. [PMID: 16339025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crino P.B., Nathanson K.L., Henske E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006;355(13):1345–1356. doi: 10.1056/NEJMra055323. [http://dx. doi.org/10.1056/NEJMra055323]. [PMID: 17005952]. [DOI] [PubMed] [Google Scholar]
- 43.Li X., Alafuzoff I., Soininen H., Winblad B., Pei J.J. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimers disease brain. FEBS J. 2005;272(16):4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [http://dx.doi.org/10.1111/j.1742-4658. 2005.04833.x]. [PMID: 16098202]. [DOI] [PubMed] [Google Scholar]
- 44.An W.L., Cowburn R.F., Li L., Braak H., Alafuzoff I., Iqbal K., Iqbal I.G., Winblad B., Pei J.J. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimers disease. Am. J. Pathol. 2003;163(2):591–607. doi: 10.1016/S0002-9440(10)63687-5. [http://dx.doi.org/10.1016/S0002-9440(10) 63687-5]. [PMID: 12875979]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffin R.J., Moloney A., Kelliher M., Johnston J.A., Ravid R., Dockery P. OConnor, R.; ONeill, C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimers disease pathology. J. Neurochem. 2005;93(1):105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [http://dx.doi.org/ 10.1111/j.1471-4159.2004.02949.x]. [PMID: 15773910]. [DOI] [PubMed] [Google Scholar]
- 46.Tramutola A., Triplett J.C., Di Domenico F., Niedowicz D.M., Murphy M.P., Coccia R., Perluigi M., Butterfield D.A. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J. Neurochem. 2015;133(5):739–749. doi: 10.1111/jnc.13037. [http://dx.doi.org/ 10.1111/jnc.13037]. [PMID: 25645581]. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A., Hoeffer C.A., Takayasu Y., Miyawaki T., McBride S.M., Klann E., Zukin R.S. Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 2010;30(2):694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [http:// dx.doi.org/10.1523/JNEUROSCI.3696-09.2010]. [PMID: 20071534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gross C., Nakamoto M., Yao X., Chan C.B., Yim S.Y., Ye K., Warren S.T., Bassell G.J. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J. Neurosci. 2010;30(32):10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [http://dx. doi.org/10.1523/JNEUROSCI.0402-10.2010]. [PMID: 20702695]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricciardi S., Boggio E.M., Grosso S., Lonetti G., Forlani G., Stefanelli G., Calcagno E., Morello N., Landsberger N., Biffo S., Pizzorusso T., Giustetto M., Broccoli V. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum. Mol. Genet. 2011;20(6):1182–1196. doi: 10.1093/hmg/ddq563. [http://dx.doi.org/10.1093/hmg/ddq563]. [PMID: 21212100]. [DOI] [PubMed] [Google Scholar]
- 50.Xu C., Zeng Q., Xu W., Jiao L., Chen Y., Zhang Z., Wu C., Jin T., Pan A., Wei R., Yang B., Sun Y. miRNA-100 inhibits human bladder urothelial carcinogenesis by directly targeting mTOR. Mol. Cancer Ther. 2013;12(2):207–219. doi: 10.1158/1535-7163.MCT-12-0273. [http://dx.doi. org/10.1158/1535-7163.MCT-12-0273]. [PMID: 23270926]. [DOI] [PubMed] [Google Scholar]
- 51.Li W., Chang J., Wang S., Liu X., Peng J., Huang D., Sun M., Chen Z., Zhang W., Guo W., Li J. miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget. 2015;6(27):24448–24462. doi: 10.18632/oncotarget.4423. [http://dx.doi.org/10. 18632/oncotarget.4423]. [PMID: 26259252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cong J., Liu R., Wang X., Jiang H., Zhang Y. MiR-634 decreases cell proliferation and induces apoptosis by targeting mTOR signaling pathway in cervical cancer cells. Artif. Cells Nanomed. Biotechnol. 2015;•••:1–8. doi: 10.3109/21691401.2015.1080171. [PMID: 26367112]. [DOI] [PubMed] [Google Scholar]
- 53.Sun D., Lee Y.S., Malhotra A., Kim H.K., Matecic M., Evans C., Jensen R.V., Moskaluk C.A., Dutta A. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 2011;71(4):1313–1324. doi: 10.1158/0008-5472.CAN-10-1031. [http://dx.doi.org/10.1158/0008-5472.CAN-10-1031]. [PMID: 21212412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsujimura K., Irie K., Nakashima H., Egashira Y., Fukao Y., Fujiwara M., Itoh M., Uesaka M., Imamura T., Nakahata Y., Yamashita Y., Abe T., Takamori S., Nakashima K. miR-199a Links MeCP2 with mTOR Signaling and Its Dysregulation Leads to Rett Syndrome Phenotypes. Cell Reports. 2015;12(11):1887–1901. doi: 10.1016/j.celrep.2015.08.028. [http://dx.doi.org/10.1016/j.celrep.2015.08.028]. [PMID: 26344767]. [DOI] [PubMed] [Google Scholar]
- 55.Nellemann C., Abell K., Nørremølle A., Løkkegaard T., Naver B., Röpke C., Rygaard J., Sørensen S.A., Hasholt L. Inhibition of Huntington synthesis by antisense oligodeoxynucleotides. Mol. Cell. Neurosci. 2000;16(4):313–323. doi: 10.1006/mcne.2000.0872. [http://dx.doi.org/10.1006/ mcne.2000.0872]. [PMID: 11085870]. [DOI] [PubMed] [Google Scholar]
- 56.Fedorov Y., Anderson E.M., Birmingham A., Reynolds A., Karpilow J., Robinson K., Leake D., Marshall W.S., Khvorova A. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12(7):1188–1196. doi: 10.1261/rna.28106. [http://dx.doi.org/10.1261/rna.28106]. [PMID: 16682561]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reynolds A., Anderson E.M., Vermeulen A., Fedorov Y., Robinson K., Leake D., Karpilow J., Marshall W.S., Khvorova A. Induction of the interferon response by siRNA is cell type- and duplex length-dependent. RNA. 2006;12(6):988–993. doi: 10.1261/rna.2340906. [http://dx. doi.org/10.1261/rna.2340906]. [PMID: 16611941]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimm D., Streetz K.L., Jopling C.L., Storm T.A., Pandey K., Davis C.R., Marion P., Salazar F., Kay M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [http://dx.doi.org/ 10.1038/nature04791]. [PMID: 16724069]. [DOI] [PubMed] [Google Scholar]
