Abstract
Given the importance of microRNAs (miRNAs) in modulating brain functions and their implications in neurocognitive disorders there are currently significant efforts devoted in the field of miRNA-based therapeutics to correct and/or to treat these brain diseases. The observation that miRNA 29a/b-1 cluster, miRNA 10b and miRNA 7, for instance, are frequently deregulated in the brains of patients with neurocognitive diseases and in animal models of Alzheimer, Huntington’s and Parkinson’s diseases, suggest that correction of miRNA expression using agonist or antagonist miRNA oligonucleotides might be a promising approach to correct or even to cure such diseases. The encouraging results from recent clinical trials allow envisioning that pharmacological approaches based on miRNAs might, in a near future, reach the requirements for successful therapeutic outcomes and will improve the healthcare of patients with brain injuries or disorders. This review will focus on the current strategies used to modulate pharmacological function of miRNA using chemically modified oligonucleotides. We will then review the recent literature on strategies to improve nucleic acid delivery across the blood-brain barrier which remains a severe obstacle to the widespread application of miRNA therapeutics to treat brain diseases. Finally, we provide a state-of-art of current preclinical research performed in animal models for the treatment of neurocognitive disorders using miRNA as therapeutic agents and discuss future developments of miRNA therapeutics.
Keywords: AMO, antagomir, LNA, miRNA, miRNA mimics, neurocognitive diseases, therapy
1. INTRODUCTION
MicroRNAs (miRNA) are a class of short non-coding RNA molecules that control the expression of target genes at the posttranscriptional level in plants and animals. They play essential roles in diverse biological processes through a Watson–Crick base pairing mechanism between, mainly, the 5’ part of miRNA and the 3′ untranslated region (UTR) of mRNAs, inducing gene silencing [1]. Since the discovery of miRNAs in the early 1990s in development timing of C.elegans [2], more 30.424 miRNAs have been recently annotated in 206 species [3]. It is now established that deregulated expression of miRNAs is a hallmark of several diseases and that manipulating expression of miRNAs in pathological tissues is sometimes sufficient to reverse the pathological state of the disease [4].
Most miRNA genes are usually transcribed by polymerase II from endogenous genes either from individual and/or polytranscriptional units (intergenic miRNAs) or in frame with host genes (intronic miRNAs) (Fig. 1) [1]. They are processed in the nucleus as long primary miRNA (pri-miRNA) transcripts before being shortened as precursor (pre-miRNA) transcripts of approximately 70 nucleotides by a first microprocessor complex consisting of Drosha (also known as RNASEN) and DGCR8 (DiGeorge syndrome critical region 8). Alternatively, a minority of pre-miRNA, called mirtrons (mitronic miRNAs) are not processed by Drosha but rather are produced from intronic hairpins deriving from splicing of protein-coding genes [5, 6]. In both pathways, the pre-miRNAs are exported into the cytoplasm by the Exportin-5/Ran-GTP complex and then cleaved by a second endoribonuclease complex, consisting of the DICER enzyme and its ribonucleoprotein binding partner TRBP (TAR RNA-binding protein 2) to produce a 20 to 22 nucleotide-long duplex RNA molecule. The miRNA duplexes are incorporated into the miRNA-induced silencing complex (miRISC) containing the Argonaute protein which initiates the unwinding of the miRNA duplex to retain the mature strand while the complementary passenger strand is degraded [7]. In the miRISC, the mature miRNA strand binds to the mRNA target sequence through base pairing mechanism occurring between the seed region (2-8 nucleotides of the 5’-end sequence of miRNAs) and on the 3’-UTR part of several target mRNAs. Alternatively, imperfect or nearly perfect base pairing between the miRNA sequence and the 3’ and/or 5’-UTR parts of mRNAs have also been described [8]. The bound mRNA is then either degraded or the translational machinery is blocked inducing mRNA deadenylation and/or decapping [1, 4] (Fig. 1).
Fig. (1).
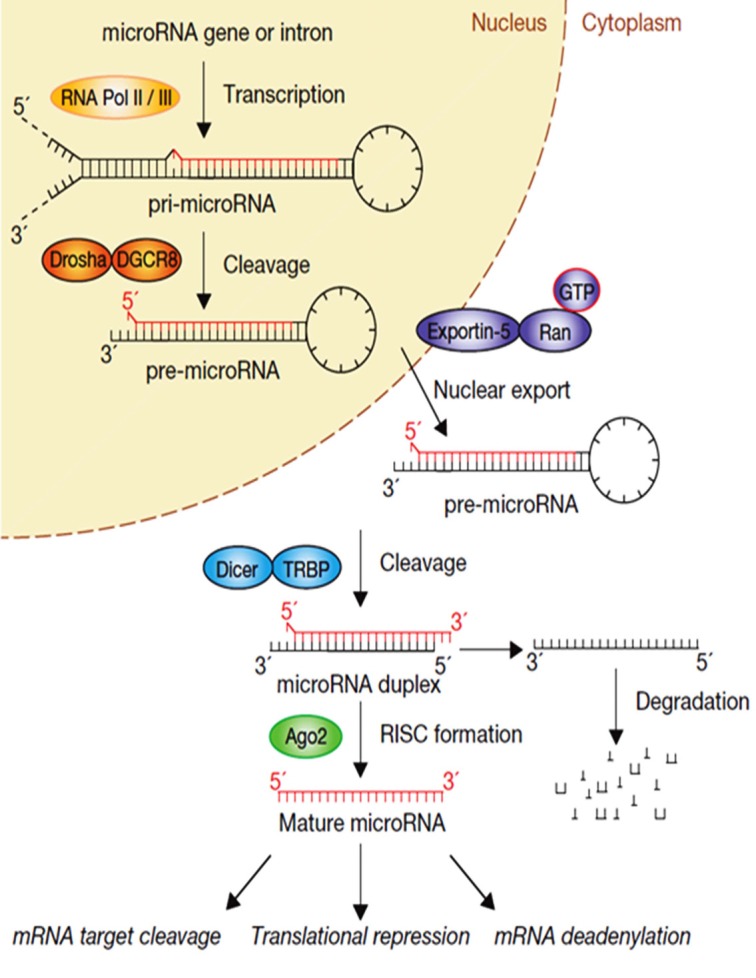
MiRNA biogenesis. In the canonical pathway, miRNA genes are transcribed by RNA polymerase II from intergenic or intronic loci to long several kilobases pri-miRNAs transcripts before being processed by the Drosha–DGCR8 endoribonuclease complex to form 70 nt pre-miRNA hairpin structures. The pre-miRNAs are then transported by exportin 5 into the cytoplasm, where they are processed by a second endoribonuclease complex consisting in Dicer–TRBP to form miRNA duplexes. The duplexes are loaded in Argonaute 2 (AGO2)-containing RNA-induced silencing complexes (miRISCs) where the single strand mature miRNA suppresses downstream target mRNAs either by translational repression and/or by mRNA degradation. Reprint and adapted with permission from [1].
Due to the short length of the seed regions that interact with mRNAs, a single miRNA can theoretically repress many closely related target genes. Furthermore, the majority of mRNAs contain multiple potential miRNA-binding sites in their 3’-UTR, meaning that one given mRNA can be regulated by multiple miRNAs. In fact there are an average of 300 conserved targets predicted for each evolutionarily conserved miRNA [9] and functional gain-and-off studies indicated that manipulating a single miRNA can alter the abundance of many downstream mRNAs [10]. Computational prediction studies estimate that at least 60% of the human transcriptome can be regulated by miRNAs. Therefore, it is not surprising that miRNA deregulation is common motif in cancer, metabolic disorders, inflammation, cardiovascular diseases and neurological disorder as neurocognitive disorders. In these latter disorders, as detailed in the previous manuscript review, the miRNA-29a/b-1 cluster was found to be a potential major suppressor of beta-site amyloid precursor protein cleaving enzyme BACE1 in Alzheimer’s disease while the miRNA-10b-5p was found to be one of the most differentially expressed miRNA in tissues of patients with Huntington’s disease. In Parkinson’s disease, miRNA-7 was found as a direct regulator of α-synuclein expression. Indeed, the ectopic expression of this miRNA significantly reduced accumulation of α-synuclein in cortical neurons [11]. Other examples include abnormal expression levels of miRNA-9, -124 and -219 in Schizophrenia. These miRNAs were found to control the expression of multiple genes involved in this neuropsychiatric disorder such as the dopamine receptor DE (DRD2), the regulator of G protein signalling 4 (RGS4) or the N-methyl-D aspartate (NMDAZ) receptor signalling.
Therefore, miRNA deregulation associated with gain-of-function studies performed in relevant cellular and animal models of cognitive disorders have paved the way for therapeutic interventions to correct neurocognitive disorders. This review will focus first on the current strategies used to modulate the pharmacological function of miRNAs using chemically modified oligonucleotides. We then review the recent literature on strategies to improve nucleic acid delivery across the blood-brain barrier that remains a significant obstacle to the widespread application of miRNA therapeutics to treat brain diseases. Finally, we provide a synopsis of current state-of-art preclinical research performed in animal model, for the treatment of neurocognitive disorders using miRNA as therapeutic agents.
2. DESIGN AND CHEMICAL MODIFICATIONS OF MIRNA OLIGONUCLEOTIDES
Currently, two approaches are employed to modulate the expression or the activity of endogenously expressed miRNAs. The first approach consists of inhibiting the function of miRNAs using single-stranded antisense miRNAs oligonucleotides (AMOs). This approach generally referred to as antagonist therapy, aims to block the interactions between miRNA and its endogenous mRNAs targets through a competitive base-pairing binding mechanism. The second approach consists of restoring the function of miRNAs using synthetic double-stranded oligonucleotides miRNAs to mimic (miRNA mimics) the endogenously expressed miRNA duplex. This approach is generally referred to as replacement and/or agonist therapy and aims to restore the functional activity of downregulated miRNAs in pathological cells or tissues.
Ideally, miRNAs therapeutic oligonucleotides would have several attributes to be functional such as (i) high affinity to targeted miRNA or mRNA nucleic acids, (ii) efficient uptake by the miRISC machinery when considering miRNA mimics and irreversible binding to endogenous miRNA molecules when considering AMO, (iii) resistance to exo- and endonucleases, (iv) low toxicity and good pharmacokinetics properties and (vi) when considering brain application, ability to diffuse through the blood brain barrier.
2.1. Design and Chemistry of Antisense miRNAs Oligonucleotides (AMO)
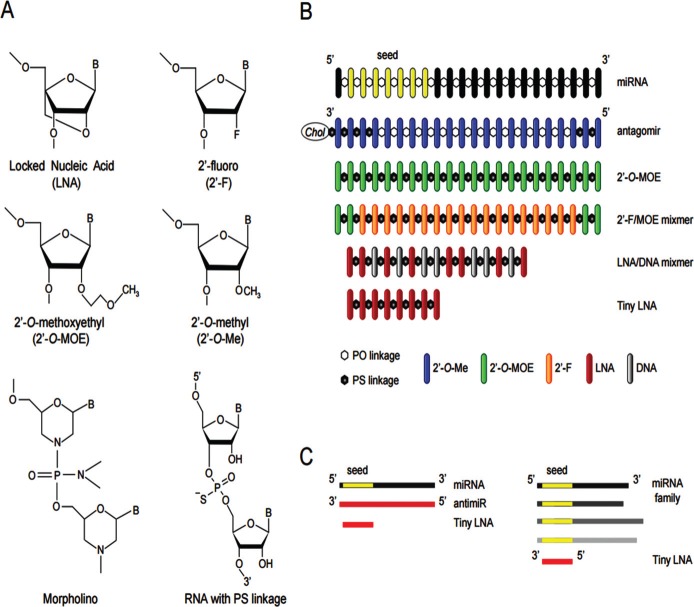
The AMO or antagonist therapy has been initially explored in early 2000 to suppress miRNA expression in Drosophila for loss-of-function studies. It was quickly appreciated that unmodified antisense oligonucleotides were prone to alter miRNA function but were, also, rapidly degraded by exonucleases and endonucleases present in serum and in the cytosolic compartment of cells [12]. Subsequently, several chemical modifications of oligonucleotides have been evaluated and are now well recognized as key steps to improve resistance to nucleases and enhance affinity and stability of AMOs to their miRNA targets. The most popular chemical modifications involve the addition of several types of chemical groups to the 2'-hydroxyl group of riboses of miRNA oligonucleotides [13].
