Abstract
BACKGROUND
Panic disorder (PD) is a disabling psychiatry condition that affects approximately 5% of the worldwide population. Currently, long-term selective serotonin reuptake inhibitors (SSRIs) are the first-line treatment for PD; however, the common side-effect profiles and drug interactions may provoke patients to abandon the treatment, leading to PD symptoms relapse. Cannabidiol (CBD) is the major non-psychotomimetic constituent of the Cannabis sativa plant with anti-anxiety properties that has been suggested as an alternative for treating anxiety disorders. The aim of the present review was to discuss the effects and mechanisms involved in the putative anti-panic effects of CBD.
METHODS
electronic database was used as source of the studies selected selected based on the studies found by crossing the following keywords: cannabidiol and panic disorder; canabidiol and anxiety, cannabidiol and 5-HT1A receptor).
RESULTS
In the present review, we included both experimental laboratory animal and human studies that have investigated the putative anti-panic properties of CBD. Taken together, the studies assessed clearly suggest an anxiolytic-like effect of CBD in both animal models and healthy volunteers.
CONCLUSION
CBD seems to be a promising drug for the treatment of PD. However, novel clinical trials involving patients with the PD diagnosis are clearly needed to clarify the specific mechanism of action of CBD and the safe and ideal therapeutic doses of this compound.
Keywords: Animal models, cannabidiol, human studies, 5-HT1A receptors, panic disorder, serotonin
1. INTRODUCTION
Panic disorder (PD) is a chronic and disabling psychiatric disorder that is characterised by unexpected and recurrent panic attacks and affects approximately 0.8-5% of all people worldwide and may vary according to socio-demographic factors [1]. PD patients experience psychosocial impairment and a high risk of psychiatric co-morbidities and suicide [2]. In the early 1960s, Donald Klein described the efficacy of the tricyclic antidepressant imipramine in blocking panic attacks [3]. Currently, selective serotonin reuptake inhibitors (SSRIs) are the first-line compounds in the treatment of PD, although other drugs, such as tricyclic antidepressants and highly potent benzodiazepines, are also indicated. Less than half of the patients who suffer from PD show complete and sustained remission of the symptoms after long-term treatments with the currently available treatments [2]. This apparent discrepancy might be due to genetic variations in PD etiology that could affect the pharmacological responses and side effects [2]. Thus, the development of more effective drugs with a better pharmacological profile than the current ones used to treat this psychiatric condition is needed.
Over the last two decades, the therapeutic potential of cannabinoids has been extensively studied. Although cannabis abuse is connected to marked anxiety, panic attacks, depersonalisation, and emotional liability (primarily due to the psychotropic effects of ∆9-THC) [4], a growing body of evidence suggests that non-psychotomimetic phytocannabinoids could be useful as therapeutic tools. The most promising of these compounds is cannabidiol (CBD), the major non-psychotomimetic constituent of Cannabis sativa. Different from the endogenous ligands anandamide and 2-arachidonylglycerol act as agonists of CB1/CB2 receptors [5, 6], CBD has a very low affinity for these receptors in vitro [7, 8] but it can facilitate endocannabinoid signalling by inhibiting the cellular uptake and enzymatic hydrolysis of endocannabinoids [7]. Finally, CBD can also promote the blockade of adenosine uptake or act as an agonist of vanilloid (TRPV1) or 5-HT1A serotonergic receptors [9-12]. Pre-clinical studies have shown that systemically administered CBD induces anxiolytic-like effects in several animal models that have been associated with generalised anxiety (GAD), such as the elevated plus maze (EPM), the Vogel conflict test and aversive conditioning [7]. Reinforcing these findings, human studies have suggested that the drug decreases generalised anxiety symptoms [7]. The specific effects and the relevance of each of these mechanisms for the putative anti-panic effects of CBD are discussed in this review. MEDLINE/PubMed (www.pubmed.com) electronic database was used as source of the studies selected for this review (from 1990 to July 2015). Works were selected based on the studies found by crossing the following keywords: cannabidiol and panic disorder; Cannabidiol and anxiety, Cannabidiol and 5-HT1A receptor.