- 59.Salta E., De Strooper B. Non-coding RNAs with essential roles in neurodegenerative disorders. Lancet Neurol. 2012;11(2):189–200. doi: 10.1016/S1474-4422(11)70286-1. [http://dx.doi.org/10.1016/S1474-4422(11)70286-1]. [PMID: 22265214]. [DOI] [PubMed] [Google Scholar]
- 60.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [http://dx.doi.org/10.1038/nature02871]. [PMID: 15372042]. [DOI] [PubMed] [Google Scholar]
- 61.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [http://dx.doi.org/10.1016/ S0092-8674(04)00045-5]. [PMID: 14744438]. [DOI] [PubMed] [Google Scholar]
- 62.Jiang X., Nardelli J. Cellular and molecular introduction to brain development. Neurobiol. Dis. 2015 doi: 10.1016/j.nbd.2015.07.007. [PMID: 26184894]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giraldez A.J., Cinalli R.M., Glasner M.E., Enright A.J., Thomson J.M., Baskerville S., Hammond S.M., Bartel D.P., Schier A.F. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308(5723):833–838. doi: 10.1126/science.1109020. [http://dx.doi.org/10.1126/ science.1109020]. [PMID: 15774722]. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein E., Kim S.Y., Carmell M.A., Murchison E.P., Alcorn H., Li M.Z., Mills A.A., Elledge S.J., Anderson K.V., Hannon G.J. Dicer is essential for mouse development. Nat. Genet. 2003;35(3):215–217. doi: 10.1038/ng1253. [http://dx.doi.org/10.1038/ng1253]. [PMID: 14528307]. [DOI] [PubMed] [Google Scholar]
- 65.Kawase-Koga Y., Otaegi G., Sun T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Dev. Dyn. 2009;238(11):2800–2812. doi: 10.1002/dvdy.22109. [http://dx.doi.org/10.1002/dvdy.22109]. [PMID: 19806666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaefer A. OCarroll, D.; Tan, C.L.; Hillman, D.; Sugimori, M.; Llinas, R.; Greengard, P. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 2007;204(7):1553–1558. doi: 10.1084/jem.20070823. [http://dx.doi.org/10.1084/jem.20070823]. [PMID: 17606634]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hébert S.S., De Strooper B. Molecular biology. miRNAs in neurodegeneration. Science. 2007;317(5842):1179–1180. doi: 10.1126/science.1148530. [http://dx.doi.org/10.1126/science.1148530]. [PMID: 17761871]. [DOI] [PubMed] [Google Scholar]
- 68.Kim J., Inoue K., Ishii J., Vanti W.B., Voronov S.V., Murchison E., Hannon G., Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–1224. doi: 10.1126/science.1140481. [http://dx.doi.org/10.1126/science.1140481]. [PMID: 17761882]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis T.H., Cuellar T.L., Koch S.M., Barker A.J., Harfe B.D., McManus M.T., Ullian E.M. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 2008;28(17):4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.4815-07.2008]. [PMID: 18434510]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonev B., Pisco A., Papalopulu N. MicroRNA-9 reveals regional diversity of neural progenitors along the anterior-posterior axis. Dev. Cell. 2011;20(1):19–32. doi: 10.1016/j.devcel.2010.11.018. [http://dx.doi.org/10.1016/j.devcel. 2010.11.018]. [PMID: 21238922]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coolen M., Thieffry D., Drivenes Ø., Becker T.S., Bally-Cuif L. miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Dev. Cell. 2012;22(5):1052–1064. doi: 10.1016/j.devcel.2012.03.003. [http://dx.doi.org/10.1016/j.devcel.2012.03.003]. [PMID: 22595676]. [DOI] [PubMed] [Google Scholar]
- 72.Leucht C., Stigloher C., Wizenmann A., Klafke R., Folchert A., Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat. Neurosci. 2008;11(6):641–648. doi: 10.1038/nn.2115. [http://dx.doi.org/10.1038/nn.2115]. [PMID: 18454145]. [DOI] [PubMed] [Google Scholar]
- 73.Shibata M., Kurokawa D., Nakao H., Ohmura T., Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J. Neurosci. 2008;28(41):10415–10421. doi: 10.1523/JNEUROSCI.3219-08.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.3219-08.2008]. [PMID: 18842901]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shibata M., Nakao H., Kiyonari H., Abe T., Aizawa S. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J. Neurosci. 2011;31(9):3407–3422. doi: 10.1523/JNEUROSCI.5085-10.2011. [http://dx.doi.org/10.1523/JNEUROSCI.5085-10.2011]. [PMID: 21368052]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Packer A.N., Xing Y., Harper S.Q., Jones L., Davidson B.L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntingtons disease. J. Neurosci. 2008;28(53):14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.2390-08.2008]. [PMID: 19118166]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballas N., Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 2005;15(5):500–506. doi: 10.1016/j.conb.2005.08.015. [http://dx.doi.org/10.1016/j.conb.2005.08.015]. [PMID: 16150588]. [DOI] [PubMed] [Google Scholar]