The first chemical modification of AMO was the incorporation of 2'-O-methyl (2'OMe) residues to the C2 of the AMOs using RNA chemistry. This modification showed better resistance to cytosolic nucleases and improved binding affinity (melting temperature or Tm) to endogenous miRNAs. Hutvagner et al. [14] described the first reported 2'OMe AMO specific to the let-7 miRNA to suppress the translation repression of lin-41 in Caenorhabditis Elegans. The authors showed that this 2'OMe AMO acts as a potent irreversible miRNA inhibitor, inducing a let-7 loss-of-function phenotype without any toxicity or animal abnormal behaviour. Although 2'OMe AMO’s were more effective, nuclease protection was not total and still problematic. The observation that endo- and exonucleases cleave the phosphate bounds between nucleotides led to the idea of replacing the phosphate backbone of the phosphodiester bonds with sulphur atoms, resulting in modified phosphorothioate (PS) nucleotide bonds. Better resistance to nuclease degradation was observed, with a significant gain in stability without interfering with the structure of RNA oligonucleotides [15]. However, it also became apparent that not all phosphodiester bonds of oligonucleotide sequence could be substituted with PS modifications but, rather, should be chosen with caution. Indeed, PS modifications reduce Tm values by 0.25°C per modified linkage. Therefore, a mixture between unmodified phosphodiester (PO) and phosphorothioate (PS) linkages in AMOs is now commonly used and should be strictly evaluated [13, 15].
Since this pioneering work, additional strategies have been developed. These include the addition of methoxyethyl (2'-MOE) or fluorine (2'-F) groups and production of locked nucleic acids (LNA) [13]. LNA based- chemistry produces a methylene linker bridging the 2'-O-oxygen to the 4'-position of the ribose. The LNA modified riboses adopt a pseudo-rotation in space similar to C3'-endo conformers. This conformation favours thermodynamic stability and increases Tm values by a factor of 2 to 8°C per number of LNA modifications [16]. All of these chemical modifications are more potent than the original 2’OMe modification. For example, LNA with 2’fluoro RNA are more stable than 2’OMe RNA counterpart in 10% of foetal bovine serum thus demonstrating their stability [17]. Interestingly, and in contrast to 2’OMe modifications, multiple LNA residues can be incorporated through the whole oligonucleotides sequence without compromising the base-pairing interaction between the AMOs and the miRNAs [16, 18]. This demonstrates that LNAs are one of the most efficient and advanced chemical modification often included in most, if not all miRNA-based oligonucleotide therapies [19]. For example, it was shown that the LNA oligonucleotide SPC3649 designed to inhibit interaction between the liver miRNA-122 and the hepatitis C virus (HCV) genome led to long-lasting suppression of HCV viremia in primates with no side effects for several weeks [20]. This SPEC3649 AMO, called miravirsen [21] is currently under evaluation in a phase IIa clinical trial to treat HCV patients. Early results indicated safety, dose-dependent and prolonged antiviral activity. This first clinical trial based on miRNA therapy is expected to open the door to novel approaches to treat many other diseases.
In parallel, another generation of AMOs have emerged, antagomiRs (Fig. 2) consisting of 2'OMe modified ribose sugar in combination with terminal phosphorothioate linkages and the presence of a cholesterol group grafted at the 3’-end of the oligonucleotide sequence [15]. Cholesterol residues extend the blood-circulation time of AMOs when injected systemically, reduce their negative charges to facilitate interaction with plasma membrane and improve their pharmacokinetics. Pioneers studies have demonstrated that intravenous injection of 80 mg/kg of antagomirs against miRNA-16, miRNA-122, miRNA-192 and miRNA-194 led to significant reduction in levels of these miRNA in liver, lung, kidney, heart, intestine, fat, skin, bone marrow, muscle, ovaries and adrenal glands.
Fig. (2).
Design of chemically modified miRNA oligonucleotides. (A) Structures of the most commonly used chemically modified miRNA oligonucleotides that include the locked nucleic acid (LNA), the 2′-O-methyl (2′- O-Me), 2′-O-methoxyethyl (2′-MOE) and 2′-fluoro (2′-F) nucleotides and the morpholino oligomers. In addition, phosphorothioate (PS) backbone linkages are also used to increase nuclease resistance and enhance the pharmacokinetic properties of miRNA oligonucleotides. (B) Design of chemically modified antimiR oligonucleotides. (C) Schematic overview of the miRNA inhibition approach using a fully complementary antimiR and a seed-targeting tiny LNA. Reprint and adapted with permissions from [22].
This approach has been found specific, efficient and long-lasting [15], and associated with specific down-regulation of corresponding mRNAs targets in mice and primates [23]. The effectiveness of this approach has been confirmed by different groups and is now considered a relevant approach to inhibit miRNA function both in vitro and in vivo [24-26].
More recently, miRNA synthetic sponges, oligonucleotides containing multiple miRNA antisense binding sites to sequester miRNA, were also found to be a powerful approach to inhibit miRNA functions [27]. Kluiver et al. [28] demonstrated that the combined inhibition of the miRNA-17-92 cluster, using sponges containing binding sites for several elements of this family, was significantly more efficient in inhibiting the proliferation of cultured B-cell lymphoma cells, than individual miRNAs.
2.2. Design and Chemistry of miRNA Mimics
In contrast with the inhition of mIRNA functions, there is also considerable interest with the restoration of miRNA functions to reverse a pathological phenotype into a normal, healthy phenotype. Synthetic miRNA duplexes are commonly used to mimic the structure and activity of endogenously expressed miRNAs molecules. However, design of synthetic miRNA mimics is different from that of AMOs. Indeed, miRNA mimics to be functionally active should be (i) efficiently uptaken by the miRISC machinery, (ii) have the same thermostability properties as the endogenous miRNA duplex, (iii) should be resistant to endo and exonucleases and (iv) have a Tm optimized for efficient binding and reduction of off-targets [29, 30].
Earlier studies have shown that unmodified passenger strands of miRNA mimics are rapidly degraded allowing the guide mature strand to be free from the duplex and processed by the miRISC machinery as for endogenous miRNA duplex [30, 31]. This inherent feature was further exploited to graft functionalized groups such as cholesterol moieties at the 3’-end of the passenger strand to improve blood stability and
cellular uptake as described for AMOs [32]. Alternatively, cell addressing molecules such as antibodies or cell targeting ligands have been used to substitute the cholesterol groups for optimal delivery in specific organs. Recently, it has been demonstrated that few of the 5’-modifications of the passenger strand totally abolished miRISC machinery uptake reducing significantly non-specific repression of non-targeted mRNA [33]. However, the chemical modifications that the guide strand might harbour are much more limited than those required for AMO stability and potency. The use of uncontrolled miRNA modifications to the guide strand might compromise uptake by the miRISC machinery and reduce the overall efficacy of the strategy. In general, the 2’-F group was found to be well tolerated by miRISC, mainly by the Argonaute proteins, providing at the same time protection against nucleases and efficient binding to the mRNA targets. The 2'-O-Me and 2'-MOE modifications could also be used but their position in the oligonucleotide sequence seemed to be detrimental and requires optimisation [31, 33]. In contrast, aromatic compounds such as 3’-benzene-pyridine were better tolerated and improved protection from nuclease degradation when grafted to the 3' end of the oligonucleotide sequence [34]. Recently Chorn et al. also demonstrated that modified single-stranded miRNA mimics can be processed by the miRISC machinery to silence gene expression [35]. However, the potency of single-stranded mimics was lower than that achieved with double-stranded oligonucleotides.
A potential issue with miRNA replacement therapy, which is less true for AMO therapy, is the challenge of restoring to a physiological level the expression of miRNA, to be biologically active without reaching supra-physiological levels that would negatively impact the expression of other non-specific mRNAs. In addition, non-specific delivery of miRNA mimics in non-desired tissues could change the phenotype of transfected cells and potentially lead to off-target effects and toxicity. Results from preclinical studies demonstrated that enforced expression of miRNA-34a mimics in several cancer types induced cell-cycle arrest, apoptosis and inhibition of tumour progression and metastasis formation [36]. The great potential of this strategy is that miRNA-34a is a tumour suppressor, down-regulated in cancer cells while well expressed in healthy cells such as brain cells. Therefore, non-specific delivery of this miRNA mimics in normal cells is not expected to compromise the cellular viability nor change their phenotype. Indeed, the delivered dose of miRNA-34a is always lower than the endogenously expressed form of this miRNA in cells [37]. Results from an ongoing clinical trial, the first miRNA replacement strategy tested in humans, are expected to be released soon and may provide important information about the relevance of this strategy for more general use.
3. CONSIDERATION OF THE ADMINISTRATION ROUTES TO DELIVER MIRNA THERAPEUTICS IN THE BRAIN
Whereas preclinical studies, reinforced by the recent clinical trials, provided evidence that miRNA therapeutics hold promise to treat various diseases, their widespread application in the clinic is constrained by the lack of tissue specificity and by their capture into the liver and the lungs. It is commonly accepted that, most of the intravascularly administered drugs are non-specifically uptaken by the cells from the reticulo-endothelial-system and mainly by Kupffer cells in the liver [38]. Furthermore, in the specific context of brain therapy, lack of permeability of the blood-brain barrier (BBB) is a serious challenge to deliver sufficient amount of therapeutic drugs to brain tissues. This is clearly illustrated by the observation that systemic injection of a dye results in the staining of all organs except the brain [39] and further by the recent observation that the systemic delivery of antagomiRs against miRNA-16, a ubiquitously expressed miRNA, suppressed function of miRNA-16 in the majority of tissues tested except the brain [15]. In contrast, the injection of the same antagomiRs into the brain results in significant reduction of functions of this miRNA in the cortex, at the site of injection [40].
The blood-brain barrier (BBB) consists in two main barriers, the vascular capillaries and the blood-cerebrospinal fluid (blood-CSF). These barriers are structurally and biologically different from blood capillaries of other tissues, allowing protection of this specialized organ against potentially toxic substances including pharmaceutical products. Indeed, the BBB serves as a selective regulatory barrier to modulate the entry of circulating blood macromolecules to CNS, to maintain a homeostatic environment in the brain and to protect brain cells against toxins and pathogens. In addition, the BBB also regulates the release of neural substances (hormones, neurotransmitters, etc) into the blood circulation to regulate the homeostasis of many different tissues (for more details, see review from Rubin et al. [41]). Structurally, BBB is composed of capillary endothelial cells, supported by a basal lamina, pericytes and astrocytic endfeet. Tight junctions between endothelial cells, lack of intracellular fenestrations and the presence of a highly compacted and dense extracellular matrix in the basal lamina literally cement the BBB into an impermeable membrane, limiting plasma infiltrate into the CNS. The BBB is also characterized by a reduced rate of pinocytosis, hampering the passive diffusion of solutes through the endothelial layers in favour of transcellular exchange. In fact circulating molecules might diffuse into the brain by two main mechanisms: (1) lipid-mediated free diffusion and (2) carrier- or receptor-mediated transport (CMT or RMT) [42]. In the latter, different transporter proteins, receptors and enzymes are responsible for the trans-diffusion of essential metabolites into the brain such as glucose, nucleosides and amino acids. Both transporter systems are selective on the basis of the physicochemical characteristics of the substances. For example, blood-circulating albumin is poorly transported to the brain although vital for brain functions, but its cationization enhances its uptake [43].
The majority of drugs used in the clinic that diffuse to the brain by the lipid-mediated free diffusion process, have a molecular weight not larger than 400 Daltons and have limited hydrogen bonding for higher lipid solubility. This means that less than 10% of all available drugs are expected to be active in the brain [44, 45]. To circumvent this problem, several approaches have been developed to overcome the lack of permeability of the BBB. Surgical approaches are the most effective route to deliver drugs into specific regions of the brain. Stereotactic apparatus [46] connected to needles and/or pumps, assisted by computed tomography and/or magnetic resonance imaging allows precise and local delivery of drugs to a specific brain region, minimizing the disruption of surrounding tissues. Although this approach is used in the clinic, the risk to alter healthy tissues is high due to the needle track, and the risk increases when repeated administrations are required. Alternative approaches have been evaluated that include the intra- venticular, [47] including the intraventicular; intrathecal and transnasal routes of administration, the delivery through active or passive transporter systems and the temporary disruption of the BBB using chemical agents such alcohols and vasodilators. Some examples of these approaches are discussed below in the context of therapeutic oligonucleotides delivery into the brain.