2. ANTI-PANIC EFFECTS OF CBD IN HUMANS
In humans, responses related to PD have been assessed in both healthy volunteers and panic patients submitted to controlled conditions of psychological or chemical-nature stimuli [15, 16]. For instance, in the simulated public speaking (SPS) test, the participant must prepare a speech and talk in front of a video camera [17]. Indices of anxiety and other emotional states during the test are obtained by applying scales, such as the Visual Analog Mood Scale (VAMS; [17]) and the Self-Statements during Public Speaking Scale [18].
Fear of public speaking is accepted to increase anxiety in healthy people, irrespective of their trait anxiety level [16]. Classical benzodiazepines decrease the VAMS indices before and after the speech, without affecting speech preparation or performance (which is associated with “fear of speaking”). Conversely, speech preparation and performance are reduced by antidepressants [15]. Based on pharmacological studies, it has been proposed that the neural networks activated by SPS are involved in anxiety disorders [16] and, thus, that the fear of speaking provoked by the SPS test can be helpful in understanding the brain areas involved and potential new drugs targets for PD.
Regarding CBD, the work of Zuardi and coworkers [14] showed that a single dose of CBD (300 mg, p.o.) decreased anxiety after the SPS test in healthy volunteers. In another study, Bergamaschi et al. [19] showed that social anxiety disorder patients presented higher anxiety, cognitive impairment and discomfort, as well as increased alertness during their speech performance when compared with healthy controls. After CBD (600 mg, p.o.) treatment, however, a significant reduction in anxiety-related measures obtained during their speech performance was observed. These results have encouraged new approaches in the study of the putative effects of CBD on PD.
3. EFFECTS OF CBD IN ANIMAL MODEL
Animal models of panic attacks are supported by the observation of Blanchard and co-workers that, depending on the presence or absence of the predator and its distance from the prey, animals display different defensive strategies [20]. According to this hypothesis, panic attacks would be related to the flight and freezing defensive responses elicited by proximal threats. Thus, the flight/escape and freezing responses generated by a stimuli, such as natural predators, open/unprotected spaces and electrical/chemical stimulation of brain areas (such as the dorsal periaqueductal grey (dPAG) or the medial hypothalamus), have been used to study several aspects of PD [21].
For instance, the encounter between the mouse and the wild snake Epicrates cenchria crassus elicited several defensive behaviours. Of note, the acute administration of CBD (0.3-30 mg/Kg, i.p.) decreased the expression of panic-related behaviours, such as defensive immobility, explosive escape and total escape of the mice [22].
The anti-panic effect of CBD was also observed in rats submitted to the open arm of the elevated T-maze or to the electrical stimulation of the dPAG. The local administration of CBD (30–60 nmol, intra-dPAG) inhibited the escape response generated by both tests [23]. Recently, the effects of CBD on experimental PD were reinforced by Campos and colleagues [24], who demonstrated that the chronic (5 mg/kg, i.p., 21 days), but not acute (5, 10 and 20 mg/kg, i.p.), peripheral administration of CBD was able to reduce the escape response of rats submitted to the elevated T-maze. These latter studies also provided new evidence regarding the brain sites and mechanisms involved in the anti-panic effects of CBD.
4. MECHANISIMS OF THE EFFECTS OF CBD ON PANIC
4.1. Brain Sites
Although the network involved in the anti-panic effects of CBD remain largely unknown, both preclinical and clinical studies have suggested some brain areas related to panic disorder [16, 25] as possible sites for the therapeutic actions of this compound (Table 1, Fig. 1).
Table 1. Putative brain sites and pharmacological mechanisms involved in the anti-panic effects of CBD.