- 77.Kapsimali M., Kloosterman W.P., de Bruijn E., Rosa F., Plasterk R.H., Wilson S.W. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8(8):R173. doi: 10.1186/gb-2007-8-8-r173. [http://dx.doi.org/ 10.1186/gb-2007-8-8-r173]. [PMID: 17711588]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makeyev E.V., Zhang J., Carrasco M.A., Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27(3):435–448. doi: 10.1016/j.molcel.2007.07.015. [http://dx.doi.org/10.1016/j.molcel.2007. 07.015]. [PMID: 17679093]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Visvanathan J., Lee S., Lee B., Lee J.W., Lee S.K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21(7):744–749. doi: 10.1101/gad.1519107. [http://dx.doi.org/10.1101/gad.1519107]. [PMID: 17403776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng L.C., Pastrana E., Tavazoie M., Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009;12(4):399–408. doi: 10.1038/nn.2294. [http://dx.doi.org/ 10.1038/nn.2294]. [PMID: 19287386]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brenes J.C., Lackinger M., Hoglinger G.U., Schratt G., Schwarting R.K., Wohr M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J. Comp. Neurol. 2015 doi: 10.1002/cne.23842. [PMID: 26132842]. [DOI] [PubMed] [Google Scholar]
- 82.Smrt R.D., Szulwach K.E., Pfeiffer R.L., Li X., Guo W., Pathania M., Teng Z.Q., Luo Y., Peng J., Bordey A., Jin P., Zhao X. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells. 2010;28(6):1060–1070. doi: 10.1002/stem.431. [http://dx.doi.org/10.1002/stem.431]. [PMID: 20506192]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun G., Ye P., Murai K., Lang M.F., Li S., Zhang H., Li W., Fu C., Yin J., Wang A., Ma X., Shi Y. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat. Commun. 2011;2:529. doi: 10.1038/ncomms1532. [http://dx.doi.org/10.1038/ ncomms1532]. [PMID: 22068596]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Szulwach K.E., Li X., Smrt R.D., Li Y., Luo Y., Lin L., Santistevan N.J., Li W., Zhao X., Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 2010;189(1):127–141. doi: 10.1083/jcb.200908151. [http://dx.doi.org/10.1083/jcb. 200908151]. [PMID: 20368621]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011;43(10):969–976. doi: 10.1038/ng.940. [http://dx.doi.org/10. 1038/ng.940]. [PMID: 21926974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guella I., Sequeira A., Rollins B., Morgan L., Torri F., van Erp T.G., Myers R.M., Barchas J.D., Schatzberg A.F., Watson S.J., Akil H., Bunney W.E., Potkin S.G., Macciardi F., Vawter M.P. Analysis of miR-137 expression and rs1625579 in dorsolateral prefrontal cortex. J. Psychiatr. Res. 2013;47(9):1215–1221. doi: 10.1016/j.jpsychires.2013.05.021. [http:// dx.doi.org/10.1016/j.jpsychires.2013.05.021]. [PMID: 23786914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cummings E., Donohoe G., Hargreaves A., Moore S., Fahey C., Dinan T.G., McDonald C. OCallaghan, E.; ONeill, F.A.; Waddington, J.L.; Murphy, K.C.; Morris, D.W.; Gill, M.; Corvin, A. Mood congruent psychotic symptoms and specific cognitive deficits in carriers of the novel schizophrenia risk variant at MIR-137. Neurosci. Lett. 2013;532:33–38. doi: 10.1016/j.neulet.2012.08.065. [http://dx.doi.org/10.1016/ j.neulet.2012.08.065]. [PMID: 22982201]. [DOI] [PubMed] [Google Scholar]
- 88.Green M.J., Cairns M.J., Wu J., Dragovic M., Jablensky A., Tooney P.A., Scott R.J., Carr V.J. Genome-wide supported variant MIR137 and severe negative symptoms predict membership of an impaired cognitive subtype of schizophrenia. Mol. Psychiatry. 2013;18(7):774–780. doi: 10.1038/mp.2012.84. [http://dx.doi.org/10.1038/mp. 2012.84]. [PMID: 22733126]. [DOI] [PubMed] [Google Scholar]
- 89.Scott H.L., Tamagnini F., Narduzzo K.E., Howarth J.L., Lee Y.B., Wong L.F., Brown M.W., Warburton E.C., Bashir Z.I., Uney J.B. MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur. J. Neurosci. 2012;36(7):2941–2948. doi: 10.1111/j.1460-9568.2012.08220.x. [http://dx.doi.org/10.1111/j.1460-9568.2012. 08220.x]. [PMID: 22845676]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kandel E.R. The biology of memory: a forty-year perspective. J. Neurosci. 2009;29(41):12748–12756. doi: 10.1523/JNEUROSCI.3958-09.2009. [http://dx.doi.org/10.1523/ JNEUROSCI.3958-09.2009]. [PMID: 19828785]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Impey S., Davare M., Lesiak A., Fortin D., Ando H., Varlamova O., Obrietan K., Soderling T.R., Goodman R.H., Wayman G.A. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol. Cell. Neurosci. 2010;43(1):146–156. doi: 10.1016/j.mcn.2009.10.005. [http://dx.doi.org/10.1016/j.mcn. 2009.10.005]. [PMID: 19850129]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vo N., Klein M.E., Varlamova O., Keller D.M., Yamamoto T., Goodman R.H., Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. USA. 2005;102(45):16426–16431. doi: 10.1073/pnas.0508448102. [http:// dx.doi.org/10.1073/pnas.0508448102]. [PMID: 16260724]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hernandez-Rapp J., Smith P.Y., Filali M., Goupil C., Planel E., Magill S.T., Goodman R.H., Hébert S.S. Memory formation and retention are affected in adult miR-132/212 knockout mice. Behav. Brain Res. 2015;287:15–26. doi: 10.1016/j.bbr.2015.03.032. [http://dx.doi.org/10.1016/j.bbr. 2015.03.032]. [PMID: 25813747]. [DOI] [PubMed] [Google Scholar]
- 94.Klein M.E., Lioy D.T., Ma L., Impey S., Mandel G., Goodman R.H. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat. Neurosci. 2007;10(12):1513–1514. doi: 10.1038/nn2010. [http://dx.doi.org/10.1038/nn2010]. [PMID: 17994015]. [DOI] [PubMed] [Google Scholar]