3.1. Transnasal Delivery
In theory, transnasal drug delivery offers the opportunity to circumvent the blood-brain barrier to gain direct access to the brain tissues [44]. This approach employs a fairly passive process relying on drug instillation from the nasal cavity to the neural cells via the olfactory route [44]. In fact, drugs are transported extracellularly or intracellularly along the olfactory or trigeminal nerve systems which initiate in the brain and terminate in the nasal cavity at the olfactory neuro-epithelium or respiratory epithelium. In humans intranasal instillation is an accepted route to deliver small phar- macological drugs such as the vitamin B12 or melatonin. It has further been shown that intranasal administration of components such as NAD+ [48] or erythropoietin [49] is reliable, simple, fast and efficient method to improve neuroprotection against ischemic brain injury in rats and in humans. Intranasal administration of insulin also ameliorates attention and functional status in AD patients [45, 50]. However, the properties governing drug distribution from the nose to the olfactory CSF are similar to those governing the transport across the BBB. In fact, small lipophilic molecules are better transported than larger hydrosoluble molecules and molecules larger than 400 Daltons are also difficult to transport through the olfactory route [47]. Consequently, when administered intranasally, the amount of drug transported is low, fewer than 0.1% [44]. Therefore, this route of delivery is not widely used and significant work is still needed to improve knowledge of axonal transport. To better define more efficient intranasal drug delivery systems.
3.2. Intraventricular and Intrathecal Delivery
Intraventricular delivery is defined as the direct administration of drugs into the brain ventricle by the use of an implanted catheter connected to a pump. As an alternative, the intrathecal (IT) route involves the administration of drugs to the subarachnoid space of the spinal cord allowing the drug pass through pia mater and enter the brain parenchyma through the cerebrospinal fluid. When compared to systemic administration, these methods avoid accumulation in the liver and the lungs and enable reduced doses of medication and subsequent systemic side effects.
Both methods are in common use for delivery of analgesic agents in patients with violent head and neck pains and also for delivery of pharmacological drugs such as chemotherapeutic agents [51-54]. In the specific context of neurocognitive disorders, intraventricular administration has been successfully employed for the infusion of bethanechol chloride, GM1 ganglioside or nerve growth factor in patients with Alzhemier’s Disease [55-57].
Therapeutic nucleic acids can be delivered directly into the brain via the intraventricular route using osmotic pump infusion [58, 59]. It has been reported to be a particularly efficient method of distribution of antisense nucleotides (ASO) into the CNS, both in rodents and monkeys [58, 59]. The observed relatively uniform distribution of the oligonucleotides into the brain suggested an active ASO uptake mechanism by the cells which latter transport the drugs to distant sites by a cell-to-cell mechanism [59]. Similarly, IT injection of antisense oligonucleotides in the cerebrospinal fluid surrounding the spinal cord resulted in uniform distribution of the oligonucleotides into the whole brain and the spinal cord [59-62]. Recently, two phase I clinical trials have been completed using IT infusion of antisense oligonucleotides for the treatment of amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA) [63, 64]. Preliminary results indicated no dose-limiting toxicities; good safety and tolerability, thus confirming the feasibility of IT administration of ASO in the treatment of ALS and SMA patients [63].
In contrast to these reports, other studies have demonstrated limited tissue penetration of different nucleic acids such as plasmid DNA [65, 66] and oligonucleotides [67, 68] to the injection site. This discrepancy may be due to the physicochemical features of the nucleic acids administrated, as suggested by Y. Yaida et al. [69]. In this study, it was shown that unmodified oligonucleotides have a low periventricular distribution while the counterpart chemically modified phosphorothioate oligonucleotide exhibited greater penetration and accumulation within the brain tissues [69].
Another potential factor responsible for the limited diffusion of the drugs in the brain is linked to the rapid rate of bulk flow of CSF [69]. The entire CSF volume in the human brain is produced and cleared every 4 to 5 hours [70]. Therefore, intraventricular and intrathecal administrated drugs are cycled and exited quickly in the systemic circulation once they reach and bath within the CSF. For example, IT infusion of interferon beta in primates resulted in the detection of this cytokine in the superficial layers of the brain hemispheres, but not in the deeper tissues. In addition a significant amount of the cytokine was detected in the blood of treated primates [71, 72]. More studies are needed to elucidate the precise mechanism of diffusion of drugs locally infused into the brain to fully exploit this route of administration.
3.3. Active Transport using Functionalized Peptides
Therapeutic nucleic acids can be transported across the BBB by active transport systems. Antisense oligonucleotides alone cannot cross the BBB but when coupled with a positively charged peptide to form a peptide nucleic acid (PNA) complex, they can be detected into the brain parenchyma. Although the mechanism of diffusion of the PNA is not yet known, it was hypothesised that peptides might protect ASOs from serum nucleases degradation and promote entry of oligonucleotides to brain cells through an active transporter mechanism [73]. Banks et al. [74] have demonstrated that antisense phosphorothiolate oligonucleotides directed against the amyloid precursor (Aβ-PS-ASO) can be transported intact across the BBB by a saturable system, termed oligonucleotide transport system-1 (OTS-1). High accumulation of the Aβ-PS-ASO was found into the parenchymal space of the brain and in the cerebrospinal fluid when a higher dose of ASO was used. Interestingly, a single intravenous injection of Aβ-PS-ASO was able to reverse the learning and memory deficits in animal models of AD mice [74]. More recently, the same group isolated for the first time a peptide transporter called PTS-6, responsible for drug efflux of the blood- brain barrier (BBB) [75]. PTS-6 was identified as beta- F1 ATPase component of efflux pumps which co-localized with neurotrophic peptide PACAP27. PACAP27 is a pituitary adenylate cyclase-activating polypeptide, known to be protective against Alzheimer's disease. Antisense oligonucleotides targeting PTS-6 inhibited PACAP27 efflux and significantly increased its uptake in the brain, improving cognition in a mouse model of Alzheimer's disease and reduced the risk of infarct development after cerebral ischemia [75].
An alternative strategy aims to develop a new class of peptides to facilitate the BBB transport into the brain. This approach arms the pharmaceutical cargo with a cell surface glycoprotein used by neurotropic viruses (e.g. rabies virus) to infect brain tissues. A short peptide derived from rabies virus glycoprotein RVG (an acetylcholine receptor expressed in neural cells), was exploited to facilitate interaction of siRNA oligonucleotides with the cell membrane surface of neural cells in vivo [76]. Intravascular administration of an RVG peptide previously linked with a monomer of cationic arginine residues (9R), used to complex the negatively charged siRNA oligonucleotide (RVG-9R-siRNA), can cross the BBB and deliver the siRNA in multiple brain regions of mice. Transgenic GFP mice demonstrated a specific silencing of the GFP reporter gene in the brain but not in the liver after the systemic administration of the RVG-9R-siRNA complex. This approach was found sufficient to protect mice from Flavivirus propagation and to delay the premature death of animals from 10 to at least 30 days [76]. This approach was also recently used to deliver siRNA against BACE1, a protease responsible for the accumulation of amyloid precursor protein (APP) in Alzheimers disease [77]. In the study, exosomes produced by dendritic cells were used as naturally occurring RNA carriers to encapsulate siRNA molecules. Intravenous injection of RVG-conjugated exosomes encapsulated with BACE1 siRNA, silenced BACE1 mRNA expression in the brain of mice and reduced the formation of β-amyloid plaques in cortical tissues [77]. The same year, Hwang et al. [78] delivered siRNA and miRNA to the mouse brain using an RVG-modified PEI nanocarrier (RVG-SSPEI).
3.4. Active Transport Using Cell-Penetrating Peptides
An alternate approach utilises synthetic oligonucleotides conjugated with cell-penetrating peptides to improve uptake by the brain cells [79]. Cell-penetrating peptides (CPPs) are a class of small cationic peptides of approximately 10 to 30 amino acids with repeated RXR motifs (R=L-arginine, X=6-aminohexanoic acid) [79]. The RXR motifs facilitate the entry into the cell and potentiate optimal intracellular trafficking. Compared with other delivery systems, cell-penetrating peptides show greater ability to carry different types of macromolecules (from nucleic acids to proteins) across cellular membranes with low cellular toxicity and high efficiency [80]. Due to their small size and amphipathic properties, CPPs have long been considered as a promising system to transport several cargo molecules through the BBB.
In an experimental approach, Du et al. [81] demonstrated that conjugation of an arginine-rich cell-penetrating peptide (RXRRBR)2XB, (R=L-arginine, X=6-aminohexanoic acid, B=beat-alanin) complexed with antisense morpholino oligonucleotides used to alter the splicing process of ATM mRNA (Ataxia telangiectasia mutated) was sufficient to restore expression of the functional form of ATM. In this study, the corrected form of ATM protein was detected in the brain up to 21 days after a unique intravenous administration. The Purkinje cells were found as the main brain cells targeted by this approach. Repeated administrations significantly increased uptake in several brain tissues, notably in the cerebellum, with no reported brain damage or cellular toxicity.
CPPs can also be employed to deliver other nucleic acids, such as antisense oligonucleotides. Caille et al. [82] demonstrated that penetratin-APP antisense oligonucleotides reduced APP expression and embryonic neural stem cell proliferation in the subventricular zone of the CNS in adult mice. In other work by Kanazawa, T., et al siRNA molecules complexed to cell-penetrating peptides encapsulated into nano-micelles formulation was evaluated in in vivo animal model [83]. It was found that intranasal delivery of the complex was superior to the intravenous route and that the presence of CPP moieties improved the delivery to the brain tissues by facilitating transport along the olfactory and trigeminal nerve pathway.
Efficacy of the brain delivery strategy by using transporter systems can be even further improved by employing biological components known to enhance the BBB permeability. Such molecules include, the histamine bradykinine, mannitol and VEGF [84, 85]. The administration of mannitol for example, increases permeability of BBB by temporarily shrinking the endothelial cells, resulting in relaxation of cellular tight junctions within five to thirty minutes after injection [86]. Hwang do et al, [78] exploited this strategy to deliver miRNA-124a mimics encapsulated in RVG-functionalized SSPEI nanoparticles to the brain. SSPEI formulation is a modified version of the well-known PEI (polyethylenimine) formulation in which disulfide bounds are cross-linked to an amine backbone to increase cell biocompatibility and reduce toxicity [78]. The authors demonstrated a greater accumulation of miRNA-124a in the brain region using the RVG-SSPEI polymeric nano-carrier than the SSPEI formulation without the RVG peptide [78]. Such miRNA-124a accumulation increased further when mannitol was added to the RVG-SSPEI complexes. This study indicates that combination of the RVG-SSPEI nano-formulation with an optimised dose of mannitol could adopted for the delivery of a large variety of oligomers for the treatment of neurological diseases. However, manipulation of these hyperosmotic solutions needs to be undertaken with caution, as suboptimal dosing of mannitol might open or damage the BBB irreversibly, inducing toxicities and even lethality [87].
4. STATE OF THE ART OF CURRENT EXPERI- MENTAL RESEARCH ON MIRNA THERAPY FOR THE TREATMENT OF NEUROCOGNITIVE DISORDERS
Currently, most neurocognitive diseases lack efficient therapies because of the relative limited understanding of the cellular and molecular mechanisms governing neurocognitive development and of the difficulty to overcome the blood brain barrier. However, miRNAs regulate expression of specific mRNAs involved in neuron plasticity, memory functionality, cognition and, importantly, in the pathogenesis of neurocognitive disorders. Different studies have now established the potency of chemically modified miRNA nucleic acids to cross the BBB either passively or actively and to treat brain diseases including brain tumours [88]. In this last section, we provide an overview of the current results generated from preclinical models of neurocognitive diseases (Table 1). Approaches based on antagonist miRNA therapy (AMOs) will be differentiated from approaches based on agonist miRNA therapy (miR mimics).