| Brain Area | Dose, Route, Schedule | CBD Effects | |
|---|---|---|---|
| Amygdala/Bed nucleus of stria terminalis-BNST | Clinical studies | ||
| 400mg, p.o., single dose | ↓ rCBF, ↓ subjective anxiety and ↑ mental sedation [30] | ||
| 600mg, p.o., single dose | ↓BOLD signal during fearful faces presentation [13, 37] | ||
| 600mg, p.o., single dose | ↓amygdala-anterior cingulated connectivity [13] | ||
| Preclinical studies | |||
| 1μg, intra-central nucleus, single dose | ↓ anxiety-like behaviors [60] | ||
| 10 mg/Kg, i.p., single dose | ↓ c-fos expression in BNST [61] | ||
| 30 nmol, intra-BNST, single dose | ↓ anxiety-like behaviors via 5-HT1A receptors [51] | ||
| Basal ganglia (nucleus accumbens, striatum, substancia nigra) |
Clinical study | ||
| 600mg, p.o., single dose | ↑ striatal activation [37] | ||
| Preclinical studies | |||
| 120mg/Kg, i.p., single dose | ↑ number of Fos immunoreactive neurons in nucleus accumbens, but not in the dorsal striatum [62] | ||
| 10μg, i.c.v., single dose | ↑ dopamine in nucleus accumbens and ↓sleep. [63] | ||
| 5mg/Kg, i.p., 5 days | prevented striatal lesion; effect not reversed by CB1, TRPV1, A2A antagonists [65] | ||
| 15, 30 or 60 mg/Kg, i.p., single dose or 14 days |
↑mitochondrial complex and creatine kinase activity in striatum [59] | ||
| 20mg/Kg, i.p., single dose | altered motor behaviors; effect reversed by peripheral 5-HT1A antagonist; no changes in 5-HT contend [50] | ||
| Cortex (cingulate, insular, prefrontal, temporal) |
Clinical studies | ||
| 400mg, p.o., single dose | ↓ rCBF in posterior cingulated cortex; ↓ subjective anxiety and ↑ mental sedation [30] | ||
| 600mg, p.o., single dose | ↓ activation left temporal and insular cortex during motor inhibition task [36] | ||
| 600mg, p.o., single dose | ↓BOLD signal in fearful faces presentation [29] and ↓amygdala-anterior cingulated connectivity during fearful faces presentation [13] | ||
| 400mg, p.o., single dose | ↑ rCBF in posterior cingulated cortex and ↓ rCBF in temporal gyrus; ↓ generalized social anxiety disorder patients [39] | ||
| Preclinical studies | |||
| 30 nmol, intra-PL and -IL cortices, single dose | ↑c-fos expression in PL and IL cortices; ↓contextual fear after PL and ↑contextual fear after IL cortex injections [61] | ||
| 10mg/Kg, i.p., 14 days | ↑ anxiety-like behaviors and ↓BDNF and phosphoERK1/2 expression in frontal cortex [65] | ||
| 0.4μg, intra-IL cortex, 3 days | facilitated fear extinction; effect reversed by peripheral CB1 receptor antagonist [66] | ||
| 15, 30 or 60 mg/Kg, i.p., single dose or 14 days |
↑ mitochondrial complex and creatine kinase activity in prefrontal cortex [59] | ||