- 95.Hansen K.F., Sakamoto K., Wayman G.A., Impey S., Obrietan K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS One. 2010;5(11):e15497. doi: 10.1371/journal.pone.0015497. [http://dx.doi.org/10.1371/journal.pone.0015497]. [PMID: 21124738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Numakawa T., Yamamoto N., Chiba S., Richards M., Ooshima Y., Kishi S., Hashido K., Adachi N., Kunugi H. Growth factors stimulate expression of neuronal and glial miR-132. Neurosci. Lett. 2011;505(3):242–247. doi: 10.1016/j.neulet.2011.10.025. [http://dx.doi.org/10.1016/j.neulet. 2011.10.025]. [PMID: 22027176]. [DOI] [PubMed] [Google Scholar]
- 97.Kawashima H., Numakawa T., Kumamaru E., Adachi N., Mizuno H., Ninomiya M., Kunugi H., Hashido K. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience. 2010;165(4):1301–1311. doi: 10.1016/j.neuroscience.2009.11.057. [http://dx.doi. org/10.1016/j.neuroscience.2009.11.057]. [PMID: 19958814]. [DOI] [PubMed] [Google Scholar]
- 98.Magill S.T., Cambronne X.A., Luikart B.W., Lioy D.T., Leighton B.H., Westbrook G.L., Mandel G., Goodman R.H. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Natl. Acad. Sci. USA. 2010;107(47):20382–20387. doi: 10.1073/pnas.1015691107. [http://dx.doi.org/10.1073/pnas. 1015691107]. [PMID: 21059906]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mellios N., Sugihara H., Castro J., Banerjee A., Le C., Kumar A., Crawford B., Strathmann J., Tropea D., Levine S.S., Edbauer D., Sur M. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nat. Neurosci. 2011;14(10):1240–1242. doi: 10.1038/nn.2909. [http://dx.doi.org/10.1038/nn.2909]. [PMID: 21892155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schratt G.M., Tuebing F., Nigh E.A., Kane C.G., Sabatini M.E., Kiebler M., Greenberg M.E. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. doi: 10.1038/nature04367. [http://dx.doi.org/10.1038/nature04367]. [PMID: 16421561]. [DOI] [PubMed] [Google Scholar]
- 101.Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P.F., Busch C.J., Kane C., Hübel K., Dekker F., Hedberg C., Rengarajan B., Drepper C., Waldmann H., Kauppinen S., Greenberg M.E., Draguhn A., Rehmsmeier M., Martinez J., Schratt G.M. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 2009;11(6):705–716. doi: 10.1038/ncb1876. [http://dx. doi.org/10.1038/ncb1876]. [PMID: 19465924]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin Q., Wei W., Coelho C.M., Li X., Baker-Andresen D., Dudley K., Ratnu V.S., Boskovic Z., Kobor M.S., Sun Y.E., Bredy T.W. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat. Neurosci. 2011;14(9):1115–1117. doi: 10.1038/nn.2891. [http://dx.doi.org/10.1038/nn.2891]. [PMID: 21841775]. [DOI] [PubMed] [Google Scholar]
- 103.Franzoni E., Booker S.A., Parthasarathy S., Rehfeld F., Grosser S., Srivatsa S., Fuchs H.R., Tarabykin V., Vida I., Wulczyn F.G. miR-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene Phf6. eLife. 2015;4:4. doi: 10.7554/eLife.04263. [http://dx.doi.org/10.7554/eLife.04263]. [PMID: 25556700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Busto G.U., Guven-Ozkan T., Fulga T.A., Van Vactor D., Davis R.L. microRNAs That Promote or Inhibit Memory Formation in Drosophila melanogaster. Genetics. 2015;200(2):569–580. doi: 10.1534/genetics.114.169623. [http:// dx.doi.org/10.1534/genetics.114.169623]. [PMID: 26088433]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guven-Ozkan T., Busto G.U., Schutte S.S., Cervantes-Sandoval I. ODowd, D.K.; Davis, R.L. MiR-980 Is a Memory Suppressor MicroRNA that Regulates the Autism-Susceptibility Gene A2bp1. Cell Reports. 2016;14(7):1698–1709. doi: 10.1016/j.celrep.2016.01.040. [http://dx.doi.org/10.1016/ j.celrep.2016.01.040]. [PMID: 26876166]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Q.S., Kagan B.L., Hirakura Y., Xie C.W. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J. Neurosci. Res. 2000;60(1):65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [http://dx.doi.org/ 10.1002/(SICI)1097-4547(20000401)60:1<65:AID-JNR7>3.0.CO; 2-Q]. [PMID: 10723069]. [DOI] [PubMed] [Google Scholar]
- 107.Hardy J.A., Higgins G.A. Alzheimers disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [http://dx.doi.org/ 10.1126/science.1566067]. [PMID: 1566067]. [DOI] [PubMed] [Google Scholar]
- 108.Mandelkow E.M., Mandelkow E. Tau in Alzheimers disease. Trends Cell Biol. 1998;8(11):425–427. doi: 10.1016/s0962-8924(98)01368-3. [http://dx.doi.org/10.1016/ S0962-8924(98)01368-3]. [PMID: 9854307]. [DOI] [PubMed] [Google Scholar]
- 109.Hébert S.S., Horré K., Nicolaï L., Papadopoulou A.S., Mandemakers W., Silahtaroglu A.N., Kauppinen S., Delacourte A., De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimers disease correlates with increased BACE1/ beta-secretase expression. Proc. Natl. Acad. Sci. USA. 2008;105(17):6415–6420. doi: 10.1073/pnas.0710263105. [http://dx.doi.org/10.1073/pnas.0710263105]. [PMID: 18434550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zovoilis A., Agbemenyah H.Y., Agis-Balboa R.C., Stilling R.M., Edbauer D., Rao P., Farinelli L., Delalle I., Schmitt A., Falkai P., Bahari-Javan S., Burkhardt S., Sananbenesi F., Fischer A. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30(20):4299–4308. doi: 10.1038/emboj.2011.327. [http://dx.doi.org/10.1038/ emboj.2011.327]. [PMID: 21946562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lau P., Bossers K., Janky R., Salta E., Frigerio C.S., Barbash S., Rothman R., Sierksma A.S., Thathiah A., Greenberg D., Papadopoulou A.S., Achsel T., Ayoubi T., Soreq H., Verhaagen J., Swaab D.F., Aerts S., De Strooper B. Alteration of the microRNA network during the progression of Alzheimers disease. EMBO Mol. Med. 2013;5(10):1613–1634. doi: 10.1002/emmm.201201974. [http://dx.doi.org/ 10.1002/emmm.201201974]. [PMID: 24014289]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith P.Y., Delay C., Girard J., Papon M.A., Planel E., Sergeant N., Buée L., Hébert S.S. MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum. Mol. Genet. 2011;20(20):4016–4024. doi: 10.1093/hmg/ddr330. [http://dx.doi.org/10.1093/ hmg/ddr330]. [PMID: 21807765]. [DOI] [PubMed] [Google Scholar]