Table 1. Overview of different therapeutic strategies used in preclinical studies to treat neurocognitive diseases using miRNA therapeutics.
| microRNA Therapeutic Strategy | Nucleic Acids | Delivery Vehicle | Diseases | Delivery Route | Refs. |
|---|---|---|---|---|---|
| inhibition | LNA miRNA-29 | Neurotropic peptide | Alzheimer’s Disease and spinocerebellar ataxias | intravenous | [91] |
| inhibition | LNA miRNA-219 | LNA | Schizophrenia | intracranial | [95] |
| inhibition | miRNA-34c | HiPerfect | Alzheimer’s Disease | intracranial | [89] |
| inhibition | miRNA-206 antagomir | AM206 | Alzheimer’s Disease | Intracranial and intranasal | [90] |
| inhibition | miRNA-101 sponge | Lentivirus | Alzheimer’s Disease | intracranial | [96] |
| inhibition | miRNA-132 sponge | Retrovirus | Alzheimer’s Disease | intracranial | [97] |
| replacement | miRNA-124a mimics | RVG-SS-PEI Nanoparticles | Proof of concept for BBB delivery | intravenous | [78] |
| replacement | miRNA-219 | Exosomes | Multiple Sclerosis | intranasal | [98] |
| replacement | miRNA-188-3p | Lentivirus | Alzheimer’s Disease | intracranial | [99] |
| replacement | miR-196 mimic | Adeno-associated virus | Bulbar muscular atrophy | intramuscular | [100] |
| Inhibition and replacement | miRNA-195 mimics /inhibitors | Lentivirus | Dementia | intracranial | [101] |
4.1. Antagonist Therapy of Neurocognitive Diseases
The adult hippocampus plays a central role in cognitive functions such as memory and learning ability. Therefore, it is not surprising that deregulation of molecular pathways involved in hippocampus functions is associated with neurocognitive disorders such as Alzheimer’s disease (AD). In a recent study, a deep sequencing of mouse hippocampal small RNA libraries was used to identify miRNA-34c as one of the most up-regulated miRNA associated with hippocampal memory faculty [89]. Elevated miRNA-34c expression was found in the hippocampus of aging mice, AD mouse models and in human AD patients. To investigate whether miRNA-34c modulation would rescue memory function, a loss-of-functions strategy was evaluated in a mouse model for neurodegeneration. The strategy adopted local delivery of AMOs directed to the miRNA-34 seed regions to block the activity of miRNA-34c and other miRNAs that share the same seed regions such as miRNA-34a, miRNA-34b, miRNA-449a/b/c and miRNA-699. It was observed that microinjections of miRNA-34 seed inhibitors into the hippocampus of AD animal model (APPPS1‐21 mice) improved the memory performance of these mice, similar to age-matched wild-type mice. In contrast, scrambled control miRNAs did not show any improvement of memory performance in the control group of animals. Similar effects were also observed when the seed miRNA-34 AMO were injected in aged mice. This rescued memory function was correlated with restored levels of sirtuin 1 gene expression in hippocampal tissues, which was found to possess two functional putative binding sites for miRNA-34c, demonstrating the specificity of this therapeutic pharmacological intervention.
Potential miRNA candidates involved in the negative regulation of the brain-derived neurotrophic factor (BDNF) have been screened in another mice model of Alzheimer’s Disease (Tg2576). As reduced level of BDNF expression is a common signature of AD, strategies to restore its expression may improve cognitive performances. A miRNONE approach combined with in silico miRNA prediction bioinformatic tools and target gene miRNA luciferase assay was able to identify miRNA-206 as a direct regulator of BDNF expression [90]. Elevated levels of miRNA-206 were found not only in the brain of Tg2576 mice but also in the temporal cortex of human AD brains. To evaluate the potential of miRNA-206 inhibition therapy, two administration delivery routes of AMO-206 antagomiR were evaluated: intraventricular and intranasal. The intraventricular injection of AMO-206 antagomiR increased BDNF expression, prevented the detrimental effects of amyloid-β and improved the memory function of Tg2576 mice [90]. A significant improvement of hippocampal synaptic density and neurogenesis in the AMO-206 antagomiR-treated mice was also noted. Remarkably, intranasal administration of AMO-206 antagomiR produced similar results, associated with an increasing level of BDNF expression and improvement of memory function. Specificity of the results was demonstrated by the detection of the intranasally administered Cy3-labelled AM206 antagomiR in the cortex, the olfactory bulb and hippocampus of the Tg2576 mice [90].
In an alternate strategy, miRNA inhibitors were administered intravenously. For this specific use, Roshan et al., [91] have functionalized an LNA based-miRNA-29 AMO with a neurotropic peptide (NP- LNA miRNA-29 AMO). The miRNA-29 is a brain-specific miRNA with reduced expression in patients and animal models of Alzheimer's and Huntington's diseases as well as spinocerebellar ataxias. The intravenous administration of NP-LNA miRNA-29 AMO induced features specific to ataxia, characterized by deficiency in learning processing and reduced step lengths in mice. This phenotype in mice was associated with the presence of massive cell death in large regions of the hippocampus and cerebellum. Furthermore, an unexpected increase in the expression of the voltage-dependent anion channel 1 (VDAC1) in the hippocampus, cerebellum, and cortex of mice was also seen. To demonstrate that inhibition of miRNA-29 is mediated through the regulation of VDAC1, siRNA directed against VDAC1 was also evaluated in miRNA-29 knockdown cells. As expected, siRNA VDAC silencing partially restored the healthy status of the cells. This study confirms that miRNA-29 is an important determinant of neuronal cell survival in the brain and suggests potential applications of either miRNA mimic-29 or siRNA directed against VDAC1 in the treatment of neurodegenerative diseases like ataxia [91].
Other examples of successful delivery of miRNA inhibitors in the brain are documented. For example, tissues from Schizophrenia patients are characterized by a reduced expression level of miRNA-219 in the prefrontal cortex [92]. In an animal model of Schizophrenia, down regulation of miRNA-219 was correlated with cognitive deficits [93] and with deregulation of the N-methyl-D-aspartate (NMDA)-mediated glutamate signalling [94]. Kocerha et al. [95] investigated the hypothesis that miRNA-219 may directly modulate NMDA-R signalling. By using in silico miRNA prediction bioinformatic tools combined with target gene miRNA luciferase assay, binding sites for miRNA-219 were found in the 3’-UTR of calcium calmodulin dependent protein kinase II gamma (CaMKIIγ) mRNA. CaMKII kinases are core regulators of NMDA signalling that can modulate NMDA-R activity to promote rapid plasticity of dendrites [102].
In vitro assay demonstrates that miRNA-219 transfection in P19 neuronal-like cells decreased expression level of CaMKIIγ at the protein level. This was followed up with experiments to monitor the effect in vivo. Stereotactic infusion of LNA-AMO-219 for 7 consecutive days, significantly increased the expression level of CaMKIIγ protein in the prefrontal cortex of the mice, supporting the hypothesis that miRNA-219 is a regulatory component of NMDA-R signalling pathway. In a transient schizophrenia animal model generated by treatment of mice with dizocilpine to disrupt the NMDA receptor signalling, LNA-AMO-219 delivery displayed markedly altered hyper-locomotion and stereotypy 30 min after dizocilpine administration, that can persist up to 1 hour [95].
4.2. Agonist Therapy of Neurocognitive Diseases
Abnormal accumulation of amyloid-β- (Aβ) in the brain is a major neuropathological hallmark of AD patients. Transcriptomic analysis combined with 3’-UTR luciferase assay has identified miRNA-188-3p as a potential regulator of BACE1 expression. BACE1 is a metabolic enzyme involved in the formation of β-amyloid peptides in brains of transgenic animal model of AD (APP mice) but also in AD patients [99]. Recently, Zang et al. [99] investigated the hypothesis that overexpression of miRNA-188-3p might decrease Aβ production in APP mice by suppressing BACE1 expression. Lentiviruses encoding for the precursor form of miRNA-188-3p were administrated by stereotaxic injection into the hippocampus of APP transgenic mice. Overexpression of miRNA-188-3p in the hippocampus was correlated with an improvement of spatial learning and memory response and a concomitant reduction of Aβ plaques. Down-regulation of human beta secretase BACE1 expression was observed in hippocampus tissues of treated animals confirming the role BACE1 in the accumulation of Aβ and highlighting the potential therapeutic value of a strategy dedicated to the inhibition of miRNA 188-3p function to treat this disease [99].
Multiple sclerosis (MS), a progressive disease of the CNS, is characterised by widespread lesions in the spinal cord and the brain [103]. This pathology involves oligodendrocyte loss-of-function, axon damage and de-myelination. The multiple sclerosis symptoms are motor, cognitive, and neuro- psychiatric, inducing deficiency in long-term memory, as well as delay in information processing and executive functioning [103]. Re-myelination is mediated by the recruitment of oligodendrocyte precursor cells to demyelinated areas where they differentiate into myelinating producing oligodendrocytes. This repairr process is impaired in MS and is less active in aging MS patients [104]. Recently, Pusic et al. [98] explored the feasibility of exosomes as miRNAs delivery vehicles to improve the remyelination process. Dual comparison of miRNAs content in exosomes produced from dendritic cells isolated from young and aged rat brains identified miRNA-219 as the most down regulated miRNA in aged rats but also in MS lesions of human patients [105]. As previous functional studies established a key role of this miRNA in maturation of oligodendrocyte precursor cells [106], it was hypothesized that miRNA-219 may participate in remyelination failure and disease progression in MS lesions. This hypothesis was first investigated in an ex vivo functional study approach using brain tissues slices treated with lysolecithin to induce demyelination. Incubation of exosomes carrying endogenous miRNA-219 to the lysolecithin-treated tissues reduced oxidative stress and improved remyelination. Notably, a single treatment of lysolecithin- and exosomes- treated tissues with an LNA AMO specific to miRNA-219-5p reversed the observed remyelination, demonstrating the dependency of the demyelination process to miRNA-219-5p. Concomitant results were also generated in an in vivo approach. Intranasal administration of exosomes carrying miRNA-219 to aging rats resulted in a significant increase of myelin in the motor cortex of animals, as compared to control animals treated with sham (UV-inactivated) exosomes [98].
These novel approaches have also been used to explore Spinal and bulbar muscular atrophy (SBMA). SBMA is a motor neuron disorder caused by the expansion of the polyglutamine (polyQ) tract of the androgen receptor (AR-polyQ). This abnormality causes muscular atrophy, weakness and cognitive disturbances [107, 108]. Studies demonstrated that pharmacological drugs [109], plasmid DNA-PEI complexes [110] and viral vectors [111-113] injected intramuscularly can be transported from the muscles into the brain. Myiazaki et al. [100] exploited this process and intramuscularly injected an adeno-associated viral vector (AAV) encoding for miRNA-196a into the hind limb skeletal muscle of mice. A biodistribution study demonstrated that this route of administration resulted in the distribution of the vector in the brain, the spinal cord and the skeletal muscle at the site of injection [100]. Overexpression of miRNA-196a in these tissues enhanced the decay of the androgen receptor mRNA by silencing both the CUGBPElav-like family member 2 (CELF2). As a consequence, the phenotype of SBMA mouse model was found to be improved. This result establishes the proof of principle that miRNA delivery through an intramuscular injection might be relevant to treat neurodegenerative diseases.
In further studies that focused on dementia therapy, both antagonist and agonist miRNA approaches were employed to investigate the functional role of miRNA-195 in this neurocognitive disorder [101]. MiRNA-195 is a tumour suppressor miRNA down regulated in many types of cancer [114, 115] which is also known to modulate the expression of brain-derived neurotrophic factor, BDNF and GABAergic transcripts [116]. Knockdown of endogenous miRNA-195 using a lentiviral vector encoding for an antisense molecule (lenti-pre-AMO-miR-195) functionally induced dementia in Sprague Dawley rats, characterized by a decrease in learning and memory ability. In contrast, dementia vulnerability was reduced when miRNA-195 expression was restored using a lentiviral vector expressing the premature form of this miRNA-195. Studies aim at understanding the molecular bases underlying this process indicated that BACE1 was the transcriptomic target of miRNA-195. From this study, it was hypothesized that the down-regulation of miRNA-195 found in this animal model but also in patients with vascular dementia might slow down the formation of amyloid-β aggregation in the brain by up-regulating the expression of APP and BACE1. Therefore as postulated by the authors, exogenous complementation of miRNA-195 may be a potential valuable approach against dementia [101].
5. CONCLUDING REMARKS
There are many examples of the potential therapeutic benefits of miRNAs drugs in preclinical animal models of diseases. Recent results from clinical studies in HCV-infected patients are clearly encouraging and push further the optimization of current used miRNAs based drugs but also the development of other miRNA therapeutic approaches based on miRNAs.