| 30nmol, intra-PL cortex, single dose | ↓ contextual fear and ↑ or ↓anxiety-like, behaviors depending on previous stressful experience via 5-HT1A [52] | ||
| Hippocampus/ Para- hippocampal gyrus |
Clinical studies | ||
| 400mg, p.o., single dose | ↓ rCBF in hippocampus and left parahippocampal gyrus, ↓ subjective anxiety and social anxiety [30, 39] | ||
| 400mg, p.o., single dose | ↑ rCBF in left parahippocampal gyrus, ↓ subjective anxiety and ↑ mental sedation [30] | ||
| Brain Area | Dose, Route, Schedule | CBD Effects | |
| Hippocampus/ Para- hippocampal gyrus |
Preclinical studies | ||
| 10mg/Kg, i.p., 15 days | ↑neurogenesis; effect blocked by peroxisome proliferator-activated receptor-ᵧ antagonist [67] | ||
| 10mg/Kg, i.p., 14 days | ↑ anxiety-like behaviors and ↓ brain derived neurotrophic factor [65] | ||
| 60 mg/Kg, i.p., single dose and 15, 30 or 60 mg/Kg, i.p., 14 days | ↑ mitochondrial complex and creatine kinase activity [59] | ||
| 30 mg/kg, i.p., 14 days | ↑ neurogenesis, ↑ AEA, prevented by CB1 antagonist [56] | ||
| Hypothalamus | Preclinical studies | ||
| 10μg, i.c.v., single dose | ↑c-fos expression in hypothalamus and ↓sleep [63] | ||
| 30, 60 and 90nM or 10 and 20μg, intra-hypothalamus, single dose | ↑ dopamine and adenosine levels in nucleus accumbens and ↓sleep after lateral hypothalamus injections [63, 68] | ||
| Periaqueductal Gray | Preclinical studies | ||
| 30 and 60nmol, intra-dPAG, single dose | ↓ anxiety-like and ↓ panic-like behaviors after intra-dPAG injection via 5-HT1A receptor [10, 23] | ||
| 3 nmol, intra-ventrolateral periaqueductal gray, single dose | ↑ endocannabinoids levels and ↓ nociception after intra-vlPAG injection via CB1, A1 and TRPA1, but not TRPV1 receptors [69] | ||
| 5 mg/Kg, i.p., 21 days | ↓ panic-like behaviors via 5-HT1A receptor without changing 5HT-1A or 5-HT-2C receptors expression and 5-HT concentrations in the dPAG [24] | ||
| Raphe nuclei | Preclinical studies | ||
| 10μg, i.c.v., single dose | ↑ c-fos expression in dorsal raphe nucleus and ↓sleep. [63] | ||
| 10 and 20μg, intra-dorsal raphe nucleus, single dose | ↓sleep [70] | ||
| 5mg/Kg, i.p., single dose | inhibited reward-facilitating effect of morphine via 5-HT1A receptor located in raphe nucleus [71] | ||
| 5mg/Kg (s.c.) or 10μg (intra-dorsal raphe nucleus), single dose | suppressed nausea via 5-HT1A [53] | ||
i.p. – intraperitoneal; i.c.v- intracerebroventricular; s.c- subcutaneal, BNST- bed nucleus striaterminalis, PAG- periaqueductal grey matter, CBF- cerebral blood flow; AEA- anandamide.
Fig. (1).
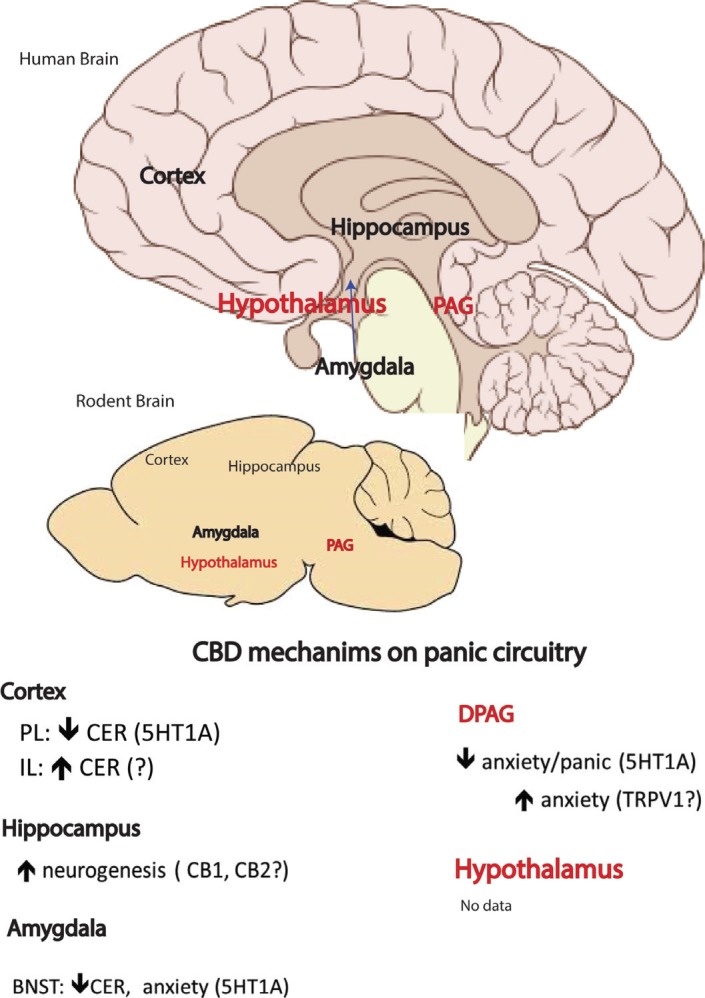
Possible neurobiology network brain sites involved in the mechanism of action of cannabidiol in panic disorder. CER: results based on Contextual Fear Conditioning test.