- 113.Wong H.K., Veremeyko T., Patel N., Lemere C.A., Walsh D.M., Esau C., Vanderburg C., Krichevsky A.M. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimers disease. Hum. Mol. Genet. 2013;22(15):3077–3092. doi: 10.1093/hmg/ddt164. [http://dx.doi.org/10.1093/hmg/ddt164]. [PMID: 23585551]. [DOI] [PubMed] [Google Scholar]
- 114.Grasso M., Piscopo P., Confaloni A., Denti M.A. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules. 2014;19(5):6891–6910. doi: 10.3390/molecules19056891. [http://dx.doi.org/10.3390/molecules 19056891]. [PMID: 24858274]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Denk J., Boelmans K., Siegismund C., Lassner D., Arlt S., Jahn H. MicroRNA Profiling of CSF Reveals Potential Biomarkers to Detect Alzheimers Disease. PLoS One. 2015;10(5):e0126423. doi: 10.1371/journal.pone.0126423. [http:// dx.doi.org/10.1371/journal.pone.0126423]. [PMID: 25992776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoeffer C.A., Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [http://dx.doi.org/10.1016/j.tins.2009.11.003]. [PMID: 19963289]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Samii A., Nutt J.G., Ransom B.R. Parkinsons disease. Lancet. 2004;363(9423):1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [http://dx.doi.org/10.1016/S0140-6736(04)16305-8]. [PMID: 15172778]. [DOI] [PubMed] [Google Scholar]
- 118.Junn E., Lee K.W., Jeong B.S., Chan T.W., Im J.Y., Mouradian M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA. 2009;106(31):13052–13057. doi: 10.1073/pnas.0906277106. [http://dx.doi.org/10.1073/pnas.0906277106]. [PMID: 19628698]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010;285(17):12726–12734. doi: 10.1074/jbc.M109.086827. [http://dx.doi.org/10.1074/jbc.M109.086827]. [PMID: 20106983]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Z., Cheng Y. miR-16-1 promotes the aberrant alphasynuclein accumulation in parkinson disease via targeting heat shock protein 70. Sci. World J., 2014. 938348. [DOI] [PMC free article] [PubMed]
- 121.Parisiadou L., Xie C., Cho H.J., Lin X., Gu X.L., Long C.X., Lobbestael E., Baekelandt V., Taymans J.M., Sun L., Cai H. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 2009;29(44):13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [http:// dx.doi.org/10.1523/JNEUROSCI.3799-09.2009]. [PMID: 19890007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taylor J.P., Mata I.F., Farrer M.J. LRRK2: a common pathway for parkinsonism, pathogenesis and prevention? Trends Mol. Med. 2006;12(2):76–82. doi: 10.1016/j.molmed.2005.12.004. [http://dx.doi.org/10.1016/j.molmed.2005.12. 004]. [PMID: 16406842]. [DOI] [PubMed] [Google Scholar]
- 123.Rajasethupathy P., Fiumara F., Sheridan R., Betel D., Puthanveettil S.V., Russo J.J., Sander C., Tuschl T., Kandel E. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63(6):803–817. doi: 10.1016/j.neuron.2009.05.029. [http://dx.doi.org/10.1016/j.neuron.2009.05. 029]. [PMID: 19778509]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Franke K., Otto W., Johannes S., Baumgart J., Nitsch R., Schumacher S. miR-124-regulated RhoG reduces neuronal process complexity via ELMO/Dock180/Rac1 and Cdc42 signalling. EMBO J. 2012;31(13):2908–2921. doi: 10.1038/emboj.2012.130. [http://dx.doi.org/10.1038/ emboj.2012.130]. [PMID: 22588079]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gu X., Meng S., Liu S., Jia C., Fang Y., Li S., Fu C., Song Q., Lin L., Wang X. miR-124 represses ROCK1 expression to promote neurite elongation through activation of the PI3K/Akt signal pathway. J. Mol. Neurosci. 2014;52(1):156–165. doi: 10.1007/s12031-013-0190-6. [http:// dx.doi.org/10.1007/s12031-013-0190-6]. [PMID: 24338057]. [DOI] [PubMed] [Google Scholar]
- 126.Bruno I.G., Karam R., Huang L., Bhardwaj A., Lou C.H., Shum E.Y., Song H.W., Corbett M.A., Gifford W.D., Gecz J., Pfaff S.L., Wilkinson M.F. Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Mol. Cell. 2011;42(4):500–510. doi: 10.1016/j.molcel.2011.04.018. [http://dx.doi.org/10.1016/ j.molcel.2011.04.018]. [PMID: 21596314]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cho H.J., Liu G., Jin S.M., Parisiadou L., Xie C., Yu J., Sun L., Ma B., Ding J., Vancraenenbroeck R., Lobbestael E., Baekelandt V., Taymans J.M., He P., Troncoso J.C., Shen Y., Cai H. MicroRNA-205 regulates the expression of Parkinsons disease-related leucine-rich repeat kinase 2 protein. Hum. Mol. Genet. 2013;22(3):608–620. doi: 10.1093/hmg/dds470. [http://dx.doi.org/10.1093/hmg/ dds470]. [PMID: 23125283]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alvarez-Erviti L., Seow Y., Schapira A.H., Rodriguez-Oroz M.C., Obeso J.A., Cooper J.M. Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinsons disease. Cell Death Dis. 2013;4:e545. doi: 10.1038/cddis.2013.73. [http://dx.doi.org/10.1038/cddis.2013.73]. [PMID: 23492776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harrison P.J. Schizophrenia: a disorder of neurodevelopment? Curr. Opin. Neurobiol. 1997;7(2):285–289. doi: 10.1016/s0959-4388(97)80018-9. [http://dx.doi.org/10. 1016/S0959-4388(97)80018-9]. [PMID: 9142763]. [DOI] [PubMed] [Google Scholar]