However, one of the greatest challenges in the field of miRNA therapeutics remains the successful delivery into target tissues of miRNAs antagonists or agonists. As for other nucleic acids such as cDNA, siRNA and mRNAs, only a small fraction (less than 5%) of administrated cargo can reach the desired tissues [117]. Therefore, the vast majority of administrated drugs are lost in body fluids, being captured by the RES system, degraded by serum nucleases and diluted in vascularized tissues. In the context of brain delivery, these issues are even more problematic particularly when considering the biochemical and biological properties of the BBB. However, as outlined here, there are many examples of efficient transport methods used to deliver therapeutic oligonucleotides into the brain even when the systemic administration is employed methods for o therapeutic oligonucleotides in the brain tissues through the systemic circulation. These strategies include peptide nucleic acids, utilization of neurotropic peptides and cell-penetrating peptides and exosomes. These emerging types of nucleic acid delivery vehicles are particularly well suited to the size and charge of short oligonucleotides. In addition, the local delivery of miRNA therapeutics directly into the brain tissues is demonstrated to be one of the most relevant delivery routes for brain treatment. However, the intracranial delivery route is not a straightforward method and it is challenging to target precisely a specific brain region without damaging the whole brain tissues. The intranasal route of delivery appears to be a viable and interesting alternative that deserves further study. This route of delivery can bypass the uptake of molecules by the RES system in favour of a better bio-availability to the brain tissues. Furthermore, intranasal instillation is a minimally invasive approach, allowing repeated administration of drugs without creating undue discomfort for patients. However, knowledge of molecular basis underlying this transport mechanism is not well understood and currently constitutes a limitation for its broad clinical application.
An important key to the development of miRNA therapeutics in the clinic is the understanding of the mechanisms governing the emergence of side effects and the evaluation of repercussions of long-term miRNA modulation in vivo. It is worth noting that although some miRNAs are differentially expressed in pathological tissues, they remain present in normal healthy cells. It will be difficult to find an exclusive miRNA signature for each cells type. Therefore, unspecific delivery of miRNA therapeutics in cells of healthy tissues might change their phenotype and may be potential for diseases initiation. Similarly, the doses of miRNA agonists or antagonists in target cells must be controlled when possible, as this might prevent the activation of internal control loops developed by the cells to compensate the dose of delivered miRNA, as well as the changes in expression of targeted mRNAs. Consequently, the therapeutic benefit of miRNAs might only be transient requiring repeated dosing and potential for more frequent side effects. Furthermore, with regard to siRNA therapy, silencing gene expression using miRNAs drugs leads to off-target effects, referred to as non-specific binding of miRNA to unwanted mRNAs. The mechanisms underlying these side effects are little understood and cannot currently be controlled. A good deal of effort is required to take this further to understand the chemistry of oligonucleotides thereby hopefully circumventing this problem. Optimized modifications of AMO were recently proved as a relevant tool to prevent the uptake of the passenger miRNA strands by the miRISC machinery. High-throughput molecular screening platforms for identification of the best combination of chemical modifications of antagonist and agonist oligonucleotides might be particularly useful to achieve this objective and also to design novel classes of RNAi oligonucleotides. Furthermore there is still work to do on facilitating the intracellular trafficking and endosomal escape of therapeutic oligonculeotides in target cells [118-120]. For example, one recent publication indicates that only 1-2% of the siRNA payload transfected in cells are released in the cytosol to interact with their target mRNAs [121].
Finally, although the knowledge of miRNA biology continues to advance daily, it is clear that miRNA expression and function is far more complex than a simple regulation of expression at the transcriptional level and a simple interaction by a base pairing mechanism [122]. Recent studies indicated that miRNA undergo complex post-transcriptional regulations such as miRNA processing, editing, accumulation and re-cycling within P-and GW-bodies [122]. All of these posttranscriptional events impact on the final biological activity of mature miRNA. This statement is supported by data generated from a functional miRNA screening platform that indicate that only 60% of the miRNome is functional in cells [123]. Furthermore, miRNAs are also dynamically regulated, spatially and temporally regulated in animal development [109, 124] but also during development of chronic diseases [125]. Underestimating these miRNA features might result in delivery of inappropriately dosed miRNA therapeutics in targeted tissues as well as inappropriate time point for drug administration.
Despite of these hurdles, there is currently significant efforts devoted to the field of miRNA therapeutics in both the academic and private sectors. With the development of novel miRNAs screening platforms and better knowledge of miRNA biology, it can be expected that the number of miRNA-based drugs will increase in a near future that will, collectively, allow miRNA therapeutics to reach the maturity required to be translated in many different clinical applications.
ACKNOWLEDGEMENT
This manuscript was supported by the INCA MARENGO N°2014-168 and ANR Contact-11-BSV-008 grant approvals and by a funding from the Ligue contre le Cancer, Loiret 45. V.S is supported by a postdoctoral fellowship from the Marengo INCA funding. We would like to thank Mark Weeks for the manuscript proofreading.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Winter J., Jung S., Keller S., Gregory R.I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009;11(3):228–234. doi: 10.1038/ncb0309-228. [http://dx.doi.org/ 10.1038/ncb0309-228]. [PMID: 19255566]. [DOI] [PubMed] [Google Scholar]
- 2.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [http://dx. doi.org/10.1016/0092-8674(93)90529-Y]. [PMID: 8252621]. [DOI] [PubMed] [Google Scholar]
- 3.Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(Database issue):D68–D73. doi: 10.1093/nar/gkt1181. [http://dx.doi.org/10. 1093/nar/gkt1181]. [PMID: 24275495]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z., Rana T.M. Therapeutic targeting of microRNAs: current status and future challenges. Nat. Rev. Drug Discov. 2014;13(8):622–638. doi: 10.1038/nrd4359. [http://dx.doi.org/10.1038/nrd4359]. [PMID: 25011539]. [DOI] [PubMed] [Google Scholar]
- 5.Berezikov E., Chung W.J., Willis J., Cuppen E., Lai E.C. Mammalian mirtron genes. Mol. Cell. 2007;28(2):328–336. doi: 10.1016/j.molcel.2007.09.028. [http:// dx.doi.org/10.1016/j.molcel.2007.09.028]. [PMID: 17964270]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruby J.G., Jan C.H., Bartel D.P. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448(7149):83–86. doi: 10.1038/nature05983. [http://dx.doi.org/10.1038/nature05983]. [PMID: 17589500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwak P.B., Tomari Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat. Struct. Mol. Biol. 2012;19(2):145–151. doi: 10.1038/nsmb.2232. [http://dx.doi.org/10.1038/nsmb.2232]. [PMID: 22233755]. [DOI] [PubMed] [Google Scholar]
- 8.Lee I., Ajay S.S., Yook J.I., Kim H.S., Hong S.H., Kim N.H., Dhanasekaran S.M., Chinnaiyan A.M., Athey B.D. New class of microRNA targets containing simultaneous 5-UTR and 3-UTR interaction sites. Genome Res. 2009;19(7):1175–1183. doi: 10.1101/gr.089367.108. [http://dx. doi.org/10.1101/gr.089367.108]. [PMID: 19336450]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman R.C., Farh K.K., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [http://dx.doi.org/10.1101/gr.082701.108]. [PMID: 18955434]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [http://dx.doi.org/ 10.1038/nature07228]. [PMID: 18668040]. [DOI] [PubMed] [Google Scholar]
- 11.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J. Biol. Chem. 2010;285(17):12726–12734. doi: 10.1074/jbc.M109.086827. [http://dx.doi.org/10.1074/jbc.M109.086827]. [PMID: 20106983]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutla A., Delidakis C., Tabler M. Developmental defects by antisense-mediated inactivation of micro-RNAs 2 and 13 in Drosophila and the identification of putative target genes. Nucleic Acids Res. 2003;31(17):4973–4980. doi: 10.1093/nar/gkg707. [http://dx.doi.org/10.1093/ nar/gkg707]. [PMID: 12930946]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lennox K.A., Behlke M.A. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18(12):1111–1120. doi: 10.1038/gt.2011.100. [http://dx.doi.org/10.1038/gt.2011.100]. [PMID: 21753793]. [DOI] [PubMed] [Google Scholar]
- 14.Hutvágner G., Simard M.J., Mello C.C., Zamore P.D. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004;2(4):E98. doi: 10.1371/journal.pbio.0020098. [http://dx.doi.org/10.1371/journal.pbio.0020098]. [PMID: 15024405]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krützfeldt J., Rajewsky N., Braich R., Rajeev K.G., Tuschl T., Manoharan M., Stoffel M. Silencing of microRNAs in vivo with antagomirs. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [http://dx.doi.org/ 10.1038/nature04303]. [PMID: 16258535]. [DOI] [PubMed] [Google Scholar]
- 16.Pande V., Nilsson L. Insights into structure, dynamics and hydration of locked nucleic acid (LNA) strand-based duplexes from molecular dynamics simulations. Nucleic Acids Res. 2008;36(5):1508–1516. doi: 10.1093/nar/gkm1182. [http://dx.doi.org/10.1093/nar/gkm1182]. [PMID: 18203740]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennox K.A., Behlke M.A. A direct comparison of anti-microRNA oligonucleotide potency. Pharm. Res. 2010;27(9):1788–1799. doi: 10.1007/s11095-010-0156-0. [http://dx.doi.org/10.1007/s11095-010-0156-0]. [PMID: 20424893]. [DOI] [PubMed] [Google Scholar]
- 18.Förster C., Eichert A., Oberthür D., Betzel C., Geßner R., Nitsche A., Fürste J.P. Features of All LNA Duplexes Showing a New Type of Nucleic Acid Geometry. J. Nucleic Acids, 2012. 156035. [http://dx.doi.org/10.1155/2012/156035] [PMID:22666550] [DOI] [PMC free article] [PubMed]