Structural differences have been described in the amygdala, hippocampus, hypothalamus, cingulated cortex and parahippocampal gyrus of PD patients [26]. Functional magnetic resonance imaging studies have revealed the activation of the amygdala during spontaneous panic attacks [27-29]. Other neuroimaging studies have presented increased activity in the hippocampus, hypothalamus and posterior cingulated cortex in PD patients or during panic anticipation [25]. These results indicate the amygdala, hippocampus, hypothalamus and cingulate cortex as possible brain sites for the panicolytic action of CBD. Crippa and colleagues [30] conducted a functional neuroimaging study to investigate the brain areas recruited after CBD administration in humans. A single dose of CBD, administered orally in Healthy volunteers, promoted a reduction in anxiety evoked by a tracer injection and scanning procedure. CBD also altered resting activity in limbic and paralimbic brain areas. Of note, CBD decreased the activity of the left amygdala-hippocampal complex, hypothalamus and posterior cingulated cortex while increasing the activity of the left parahippocampal gyrus compared with placebo.
Regarding the parahippocampal gyrus, although its deactivation has been observed after panic attacks induced by lactate [31] or cholecystokinin-4 [32], spontaneous panic attacks have also been associated with the activation of this area [25]. Because the study of Crippa and colleagues [30]
was conducted in healthy volunteers, it would be of interest to test whether CBD would also alter the activity of those limbic and paralimbic areas in PD patients.
In healthy volunteers treated with CBD and submitted to a model of the presentation of fearful faces, a decreasing of the amygdala and anterior and posterior cingulate cortex activities and a disruption in the connections between the amygdala and the anterior cingulated cortex were observed [13, 33]. Interestingly, previous studies have suggested that the anterior cingulated cortex is connected to the amygdala in response to fear and anxiety [30], suggesting these brain areas as candidates for the action of CBD on PD. However, as noted by Kowal and colleagues [35], the effects of CBD on anterior cingulate cortex activity might depend on the nature of the task used in imaging studies. While verbal paired associate learning is associated with cognition, facial expressions task seems to be more related to emotion. These different tasks might differently recruit cingulate cortex. Thus, the effects of CBD in this area might be dependent on the task (emotion vs cognition). In addition to the attenuation of anterior cingulate cortex activity [13, 33], other studies have failed to detect changes in the activity of the anterior cingulate cortex by CBD [36- 38].
These apparent discrepancies, however, could be explained by the fact that CBD may increase the activity in a brain area depending on the stimuli presented to the volunteers and on the anxiety disorder under observation. Preliminary data involving generalized social anxiety disorder patients suggested that CBD decreased VAMS anxiety scores with a concomitant reduction in the left parahippocampal gyrus activation and an increase in the right posterior cingulate gyrus activation, in contradistinction to the previously described results [30]. According to the authors, these discrepancies might be related to differences in the activity of the parahippocampal gyrus and cingulated gyrus in healthy individuals versus patients with anxiety disorders [39].
Imaging studies have also demonstrated that CBD can alter the activity of brain areas such as the medial and left temporal lobes, prefrontal cortex and insula [36, 40], regions that were also found to be modified in PD patients [25]. Moreover, the participation of other brain structures also related to PD [41], such as the midbrain areas, caudal pons and medulla, on the anti-panic effects of CBD cannot be ruled out. However, these brain areas are significantly smaller, and the detection of small alterations during neuroimaging studies could be difficult [42]. One example of a small area that is strongly related to panic responses is the dPAG [42]. Previous studies conducted in humans demonstrated that midbrain stimulation, which was evaluated many years ago while attempting to produce pain relief in patients, induced neurovegetative changes (such as increases in the heart beat and respiratory frequency), followed by feelings of imminent death and suffocation, which is very similar to a panorama described by patients during a panic attack [43].