- 130.Roth T.L., Lubin F.D., Sodhi M., Kleinman J.E. Epigenetic mechanisms in schizophrenia. Biochim. Biophys. Acta. 2009;1790(9):869–877. doi: 10.1016/j.bbagen.2009.06.009. [http://dx.doi.org/10.1016/j.bbagen.2009.06.009]. [PMID: 19559755]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arnold S.E., Franz B.R., Gur R.C., Gur R.E., Shapiro R.M., Moberg P.J., Trojanowski J.Q. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am. J. Psychiatry. 1995;152(5):738–748. doi: 10.1176/ajp.152.5.738. [http://dx.doi.org/10.1176/ajp.152.5.738]. [PMID: 7726314]. [DOI] [PubMed] [Google Scholar]
- 132.Falkai P., Bogerts B. Cell loss in the hippocampus of schizophrenics. Eur. Arch. Psychiatry Neurol. Sci. 1986;236(3):154–161. doi: 10.1007/BF00380943. [http://dx.doi.org/10.1007/BF00380943]. [PMID: 3803399]. [DOI] [PubMed] [Google Scholar]
- 133.Jeste D.V., Lohr J.B. Hippocampal pathologic findings in schizophrenia. A morphometric study. Arch. Gen. Psychiatry. 1989;46(11):1019–1024. doi: 10.1001/archpsyc.1989.01810110061009. [http://dx.doi.org/10.1001/archpsyc.1989. 01810110061009]. [PMID: 2818139]. [DOI] [PubMed] [Google Scholar]
- 134.Jönsson S.A., Luts A., Guldberg-Kjaer N., Brun A. Hippocampal pyramidal cell disarray correlates negatively to cell number: implications for the pathogenesis of schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 1997;247(3):120–127. doi: 10.1007/BF03033065. [http:// dx.doi.org/10.1007/BF03033065]. [PMID: 9224904]. [DOI] [PubMed] [Google Scholar]
- 135.Bowie C.R., Harvey P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006;2(4):531–536. doi: 10.2147/nedt.2006.2.4.531. [http://dx.doi.org/10.2147/nedt.2006.2.4.531]. [PMID: 19412501]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim A.H., Reimers M., Maher B., Williamson V., McMichael O., McClay J.L., van den Oord E.J., Riley B.P., Kendler K.S., Vladimirov V.I. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr. Res. 2010;124(1-3):183–191. doi: 10.1016/j.schres.2010.07.002. [http://dx.doi. org/10.1016/j.schres.2010.07.002]. [PMID: 20675101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moreau M.P., Bruse S.E., David-Rus R., Buyske S., Brzustowicz L.M. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol. Psychiatry. 2011;69(2):188–193. doi: 10.1016/j.biopsych.2010.09.039. [http://dx. doi.org/10.1016/j.biopsych.2010.09.039]. [PMID: 21183010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Santarelli D.M., Beveridge N.J., Tooney P.A., Cairns M.J. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol. Psychiatry. 2011;69(2):180–187. doi: 10.1016/j.biopsych.2010.09.030. [http://dx.doi.org/10.1016/ j.biopsych.2010.09.030]. [PMID: 21111402]. [DOI] [PubMed] [Google Scholar]
- 139.Perkins D.O., Jeffries C., Sullivan P. Expanding the central dogma: the regulatory role of nonprotein coding genes and implications for the genetic liability to schizophrenia. Mol. Psychiatry. 2005;10(1):69–78. doi: 10.1038/sj.mp.4001577. [http://dx.doi.org/10.1038/sj.mp.4001577]. [PMID: 15381925]. [DOI] [PubMed] [Google Scholar]
- 140.Perkins D.O., Jeffries C.D., Jarskog L.F., Thomson J.M., Woods K., Newman M.A., Parker J.S., Jin J., Hammond S.M. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. [http://dx.doi.org/10.1186/gb-2007-8-2-r27]. [PMID: 17326821]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miller B.H., Zeier Z., Xi L., Lanz T.A., Deng S., Strathmann J., Willoughby D., Kenny P.J., Elsworth J.D., Lawrence M.S., Roth R.H., Edbauer D., Kleiman R.J., Wahlestedt C. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc. Natl. Acad. Sci. USA. 2012;109(8):3125–3130. doi: 10.1073/pnas.1113793109. [http://dx.doi.org/10.1073/pnas. 1113793109]. [PMID: 22315408]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beveridge N.J., Tooney P.A., Carroll A.P., Gardiner E., Bowden N., Scott R.J., Tran N., Dedova I., Cairns M.J. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum. Mol. Genet. 2008;17(8):1156–1168. doi: 10.1093/hmg/ddn005. [http://dx.doi.org/10.1093/ hmg/ddn005]. [PMID: 18184693]. [DOI] [PubMed] [Google Scholar]
- 143.Beveridge N.J., Gardiner E., Carroll A.P., Tooney P.A., Cairns M.J. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010;15(12):1176–1189. doi: 10.1038/mp.2009.84. [http://dx.doi.org/10.1038/mp.2009.84]. [PMID: 19721432]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hansen T., Olsen L., Lindow M., Jakobsen K.D., Ullum H., Jonsson E., Andreassen O.A., Djurovic S., Melle I., Agartz I., Hall H., Timm S., Wang A.G., Werge T. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS One. 2007;2(9):e873. doi: 10.1371/journal.pone.0000873. [http://dx.doi.org/10.1371/journal.pone.0000873]. [PMID: 17849003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aston C., Jiang L., Sokolov B.P. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J. Neurosci. Res. 2004;77(6):858–866. doi: 10.1002/jnr.20208. [http://dx.doi.org/10.1002/ jnr.20208]. [PMID: 15334603]. [DOI] [PubMed] [Google Scholar]
- 146.Kwon E., Wang W., Tsai L.H. Validation of schizophrenia-associated genes CSMD1, C10orf26, CACNA1C and TCF4 as miR-137 targets. Mol. Psychiatry. 2013;18(1):11–12. doi: 10.1038/mp.2011.170. [http://dx.doi.org/ 10.1038/mp.2011.170]. [PMID: 22182936]. [DOI] [PubMed] [Google Scholar]