- 19.Broderick J.A., Zamore P.D. MicroRNA therapeutics. Gene Ther. 2011;18(12):1104–1110. doi: 10.1038/gt.2011.50. [http://dx.doi.org/10.1038/gt.2011.50]. [PMID: 21525952]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanford R.E., Hildebrandt-Eriksen E.S., Petri A., Persson R., Lindow M., Munk M.E., Kauppinen S., Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327(5962):198–201. doi: 10.1126/science.1178178. [http://dx.doi. org/10.1126/science.1178178]. [PMID: 19965718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindow M., Kauppinen S. Discovering the first microRNA-targeted drug. J. Cell Biol. 2012;199(3):407–412. doi: 10.1083/jcb.201208082. [http://dx.doi. org/10.1083/jcb.201208082]. [PMID: 23109665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenvang J., Petri A., Lindow M., Obad S., Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3(1):1. doi: 10.1186/1758-907X-3-1. [http://dx.doi.org/10.1186/1758-907X-3-1]. [PMID: 22230293]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmén J., Lindow M., Schütz S., Lawrence M., Petri A., Obad S., Lindholm M., Hedtjärn M., Hansen H.F., Berger U., Gullans S., Kearney P., Sarnow P., Straarup E.M., Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452(7189):896–899. doi: 10.1038/nature06783. [http://dx.doi.org/10.1038/nature06783]. [PMID: 18368051]. [DOI] [PubMed] [Google Scholar]
- 24.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., Castoldi M., Soutschek J., Koteliansky V., Rosenwald A., Basson M.A., Licht J.D., Pena J.T., Rouhanifard S.H., Muckenthaler M.U., Tuschl T., Martin G.R., Bauersachs J., Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456(7224):980–984. doi: 10.1038/nature07511. [http:// dx.doi.org/10.1038/nature07511]. [PMID: 19043405]. [DOI] [PubMed] [Google Scholar]
- 25.Ma L., Reinhardt F., Pan E., Soutschek J., Bhat B., Marcusson E.G., Teruya-Feldstein J., Bell G.W., Weinberg R.A. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat. Biotechnol. 2010;28(4):341–347. doi: 10.1038/nbt.1618. [http://dx. doi.org/10.1038/nbt.1618]. [PMID: 20351690]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H., Shykind B., Sun T. Approaches to manipulating microRNAs in neurogenesis. Front. Neurosci. 2013;6:196. doi: 10.3389/fnins.2012.00196. [http://dx.doi.org/10.3389/fnins.2012.00196]. [PMID: 23335878]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y., Xiao J., Lin H., Bai Y., Luo X., Wang Z., Yang B. A single anti-microRNA antisense oligodeoxyribonucleotide (AMO) targeting multiple microRNAs offers an improved approach for microRNA interference. Nucleic Acids Res. 2009;37(3):e24. doi: 10.1093/nar/gkn1053. [http://dx.doi.org/10.1093/nar/gkn1053]. [PMID: 19136465]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluiver J., Gibcus J.H., Hettinga C., Adema A., Richter M.K., Halsema N., Slezak-Prochazka I., Ding Y., Kroesen B.J., van den Berg A. Rapid generation of microRNA sponges for microRNA inhibition. PLoS One. 2012;7(1):e29275. doi: 10.1371/journal.pone.0029275. [http://dx. doi.org/10.1371/journal.pone.0029275]. [PMID: 22238599]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bader A.G., Brown D., Winkler M. The promise of microRNA replacement therapy. Cancer Res. 2010;70(18):7027–7030. doi: 10.1158/0008-5472.CAN-10-2010. [http://dx.doi.org/10.1158/0008-5472.CAN-10-2010]. [PMID: 20807816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rooij E., Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014;6(7):851–864. doi: 10.15252/emmm.201100899. [http://dx.doi.org/10.15252/emmm.201100899]. [PMID: 24935956]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash T.P., Allerson C.R., Dande P., Vickers T.A., Sioufi N., Jarres R., Baker B.F., Swayze E.E., Griffey R.H., Bhat B. Positional effect of chemical modifications on short interference RNA activity in mammalian cells. J. Med. Chem. 2005;48(13):4247–4253. doi: 10.1021/jm050044o. [http://dx.doi.org/10.1021/jm050044o]. [PMID: 15974578]. [DOI] [PubMed] [Google Scholar]
- 32.Choung S., Kim Y.J., Kim S., Park H.O., Choi Y.C. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun. 2006;342(3):919–927. doi: 10.1016/j.bbrc.2006.02.049. [http://dx.doi.org/10.1016/j.bbrc.2006.02.049]. [PMID: 16598842]. [DOI] [PubMed] [Google Scholar]
- 33.Chen P.Y., Weinmann L., Gaidatzis D., Pei Y., Zavolan M., Tuschl T., Meister G. Strand-specific 5-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity. RNA. 2008;14(2):263–274. doi: 10.1261/rna.789808. [http://dx.doi.org/10.1261/rna.789808]. [PMID: 18094121]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitade Y., Akao Y. MicroRNAs and their therapeutic potential for human diseases: microRNAs, miR-143 and -145, function as anti-oncomirs and the application of chemically modified miR-143 as an anti-cancer drug. J. Pharmacol. Sci. 2010;114(3):276–280. doi: 10.1254/jphs.10r12fm. [http://dx.doi.org/10.1254/jphs.10R12FM]. [PMID: 20953119]. [DOI] [PubMed] [Google Scholar]
- 35.Chorn G., Klein-McDowell M., Zhao L., Saunders M.A., Flanagan W.M., Willingham A.T., Lim L.P. Single-stranded microRNA mimics. RNA. 2012;18(10):1796–1804. doi: 10.1261/rna.031278.111. [http://dx.doi. org/10.1261/rna.031278.111]. [PMID: 22912485]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misso G., Di Martino M.T., De Rosa G., Farooqi A.A., Lombardi A., Campani V., Zarone M.R., Gullà A., Tagliaferri P., Tassone P., Caraglia M. Mir-34: a new weapon against cancer? Mol. Ther. Nucleic Acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [http://dx.doi.org/ 10.1038/mtna.2014.47]. [PMID: 25247240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bader A.G. miR-34 - a microRNA replacement therapy is headed to the clinic. Front. Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [http://dx.doi.org/10. 3389/fgene.2012.00120]. [PMID: 22783274]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petros R.A., DeSimone J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [http://dx.doi.org/10.1038/nrd2591]. [PMID: 20616808]. [DOI] [PubMed] [Google Scholar]
- 39.Hue J.J., Lee H.J., Jon S., Nam S.Y., Yun Y.W., Kim J.S., Lee B.J. Distribution and accumulation of Cy5.5-labeled thermally cross-linked superparamagnetic iron oxide nanoparticles in the tissues of ICR mice. J. Vet. Sci. 2013;14(4):473–479. doi: 10.4142/jvs.2013.14.4.473. [http://dx.doi.org/10.4142/jvs.2013.14.4.473]. [PMID: 24366671]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krützfeldt J., Kuwajima S., Braich R., Rajeev K.G., Pena J., Tuschl T., Manoharan M., Stoffel M. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35(9):2885–2892. doi: 10.1093/nar/gkm024. [http://dx.doi.org/10.1093/nar/ gkm024]. [PMID: 17439965]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubin L.L., Staddon J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [http://dx.doi.org/ 10.1146/annurev.neuro.22.1.11]. [PMID: 10202530]. [DOI] [PubMed] [Google Scholar]
- 42.Pardridge W.M. Blood-brain barrier biology and methodology. J. Neurovirol. 1999;5(6):556–569. doi: 10.3109/13550289909021285. [http://dx.doi.org/10.3109/ 13550289909021285]. [PMID: 10602397]. [DOI] [PubMed] [Google Scholar]
- 43.Smith K.R., Borchardt R.T. Permeability and mechanism of albumin, cationized albumin, and glycosylated albumin transcellular transport across monolayers of cultured bovine brain capillary endothelial cells. Pharm. Res. 1989;6(6):466–473. doi: 10.1023/a:1015960205409. [http://dx.doi.org/10.1023/A:1015960205409]. [PMID: 2762222]. [DOI] [PubMed] [Google Scholar]
- 44.Dhuria S.V., Hanson L.R., Frey W.H., II Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J. Pharm. Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [http://dx.doi.org/10.1002/ jps.21924]. [PMID: 19877171]. [DOI] [PubMed] [Google Scholar]
- 45.Dhamoon M.S., Noble J.M., Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2009;72(3):292–293. doi: 10.1212/01.wnl.0000344246.91081.2c. [http://dx.doi.org/10.1212/01. wnl.0000344246.91081.2c]. [PMID: 19153380]. [DOI] [PubMed] [Google Scholar]
- 46.Leksell L. Stereotactic radiosurgery. J. Neurol. Neurosurg. Psychiatry. 1983;46(9):797–803. [http://dx.doi.org/10.1136/jnnp. 46.9.797]. [PMID: 6352865]. [Google Scholar]
- 47.Chen Y., Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012;64(7):640–665. doi: 10.1016/j.addr.2011.11.010. [http://dx.doi.org/10.1016/j.addr.2011.11.010]. [PMID: 22154620]. [DOI] [PubMed] [Google Scholar]
- 48.Wei G., Wang D., Lu H., Parmentier S., Wang Q., Panter S.S., Frey W.H., II, Ying W. Intranasal administration of a PARG inhibitor profoundly decreases ischemic brain injury. Front. Biosci. 2007;12:4986–4996. doi: 10.2741/2443. [http://dx.doi.org/10.2741/2443]. [PMID: 17569625]. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Rodriguez J.C., Sosa-Teste I. The nasal route as a potential pathway for delivery of erythropoietin in the treatment of acute ischemic stroke in humans. ScientificWorldJournal. 2009;9:970–981. doi: 10.1100/tsw.2009.103. [http://dx.doi.org/10.1100/tsw.2009.103]. [PMID: 19768354]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tosi G., Ruozi B., Belletti D., Vilella A., Zoli M., Vandelli M.A., Forni F. Brain-targeted polymeric nanoparticles: in vivo evidence of different routes of administration in rodents. Nanomedicine (Lond.) 2013;8(9):1373–1383. doi: 10.2217/nnm.12.172. [http://dx.doi.org/ 10.2217/nnm.12.172]. [PMID: 23565661]. [DOI] [PubMed] [Google Scholar]
- 51.Saulino M., Kim P.S., Shaw E. Practical considerations and patient selection for intrathecal drug delivery in the management of chronic pain. J. Pain Res. 2014;7:627–638. doi: 10.2147/JPR.S65441. [http://dx.doi.org/ 10.2147/JPR.S65441]. [PMID: 25419158]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah R., Baqai-Stern A., Gulati A. Managing intrathecal drug delivery (ITDD) in cancer patients. Curr. Pain Headache Rep. 2015;19(6):20. doi: 10.1007/s11916-015-0488-x. [http://dx.doi.org/10.1007/s11916-015-0488-x]. [PMID: 26021753]. [DOI] [PubMed] [Google Scholar]
- 53.Lobato R.D., Madrid J.L., Fatela L.V., Rivas J.J., Reig E., Lamas E. Intraventricular morphine for control of pain in terminal cancer patients. J. Neurosurg. 1983;59(4):627–633. doi: 10.3171/jns.1983.59.4.0627. [http://dx. doi.org/10.3171/jns.1983.59.4.0627]. [PMID: 6886783]. [DOI] [PubMed] [Google Scholar]
- 54.Blond S., Meynadier J., Dupard T., Assaker R., Brichard C., Christiaens J.L., Demaille A. Neurochirurgie. 1989;35(1):52–57. [Intracerebroventricular morphine therapy. Apropos of 79 patients]. [Intracerebroventricular morphine therapy. Apropos of 79 patients]. [PMID: 2716939]. [PubMed] [Google Scholar]
- 55.Harbaugh R.E., Reeder T.M., Senter H.J., Knopman D.S., Baskin D.S., Pirozzolo F., Chui H.C., Shetter A.G., Bakay R.A., Leblanc R. Intracerebroventricular bethanechol chloride infusion in Alzheimers disease. Results of a collaborative double-blind study. J. Neurosurg. 1989;71(4):481–486. doi: 10.3171/jns.1989.71.4.0481. [http://dx.doi.org/10. 3171/jns.1989.71.4.0481]. [PMID: 2571689]. [DOI] [PubMed] [Google Scholar]
- 56.Eriksdotter Jönhagen M., Nordberg A., Amberla K., Bäckman L., Ebendal T., Meyerson B., Olson L. Seiger; Shigeta, M.; Theodorsson, E.; Viitanen, M.; Winblad, B.; Wahlund, L.O. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimers disease. Dement. Geriatr. Cogn. Disord. 1998;9(5):246–257. doi: 10.1159/000017069. [http://dx.doi.org/10.1159/000017069]. [PMID: 9701676]. [DOI] [PubMed] [Google Scholar]
- 57.Augustinsson L.E., Blennow K., Blomstrand C., Bråne G., Ekman R., Fredman P., Karlsson I., Kihlgren M., Lehmann W., Lekman A., Månsson J.E., Ramström I., Wallin A., Wikkelsö C., Gottfries C.G., Svennerholm L. Intracerebroventricular administration of GM1 ganglioside to presenile Alzheimer patients. Dement. Geriatr. Cogn. Disord. 1997;8(1):26–33. doi: 10.1159/000106597. [http://dx.doi. org/10.1159/000106597]. [PMID: 8997549]. [DOI] [PubMed] [Google Scholar]
- 58.DeVos S.L., Miller T.M. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp. 2013;(75):e50326. doi: 10.3791/50326. [PMID: 23712122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith R.A., Miller T.M., Yamanaka K., Monia B.P., Condon T.P., Hung G., Lobsiger C.S., Ward C.M., McAlonis-Downes M., Wei H., Wancewicz E.V., Bennett C.F., Cleveland D.W. Antisense oligonucleotide therapy for neurodegenerative disease. J. Clin. Invest. 2006;116(8):2290–2296. doi: 10.1172/JCI25424. [http://dx.doi.org/10.1172/ JCI25424]. [PMID: 16878173]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kordasiewicz H.B., Stanek L.M., Wancewicz E.V., Mazur C., McAlonis M.M., Pytel K.A., Artates J.W., Weiss A., Cheng S.H., Shihabuddin L.S., Hung G., Bennett C.F., Cleveland D.W. Sustained therapeutic reversal of Huntingtons disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–1044. doi: 10.1016/j.neuron.2012.05.009. [http://dx.doi.org/10.1016/j.neuron.2012.05.009]. [PMID: 22726834]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passini M.A., Bu J., Richards A.M., Kinnecom C., Sardi S.P., Stanek L.M., Hua Y., Rigo F., Matson J., Hung G., Kaye E.M., Shihabuddin L.S., Krainer A.R., Bennett C.F., Cheng S.H. Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med. 2011;3(72):72ra18. doi: 10.1126/scitranslmed.3001777. [http://dx.doi.org/10.1126/scitranslmed. 3001777]. [PMID: 21368223]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rigo F., Chun S.J., Norris D.A., Hung G., Lee S., Matson J., Fey R.A., Gaus H., Hua Y., Grundy J.S., Krainer A.R., Henry S.P., Bennett C.F. Pharmacology of a central nervous system delivered 2-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J. Pharmacol. Exp. Ther. 2014;350(1):46–55. doi: 10.1124/jpet.113.212407. [http://dx.doi.org/10. 1124/jpet.113.212407]. [PMID: 24784568]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller T.M., Pestronk A., David W., Rothstein J., Simpson E., Appel S.H., Andres P.L., Mahoney K., Allred P., Alexander K., Ostrow L.W., Schoenfeld D., Macklin E.A., Norris D.A., Manousakis G., Crisp M., Smith R., Bennett C.F., Bishop K.M., Cudkowicz M.E. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 2013;12(5):435–442. doi: 10.1016/S1474-4422(13)70061-9. [http://dx.doi.org/10.1016/S1474-4422(13)70061-9]. [PMID: 23541756]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanetta C., Nizzardo M., Simone C., Monguzzi E., Bresolin N., Comi G.P., Corti S. Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials. Clin. Ther. 2014;36(1):128–140. doi: 10.1016/j.clinthera.2013.11.006. [http://dx.doi.org/10.1016/j.clinthera.2013. 11.006]. [PMID: 24360800]. [DOI] [PubMed] [Google Scholar]
- 65.Hagihara Y., Saitoh Y., Kaneda Y., Kohmura E., Yoshimine T. Widespread gene transfection into the central nervous system of primates. Gene Ther. 2000;7(9):759–763. doi: 10.1038/sj.gt.3301169. [http://dx.doi.org/10. 1038/sj.gt.3301169]. [PMID: 10822302]. [DOI] [PubMed] [Google Scholar]
- 66.Meuli-Simmen C., Liu Y., Yeo T.T., Liggitt D., Tu G., Yang T., Meuli M., Knauer S., Heath T.D., Longo F.M., Debs R.J. Gene expression along the cerebral-spinal axis after regional gene delivery. Hum. Gene Ther. 1999;10(16):2689–2700. doi: 10.1089/10430349950016735. [http://dx. doi.org/10.1089/10430349950016735]. [PMID: 10566897]. [DOI] [PubMed] [Google Scholar]
- 67.Porritt M.J., Batchelor P.E., Howells D.W. Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp. Neurol. 2005;192(1):226–234. doi: 10.1016/j.expneurol.2004.11.030. [http://dx.doi.org/10.1016/j.expneurol.2004.11.030]. [PMID: 15698637]. [DOI] [PubMed] [Google Scholar]
- 68.Grzanna R., Dubin J.R., Dent G.W., Ji Z., Zhang W., Ho S.P., Hartig P.R. Intrastriatal and intraventricular injections of oligodeoxynucleotides in the rat brain: tissue penetration, intracellular distribution and c-fos antisense effects. Brain Res. Mol. Brain Res. 1998;63(1):35–52. doi: 10.1016/s0169-328x(98)00238-1. [http://dx.doi.org/10.1016/ S0169-328X(98)00238-1]. [PMID: 9838035]. [DOI] [PubMed] [Google Scholar]
- 69.Yaida Y., Nowak T.S., Jr Distribution of phosphodiester and phosphorothioate oligonucleotides in rat brain after intraventricular and intrahippocampal administration determined by in situ hybridization. Regul. Pept. 1995;59(2):193–199. doi: 10.1016/0167-0115(95)00100-p. [http://dx.doi. org/10.1016/0167-0115(95)00100-P]. [PMID: 8584754]. [DOI] [PubMed] [Google Scholar]
- 70.Pardridge W.M. Drug delivery to the brain. J. Cereb. Blood Flow Metab. 1997;17(7):713–731. doi: 10.1097/00004647-199707000-00001. [http://dx.doi.org/10.1097/00004647-199707000-00001]. [PMID: 9270488]. [DOI] [PubMed] [Google Scholar]
- 71.Billiau A., Heremans H., Ververken D., van Damme J., Carton H., de Somer P. Tissue distribution of human interferons after exogenous administration in rabbits, monkeys, and mice. Arch. Virol. 1981;68(1):19–25. doi: 10.1007/BF01315163. [http://dx.doi.org/10.1007/BF01315163]. [PMID: 6166278]. [DOI] [PubMed] [Google Scholar]
- 72.de Lange E.C., Danhof M., de Boer A.G., Breimer D.D. Critical factors of intracerebral microdialysis as a technique to determine the pharmacokinetics of drugs in rat brain. Brain Res. 1994;666(1):1–8. doi: 10.1016/0006-8993(94)90276-3. [http://dx.doi.org/10.1016/0006-8993(94)90276-3]. [PMID: 7889356]. [DOI] [PubMed] [Google Scholar]
- 73.Tyler B.M., Jansen K., McCormick D.J., Douglas C.L., Boules M., Stewart J.A., Zhao L., Lacy B., Cusack B., Fauq A., Richelson E. Peptide nucleic acids targeted to the neurotensin receptor and administered i.p. cross the blood-brain barrier and specifically reduce gene expression. Proc. Natl. Acad. Sci. USA. 1999;96(12):7053–7058. doi: 10.1073/pnas.96.12.7053. [http://dx.doi.org/10.1073/pnas.96.12. 7053]. [PMID: 10359837]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banks W.A., Farr S.A., Butt W., Kumar V.B., Franko M.W., Morley J.E. Delivery across the blood-brain barrier of antisense directed against amyloid beta: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J. Pharmacol. Exp. Ther. 2001;297(3):1113–1121. [PMID: 11356936]. [PubMed] [Google Scholar]
- 75.Dogrukol-Ak D., Kumar V.B., Ryerse J.S., Farr S.A., Verma S., Nonaka N., Nakamachi T., Ohtaki H., Niehoff M.L., Edwards J.C., Shioda S., Morley J.E., Banks W.A. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J. Cereb. Blood Flow Metab. 2009;29(2):411–422. doi: 10.1038/jcbfm.2008.131. [http://dx.doi.org/10. 1038/jcbfm.2008.131]. [PMID: 19002200]. [DOI] [PubMed] [Google Scholar]
- 76.Kumar P., Wu H., McBride J.L., Jung K.E., Kim M.H., Davidson B.L., Lee S.K., Shankar P., Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448(7149):39–43. doi: 10.1038/nature05901. [http://dx.doi.org/ 10.1038/nature05901]. [PMID: 17572664]. [DOI] [PubMed] [Google Scholar]
- 77.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [http:// dx.doi.org/10.1038/nbt.1807]. [PMID: 21423189]. [DOI] [PubMed] [Google Scholar]
- 78.Hwang D.W., Son S., Jang J., Youn H., Lee S., Lee D., Lee Y.S., Jeong J.M., Kim W.J., Lee D.S. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials. 2011;32(21):4968–4975. doi: 10.1016/j.biomaterials.2011.03.047. [http://dx.doi.org/10.1016/j.biomaterials.2011.03.047]. [PMID: 21489620]. [DOI] [PubMed] [Google Scholar]
- 79.Zou L.L., Ma J.L., Wang T., Yang T.B., Liu C.B. Cell-penetrating Peptide-mediated therapeutic molecule delivery into the central nervous system. Curr. Neuropharmacol. 2013;11(2):197–208. doi: 10.2174/1570159X11311020006. [http://dx.doi.org/10.2174/1570159X11311020006]. [PMID: 23997754]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Derossi D., Joliot A.H., Chassaing G., Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269(14):10444–10450. [PMID: 8144628]. [PubMed] [Google Scholar]
- 81.Du L., Kayali R., Bertoni C., Fike F., Hu H., Iversen P.L., Gatti R.A. Arginine-rich cell-penetrating peptide dramatically enhances AMO-mediated ATM aberrant splicing correction and enables delivery to brain and cerebellum. Hum. Mol. Genet. 2011;20(16):3151–3160. doi: 10.1093/hmg/ddr217. [http://dx.doi.org/10.1093/hmg/ddr217]. [PMID: 21576124]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caillé I., Allinquant B., Dupont E., Bouillot C., Langer A., Müller U., Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventri- cular zone. Development. 2004;131(9):2173–2181. doi: 10.1242/dev.01103. [http://dx.doi. org/10.1242/dev.01103]. [PMID: 15073156]. [DOI] [PubMed] [Google Scholar]
- 83.Kanazawa T., Akiyama F., Kakizaki S., Takashima Y., Seta Y. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials. 2013;34(36):9220–9226. doi: 10.1016/j.biomaterials.2013.08.036. [http://dx.doi.org/10.1016/ j.biomaterials.2013.08.036]. [PMID: 23992922]. [DOI] [PubMed] [Google Scholar]
- 84.On N.H., Savant S., Toews M., Miller D.W. Rapid and reversible enhancement of blood-brain barrier permeability using lysophosphatidic acid. J. Cereb. Blood Flow Metab. 2013;33(12):1944–1954. doi: 10.1038/jcbfm.2013.154. [http://dx.doi.org/10.1038/jcbfm.2013.154]. [PMID: 24045401]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang S., Xia R., Jiang Y., Wang L., Gao F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS One. 2014;9(2):e86407. doi: 10.1371/journal.pone.0086407. [http://dx.doi. org/10.1371/journal.pone.0086407]. [PMID: 24551038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang M., Etu J., Joshi S. Enhanced disruption of the blood brain barrier by intracarotid mannitol injection during transient cerebral hypoperfusion in rabbits. J. Neurosurg. Anesthesiol. 2007;19(4):249–256. doi: 10.1097/ANA.0b013e3181453851. [http://dx.doi.org/10.1097/ANA.0b013e3181453851]. [PMID: 17893577]. [DOI] [PubMed] [Google Scholar]
- 87.Rapoport S.I. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin. Investig. Drugs. 2001;10(10):1809–1818. doi: 10.1517/13543784.10.10.1809. [http://dx.doi.org/10.1517/13543784. 10.10.1809]. [PMID: 11772287]. [DOI] [PubMed] [Google Scholar]
- 88.Alsidawi S., Malek E., Driscoll J.J. MicroRNAs in brain metastases: potential role as diagnostics and therapeutics. Int. J. Mol. Sci. 2014;15(6):10508–10526. doi: 10.3390/ijms150610508. [http://dx.doi.org/10.3390/ ijms150610508]. [PMID: 24921708]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zovoilis A., Agbemenyah H.Y., Agis-Balboa R.C., Stilling R.M., Edbauer D., Rao P., Farinelli L., Delalle I., Schmitt A., Falkai P., Bahari-Javan S., Burkhardt S., Sananbenesi F., Fischer A. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30(20):4299–4308. doi: 10.1038/emboj.2011.327. [http://dx.doi.org/10.1038/ emboj.2011.327]. [PMID: 21946562]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee S.T., Chu K., Jung K.H., Kim J.H., Huh J.Y., Yoon H., Park D.K., Lim J.Y., Kim J.M., Jeon D., Ryu H., Lee S.K., Kim M., Roh J.K. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann. Neurol. 2012;72(2):269–277. doi: 10.1002/ana.23588. [http://dx.doi.org/10.1002/ana.23588]. [PMID: 22926857]. [DOI] [PubMed] [Google Scholar]