- 147.Kim A.H., Parker E.K., Williamson V., McMichael G.O., Fanous A.H., Vladimirov V.I. Experimental validation of candidate schizophrenia gene ZNF804A as target for hsa-miR-137. Schizophr. Res. 2012;141(1):60–64. doi: 10.1016/j.schres.2012.06.038. [http://dx.doi.org/10.1016/j.schres.2012. 06.038]. [PMID: 22883350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi S., Leites C., He D., Schwartz D., Moy W., Shi J., Duan J. MicroRNA-9 and microRNA-326 regulate human dopamine D2 receptor expression, and the microRNA-mediated expression regulation is altered by a genetic variant. J. Biol. Chem. 2014;289(19):13434–13444. doi: 10.1074/jbc.M113.535203. [http://dx.doi.org/10.1074/jbc.M113.535203]. [PMID: 24675081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gong Y., Wu C.N., Xu J., Feng G., Xing Q.H., Fu W., Li C., He L., Zhao X.Z. Polymorphisms in microRNA target sites influence susceptibility to schizophrenia by altering the binding of miRNAs to their targets. Eur. Neuropsychopharmacol. 2013;23(10):1182–1189. doi: 10.1016/j.euroneuro.2012.12.002. [http://dx.doi.org/10.1016/j.euroneuro.2012.12. 002]. [PMID: 23332465]. [DOI] [PubMed] [Google Scholar]
- 150.Kocerha J., Faghihi M.A., Lopez-Toledano M.A., Huang J., Ramsey A.J., Caron M.G., Sales N., Willoughby D., Elmen J., Hansen H.F., Orum H., Kauppinen S., Kenny P.J., Wahlestedt C. MicroRNA-219 modulates NMDA receptor-mediated neuro- behavioral dysfunction. Proc. Natl. Acad. Sci. USA. 2009;106(9):3507–3512. doi: 10.1073/pnas.0805854106. [http://dx.doi.org/10.1073/pnas.0805854106]. [PMID: 19196972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Levy S.E., Mandell D.S., Schultz R.T. Autism. Lancet. 2009;374(9701):1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [http://dx.doi.org/10.1016/S0140-6736(09) 61376-3]. [PMID: 19819542]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li X., Zou H., Brown W.T. Genes associated with autism spectrum disorder. Brain Res. Bull. 2012;88(6):543–552. doi: 10.1016/j.brainresbull.2012.05.017. [http:// dx.doi.org/10.1016/j.brainresbull.2012.05.017]. [PMID: 22688012]. [DOI] [PubMed] [Google Scholar]
- 153.Pardo C.A., Eberhart C.G. The neurobiology of autism. Brain Pathol. 2007;17(4):434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [http://dx.doi.org/10.1111/j.1750-3639.2007.00102.x]. [PMID: 17919129]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sellier C., Hwang V.J., Dandekar R., Durbin-Johnson B., Charlet-Berguerand N., Ander B.P., Sharp F.R., Angkustsiri K., Simon T.J., Tassone F. Decreased DGCR8 expression and miRNA dysregulation in individuals with 22q11.2 deletion syndrome. PLoS One. 2014;9(8):e103884. doi: 10.1371/journal.pone.0103884. [http://dx.doi.org/10.1371/journal.pone. 0103884]. [PMID: 25084529]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vaishnavi V., Manikandan M., Tiwary B.K., Munirajan A.K. Insights on the functional impact of microRNAs present in autism-associated copy number variants. PLoS One. 2013;8(2):e56781. doi: 10.1371/journal.pone.0056781. [http://dx.doi.org/10.1371/journal.pone.0056781]. [PMID: 23451085]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abu-Elneel K., Liu T., Gazzaniga F.S., Nishimura Y., Wall D.P., Geschwind D.H., Lao K., Kosik K.S. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9(3):153–161. doi: 10.1007/s10048-008-0133-5. [http://dx.doi.org/10.1007/ s10048-008-0133-5]. [PMID: 18563458]. [DOI] [PubMed] [Google Scholar]
- 157.Sarachana T., Zhou R., Chen G., Manji H.K., Hu V.W. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010;2(4):23. doi: 10.1186/gm144. [http://dx.doi.org/10.1186/gm144]. [PMID: 20374639]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ghahramani Seno M.M., Hu P., Gwadry F.G., Pinto D., Marshall C.R., Casallo G., Scherer S.W. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res. 2011;1380:85–97. doi: 10.1016/j.brainres.2010.09.046. [http://dx.doi.org/10.1016/j.brainres.2010.09.046]. [PMID: 20868653]. [DOI] [PubMed] [Google Scholar]
- 159.Willemsen M.H., Vallès A., Kirkels L.A., Mastebroek M., Olde Loohuis N., Kos A., Wissink-Lindhout W.M., de Brouwer A.P., Nillesen W.M., Pfundt R., Holder-Espinasse M., Vallée L., Andrieux J., Coppens-Hofman M.C., Rensen H., Hamel B.C., van Bokhoven H., Aschrafi A., Kleefstra T. Chromosome 1p21.3 microdeletions comprising DPYD and MIR137 are associated with intellectual disability. J. Med. Genet. 2011;48(12):810–818. doi: 10.1136/jmedgenet-2011-100294. [http://dx.doi.org/10.1136/jmedgenet-2011-100294]. [PMID: 22003227]. [DOI] [PubMed] [Google Scholar]
- 160.Nguyen A., Rauch T.A., Pfeifer G.P., Hu V.W. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010;24(8):3036–3051. doi: 10.1096/fj.10-154484. [http://dx. doi.org/10.1096/fj.10-154484]. [PMID: 20375269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Devanna P., Vernes S.C. A direct molecular link between the autism candidate gene RORa and the schizophrenia candidate MIR137. Sci. Rep. 2014;4:3994. doi: 10.1038/srep03994. [http://dx.doi.org/10.1038/ srep03994]. [PMID: 24500708]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gusella J.F., MacDonald M.E. Huntingtons disease. Semin. Cell Biol. 1995;6(1):21–28. doi: 10.1016/1043-4682(95)90011-x. [http://dx.doi.org/10.1016/1043-4682(95) 90011-X]. [PMID: 7620118]. [DOI] [PubMed] [Google Scholar]
- 163.Penney J.B., Jr, Young A.B., Shoulson I., Starosta-Rubenstein S., Snodgrass S.R., Sanchez-Ramos J., Ramos-Arroyo M., Gomez F., Penchaszadeh G., Alvir J. Huntingtons disease in Venezuela: 7 years of follow-up on symptomatic and asymptomatic individuals. Mov. Disord. 1990;5(2):93–99. doi: 10.1002/mds.870050202. [http://dx.doi.org/ 10.1002/mds.870050202]. [PMID: 2139171]. [DOI] [PubMed] [Google Scholar]
- 164.Roos R.A. Huntingtons disease: a clinical review. Orphanet J. Rare Dis. 2010;5:40. doi: 10.1186/1750-1172-5-40. [http://dx.doi.org/10.1186/1750-1172-5-40]. [PMID: 21171977]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Albin R.L. Selective neurodegeneration in Huntingtons disease. Ann. Neurol. 1995;38(6):835–836. doi: 10.1002/ana.410380602. [http://dx.doi.org/10.1002/ana. 410380602]. [PMID: 8526454]. [DOI] [PubMed] [Google Scholar]
- 166.Vonsattel J.P., Myers R.H., Stevens T.J., Ferrante R.J., Bird E.D., Richardson E.P. Jr Neuropathological classification of Huntingtons disease. J. Neuropathol. Exp. Neurol. 1985;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [http://dx.doi.org/10.1097/00005072-198511000-00003]. [PMID: 2932539]. [DOI] [PubMed] [Google Scholar]
- 167.Guo Z., Rudow G., Pletnikova O., Codispoti K.E., Orr B.A., Crain B.J., Duan W., Margolis R.L., Rosenblatt A., Ross C.A., Troncoso J.C. Striatal neuronal loss correlates with clinical motor impairment in Huntingtons disease. Mov. Disord. 2012;27(11):1379–1386. doi: 10.1002/mds.25159. [http://dx.doi.org/10.1002/mds.25159]. [PMID: 22975850]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walker F.O. Huntingtons disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [http://dx.doi.org/10.1016/S0140-6736(07)60111-1]. [PMID: 17240289]. [DOI] [PubMed] [Google Scholar]
- 169.Adam O.R., Jankovic J. Symptomatic treatment of Huntington disease. Neurotherapeutics. 2008;5(2):181–197. doi: 10.1016/j.nurt.2008.01.008. [http://dx.doi. org/10.1016/j.nurt.2008.01.008]. [PMID: 18394562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Frank S., Jankovic J. Advances in the pharmacological management of Huntingtons disease. Drugs. 2010;70(5):561–571. doi: 10.2165/11534430-000000000-00000. [http://dx. doi.org/10.2165/11534430-000000000-00000]. [PMID: 20329804]. [DOI] [PubMed] [Google Scholar]
- 171.Lauterbach E.C. Neuroprotective effects of psychotropic drugs in Huntingtons disease. Int. J. Mol. Sci. 2013;14(11):22558–22603. doi: 10.3390/ijms141122558. [http://dx.doi.org/10.3390/ijms141122558]. [PMID: 24248060]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson R., Zuccato C., Belyaev N.D., Guest D.J., Cattaneo E., Buckley N.J. A microRNA-based gene dysregulation pathway in Huntingtons disease. Neurobiol. Dis. 2008;29(3):438–445. doi: 10.1016/j.nbd.2007.11.001. [http://dx.doi.org/10.1016/j.nbd.2007.11.001]. [PMID: 18082412]. [DOI] [PubMed] [Google Scholar]
- 173.Lee S.T., Chu K. Im, W.S.; Yoon, H.J.; Im, J.Y.; Park, J.E.; Park, K.H.; Jung, K.H.; Lee, S.K.; Kim, M.; Roh, J.K. Altered microRNA regulation in Huntingtons disease models. Exp. Neurol. 2011;227(1):172–179. doi: 10.1016/j.expneurol.2010.10.012. [http://dx.doi.org/10.1016/j.expneurol.2010. 10.012]. [PMID: 21035445]. [DOI] [PubMed] [Google Scholar]
- 174.Jin J., Cheng Y., Zhang Y., Wood W., Peng Q., Hutchison E., Mattson M.P., Becker K.G., Duan W. Interrogation of brain miRNA and mRNA expression profiles reveals a molecular regulatory network that is perturbed by mutant huntingtin. J. Neurochem. 2012;123(4):477–490. doi: 10.1111/j.1471-4159.2012.07925.x. [http://dx.doi.org/10.1111/j. 1471-4159.2012.07925.x]. [PMID: 22906125]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cheng P.H., Li C.L., Chang Y.F., Tsai S.J., Lai Y.Y., Chan A.W., Chen C.M., Yang S.H. miR-196a ameliorates phenotypes of Huntington disease in cell, transgenic mouse, and induced pluripotent stem cell models. Am. J. Hum. Genet. 2013;93(2):306–312. doi: 10.1016/j.ajhg.2013.05.025. [http://dx.doi.org/10.1016/j.ajhg.2013.05.025]. [PMID: 23810380]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hoss A.G., Labadorf A., Latourelle J.C., Kartha V.K., Hadzi T.C., Gusella J.F., MacDonald M.E., Chen J.F., Akbarian S., Weng Z., Vonsattel J.P., Myers R.H. miR-10b-5p expression in Huntingtons disease brain relates to age of onset and the extent of striatal involvement. BMC Med. Genomics. 2015;8(1):10. doi: 10.1186/s12920-015-0083-3. [http:// dx.doi.org/10.1186/s12920-015-0083-3]. [PMID: 25889241]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J. OBriant, K.C.; Allen, A.; Lin, D.W.; Urban, N.; Drescher, C.W.; Knudsen, B.S.; Stirewalt, D.L.; Gentleman, R.; Vessella, R.L.; Nelson, P.S.; Martin, D.B.; Tewari, M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [http://dx.doi.org/10.1073/pnas. 0804549105]. [PMID: 18663219]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., Li Q., Li X., Wang W., Zhang Y., Wang J., Jiang X., Xiang Y., Xu C., Zheng P., Zhang J., Li R., Zhang H., Shang X., Gong T., Ning G., Wang J., Zen K., Zhang J., Zhang C.Y. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [http://dx.doi.org/ 10.1038/cr.2008.282]. [PMID: 18766170]. [DOI] [PubMed] [Google Scholar]
- 179.Gaughwin P.M., Ciesla M., Lahiri N., Tabrizi S.J., Brundin P., Björkqvist M. Hsa-miR-34b is a plasma-stable microRNA that is elevated in pre-manifest Huntingtons disease. Hum. Mol. Genet. 2011;20(11):2225–2237. doi: 10.1093/hmg/ddr111. [http://dx.doi.org/10.1093/hmg/ddr111]. [PMID: 21421997]. [DOI] [PubMed] [Google Scholar]
- 180.Miñones-Moyano E., Porta S., Escaramís G., Rabionet R., Iraola S., Kagerbauer B., Espinosa-Parrilla Y., Ferrer I., Estivill X., Martí E. MicroRNA profiling of Parkinsons disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet. 2011;20(15):3067–3078. doi: 10.1093/hmg/ddr210. [http://dx.doi.org/10.1093/hmg/ddr210]. [PMID: 21558425]. [DOI] [PubMed] [Google Scholar]