- 91.Roshan R., Shridhar S., Sarangdhar M.A., Banik A., Chawla M., Garg M., Singh V.P., Pillai B. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA. 2014;20(8):1287–1297. doi: 10.1261/rna.044008.113. [http://dx.doi.org/10.1261/rna.044008. 113]. [PMID: 24958907]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smalheiser N.R., Lugli G., Zhang H., Rizavi H., Cook E.H., Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS One. 2014;9(1):e86469. doi: 10.1371/journal.pone.0086469. [http://dx.doi.org/10.1371/ journal.pone.0086469]. [PMID: 24475125]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beveridge N.J., Gardiner E., Carroll A.P., Tooney P.A., Cairns M.J. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol. Psychiatry. 2010;15(12):1176–1189. doi: 10.1038/mp.2009.84. [http://dx.doi.org/10.1038/mp.2009.84]. [PMID: 19721432]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Javitt D.C. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [http://dx.doi.org/10.1016/ S0074-7742(06)78003-5]. [PMID: 17349858]. [DOI] [PubMed] [Google Scholar]
- 95.Kocerha J., Faghihi M.A., Lopez-Toledano M.A., Huang J., Ramsey A.J., Caron M.G., Sales N., Willoughby D., Elmen J., Hansen H.F., Orum H., Kauppinen S., Kenny P.J., Wahlestedt C. MicroRNA-219 modulates NMDA receptor-mediated neuro- behavioral dysfunction. Proc. Natl. Acad. Sci. USA. 2009;106(9):3507–3512. doi: 10.1073/pnas.0805854106. [http://dx.doi.org/10.1073/pnas.0805854106]. [PMID: 19196972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barbato C., Pezzola S., Caggiano C., Antonelli M., Frisone P., Ciotti M.T., Ruberti F. A lentiviral sponge for miR-101 regulates RanBP9 expression and amyloid precursor protein metabolism in hippocampal neurons. Front. Cell. Neurosci. 2014;8:37. doi: 10.3389/fncel.2014.00037. [http://dx.doi.org/10.3389/fncel.2014.00037]. [PMID: 24592211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luikart B.W., Bensen A.L., Washburn E.K., Perederiy J.V., Su K.G., Li Y., Kernie S.G., Parada L.F., Westbrook G.L. miR-132 mediates the integration of newborn neurons into the adult dentate gyrus. PLoS One. 2011;6(5):e19077. doi: 10.1371/journal.pone.0019077. [http://dx.doi.org/10.1371/ journal.pone.0019077]. [PMID: 21611182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pusic A.D., Kraig R.P. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia. 2014;62(2):284–299. doi: 10.1002/glia.22606. [http://dx.doi.org/10.1002/ glia.22606]. [PMID: 24339157]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang J., Hu M., Teng Z., Tang Y.P., Chen C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-1883p in a mouse model of Alzheimers disease. J. Neurosci. 2014;34(45):14919–14933. doi: 10.1523/JNEUROSCI.1165-14.2014. [http://dx.doi.org/10.1523/JNEUROSCI.1165-14.2014]. [PMID: 25378159]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyazaki Y., Adachi H., Katsuno M., Minamiyama M., Jiang Y.M., Huang Z., Doi H., Matsumoto S., Kondo N., Iida M., Tohnai G., Tanaka F., Muramatsu S., Sobue G. Viral delivery of miR-196a ameliorates the SBMA phenotype via the silencing of CELF2. Nat. Med. 2012;18(7):1136–1141. doi: 10.1038/nm.2791. [http://dx.doi.org/ 10.1038/nm.2791]. [PMID: 22660636]. [DOI] [PubMed] [Google Scholar]
- 101.Ai J., Sun L.H., Che H., Zhang R., Zhang T.Z., Wu W.C., Su X.L., Chen X., Yang G., Li K., Wang N., Ban T., Bao Y.N., Guo F., Niu H.F., Zhu Y.L., Zhu X.Y., Zhao S.G., Yang B.F. MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J. Neurosci. 2013;33(9):3989–4001. doi: 10.1523/JNEUROSCI.1997-12.2013. [http://dx.doi.org/10.1523/ JNEUROSCI.1997-12.2013]. [PMID: 23447608]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller S., Yasuda M., Coats J.K., Jones Y., Martone M.E., Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36(3):507–519. doi: 10.1016/s0896-6273(02)00978-9. [http://dx.doi.org/ 10.1016/S0896-6273(02)00978-9]. [PMID: 12408852]. [DOI] [PubMed] [Google Scholar]
- 103.Chiaravalloti N.D., DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [http://dx.doi. org/10.1016/S1474-4422(08)70259-X]. [PMID: 19007738]. [DOI] [PubMed] [Google Scholar]
- 104.Kuhlmann T., Miron V., Cui Q., Wegner C., Antel J., Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–1758. doi: 10.1093/brain/awn096. [http://dx.doi.org/10.1093/brain/ awn096]. [PMID: 18515322]. [DOI] [PubMed] [Google Scholar]
- 105.Junker A., Krumbholz M., Eisele S., Mohan H., Augstein F., Bittner R., Lassmann H., Wekerle H., Hohlfeld R., Meinl E. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;132(Pt 12):3342–3352. doi: 10.1093/brain/awp300. [http://dx.doi.org/10.1093/brain/awp300]. [PMID: 19952055]. [DOI] [PubMed] [Google Scholar]
- 106.Li J.S., Yao Z.X. MicroRNAs: novel regulators of oligodendrocyte differentiation and potential therapeutic targets in demyelination-related diseases. Mol. Neurobiol. 2012;45(1):200–212. doi: 10.1007/s12035-011-8231-z. [http://dx.doi.org/10.1007/s12035-011-8231-z]. [PMID: 22218763]. [DOI] [PubMed] [Google Scholar]
- 107.Soukup G.R., Sperfeld A.D., Uttner I., Karitzky J., Ludolph A.C., Kassubek J., Schreiber H. Frontotemporal cognitive function in X-linked spinal and bulbar muscular atrophy (SBMA): a controlled neuropsychological study of 20 patients. J. Neurol. 2009;256(11):1869–1875. doi: 10.1007/s00415-009-5212-5. [http://dx.doi.org/10.1007/s00415-009-5212-5]. [PMID: 19572162]. [DOI] [PubMed] [Google Scholar]
- 108.Kasper E., Wegrzyn M., Marx I., Korp C., Kress W., Benecke R., Teipel S.J., Prudlo J. Minor cognitive disturbances in X-linked spinal and bulbar muscular atrophy, Kennedys disease. Amyotroph. Lateral Scler. Frontotemporal Degener. 2014;15(1-2):15–20. doi: 10.3109/21678421.2013.837927. [http:// dx.doi.org/10.3109/21678421.2013.837927]. [PMID: 24200287]. [DOI] [PubMed] [Google Scholar]
- 109.Wang A., Wang L., Sun K., Liu W., Sha C., Li Y. Preparation of rotigotine-loaded microspheres and their combination use with L-DOPA to modify dyskinesias in 6-OHDA-lesioned rats. Pharm. Res. 2012;29(9):2367–2376. doi: 10.1007/s11095-012-0762-0. [http://dx.doi.org/10.1007/s11095-012-0762-0]. [PMID: 22549738]. [DOI] [PubMed] [Google Scholar]
- 110.Wang S., Ma N., Gao S.J., Yu H., Leong K.W. Transgene expression in the brain stem effected by intramuscular injection of polyethylenimine/DNA complexes. Mol. Ther. 2001;3(5 Pt 1):658–664. doi: 10.1006/mthe.2001.0324. [http://dx.doi.org/10.1006/mthe.2001.0324]. [PMID: 11356070]. [DOI] [PubMed] [Google Scholar]
- 111.Kaspar B.K., Lladó J., Sherkat N., Rothstein J.D., Gage F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301(5634):839–842. doi: 10.1126/science.1086137. [http://dx.doi. org/10.1126/science.1086137]. [PMID: 12907804]. [DOI] [PubMed] [Google Scholar]
- 112.Towne C., Schneider B.L., Kieran D., Redmond D.E., Jr, Aebischer P. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 2010;17(1):141–146. doi: 10.1038/gt.2009.119. [http://dx.doi.org/10.1038/ gt.2009.119]. [PMID: 19727139]. [DOI] [PubMed] [Google Scholar]
- 113.Benkhelifa-Ziyyat S., Besse A., Roda M., Duque S., Astord S., Carcenac R., Marais T., Barkats M. Intramuscular scAAV9-SMN injection mediates widespread gene delivery to the spinal cord and decreases disease severity in SMA mice. Mol. Ther. 2013;21(2):282–290. doi: 10.1038/mt.2012.261. [http://dx.doi.org/10.1038/mt.2012.261]. [PMID: 23295949]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ujifuku K., Mitsutake N., Takakura S., Matsuse M., Saenko V., Suzuki K., Hayashi K., Matsuo T., Kamada K., Nagata I., Yamashita S. miR-195, miR-4553p and miR-10a(*) are implicated in acquired temozolomide resistance in glioblastoma multiforme cells. Cancer Lett. 2010;296(2):241–248. doi: 10.1016/j.canlet.2010.04.013. [http://dx.doi.org/ 10.1016/j.canlet.2010.04.013]. [PMID: 20444541]. [DOI] [PubMed] [Google Scholar]
- 115.Soon P.S., Tacon L.J., Gill A.J., Bambach C.P., Sywak M.S., Campbell P.R., Yeh M.W., Wong S.G., Clifton-Bligh R.J., Robinson B.G., Sidhu S.B. miR-195 and miR-4835p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. Clin. Cancer Res. 2009;15(24):7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [http://dx.doi.org/10.1158/ 1078-0432.CCR-09-1587]. [PMID: 19996210]. [DOI] [PubMed] [Google Scholar]
- 116.Mellios N., Huang H.S., Grigorenko A., Rogaev E., Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum. Mol. Genet. 2008;17(19):3030–3042. doi: 10.1093/hmg/ddn201. [http://dx.doi.org/10. 1093/hmg/ddn201]. [PMID: 18632683]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maeda H., Wu J., Sawa T., Matsumura Y., Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65(1-2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [http://dx.doi.org/10.1016/S0168-3659(99)00248-5]. [PMID: 10699287]. [DOI] [PubMed] [Google Scholar]
- 118.Montier T., Pichon C., Blondel M., Midoux P. Editorial: Current status and prospects on nucleic acid transfer. Biotechnol. J. 2014;9(11):1363–1364. doi: 10.1002/biot.201300510. [http://dx.doi.org/10.1002/biot.201300510]. [PMID: 25367631]. [DOI] [PubMed] [Google Scholar]
- 119.Asseline U., Gonçalves C., Pichon C., Midoux P. Improved nuclear delivery of antisense 2-Ome RNA by conjugation with the histidine-rich peptide H5WYG. J. Gene Med. 2014;16(7-8):157–165. doi: 10.1002/jgm.2773. [PMID: 25044540]. [DOI] [PubMed] [Google Scholar]
- 120.Zhao F., Zhao Y., Liu Y., Chang X., Chen C., Zhao Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7(10):1322–1337. doi: 10.1002/smll.201100001. [http://dx.doi.org/10.1002/smll. 201100001]. [PMID: 21520409]. [DOI] [PubMed] [Google Scholar]
- 121.Gilleron J., Querbes W., Zeigerer A., Borodovsky A., Marsico G., Schubert U., Manygoats K., Seifert S., Andree C., Stöter M., Epstein-Barash H., Zhang L., Koteliansky V., Fitzgerald K., Fava E., Bickle M., Kalaidzidis Y., Akinc A., Maier M., Zerial M. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013;31(7):638–646. doi: 10.1038/nbt.2612. [http://dx.doi.org/10.1038/ nbt.2612]. [PMID: 23792630]. [DOI] [PubMed] [Google Scholar]
- 122.Baril P., Ezzine S., Pichon C. Monitoring the spatiotemporal activities of miRNAs in small animal models using molecular imaging modalities. Int. J. Mol. Sci. 2015;16(3):4947–4972. doi: 10.3390/ijms16034947. [http://dx.doi.org/10.3390/ijms16034947]. [PMID: 25749473]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mullokandov G., Baccarini A., Ruzo A., Jayaprakash A.D., Tung N., Israelow B., Evans M.J., Sachidanandam R., Brown B.D. High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nat. Methods. 2012;9(8):840–846. doi: 10.1038/nmeth.2078. [http://dx.doi.org/10.1038/nmeth. 2078]. [PMID: 22751203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sayed D., Abdellatif M. MicroRNAs in development and disease. Physiol. Rev. 2011;91(3):827–887. doi: 10.1152/physrev.00006.2010. [http://dx.doi.org/10.1152/ physrev.00006.2010]. [PMID: 21742789]. [DOI] [PubMed] [Google Scholar]
- 125.Ezzine S., Vassaux G., Pitard B., Barteau B., Malinge J.M., Midoux P., Pichon C., Baril P. RILES, a novel method for temporal analysis of the in vivo regulation of miRNA expression. Nucleic Acids Res. 2013;41(20):e192. doi: 10.1093/nar/gkt797. [http://dx.doi.org/10.1093/ nar/gkt797]. [PMID: 24013565]. [DOI] [PMC free article] [PubMed] [Google Scholar]