Aiming to investigate the role of dPAG in the panicolytic-like effect of CBD, Soares and colleagues [23] injected different doses of CBD (30–60 nmol, 0.2 μL) into the dPAG and successfully inhibited escape responses generated in two animal models: the electrical stimulation of the dPAG and the exposure to the open arm of the elevated T-maze. In addition, the anti-panic effects of the chronic treatment of CBD, observed in animals tested in the elevated-T maze, are mediated by serotonergic mechanisms located in the dPAG [24].
4.2. Pharmacological Mechanisms of CBD on PD
So far, several mechanisms of action have been associated with CBD effects [7]. Although many of the described mechanisms are not directly connected to anxiety disorders, the type 1A serotoninergic receptor has been extensively reported to be a key partner in the anti-anxiety and anti-panic effects of CBD [7, 23, 24, 44].
In mice, the panicolytic-like effect of CBD was prevented by the peripheral administration of a 5-HT1A receptor antagonist, WAY-100635 (0.1–0.9 mg/kg, i.p.) [44]. Accordingly, in rats, intra-dPAG CBD administration impaired escape responses generated by the open arm of the elevated T-maze and by the electrical stimulation of the dPAG; in both cases, the effects were prevented by the administration of WAY-100635 (0.37 nmol) [23]. Later, Campos and coworkers [24] suggested that the panicolytic-like effect promoted by the repeated peripheral administra- tion of CBD in rats was also mediated by 5-TH1A receptors located in the dPAG. Interestingly, the effect of CBD on the dPAG seems to be dependent on its actions on the 5-HT1A receptor rather than the modulation of serotonin release or 5-HT1A and 5-HT2C expression, as observed after antidepressant treatment.
In fact, the activation of 5-HT1A receptors in the dPAG has been proposed to reduce panic-like responses and to be involved in the mechanism of action of antidepressants [45]. In addition to the dPAG, preclinical studies have noted the amygdala and the dorsomedial hypothalamic nucleus as other brain sites for the panicolytic-like effect mediated by 5-HT1A receptor activation [46, 47]. In patients with a PD diagnosis, studies have shown that both presynaptic and postsynaptic 5-HT1A receptor binding is reduced in the anterior cingulate, posterior cingulate, raphe, orbitofrontal cortex, temporal cortex and amygdala [48]. Other genetic studies propose an association between 5-HT1A receptor gene polymorphisms and PD [49].
CBD has been suggested to activate 5-HT1A receptors in several brain regions, such as the basal ganglia [50], the bed nucleus of stria terminalis [51], the prelimbic prefrontal cortex [52] and the dorsal raphe nucleus [53, 54]. Although the molecular mechanism by which CBD favours 5-HT1A activation remains unclear, it might involve the allosteric modulation of this receptor, the ability of CBD to facilitate the 5-HT1A agonist-related stimulation of [35S]GTPᵧS binding [12] or the indirect recruitment or formation of heterodimers consisting of 5-HT1A and other receptors, such as CB1 [55].
In addition to serotonin, other mechanisms might be involved in the anti-panic effects of CBD. For instance, chronic treatment with CBD can increase the anandamide levels within the hippocampus with concomitant increases of hippocampal neurogenesis [56]. CBD may also favour neurogenesis during the aging process by activating PPARγ [57]. Other receptors may also be involved in the actions of CBD, such as CB1, adenosine 1, TRPV1 and TRPA1 [7]. For instance, after chronic (21 days) treatment, CBD reduced 5-HT2A receptor binding in the substantia nigra [58] and increased mitochondrial complex and creatine kinase activity in the prefrontal cortex, hippocampus and striatum of rats [59]. However, the contribution of the aforementioned mechanisms in the anti-panic actions of CBD has yet to be investigated.
5. CONCLUSIONS AND PERSPECTIVE:
Taken together, the results presented in this chapter, which were derived from both laboratory animal and human studies, support the notion that CBD exhibits anti-panic properties. Despite the described panic response reported as a result of cannabis use, it is important to note that CBD does not present psychoactive effects; it is safe and well-tolerated via the oral route (up to 1,500 mg/day) [19]. Moreover, because this compound does not induce dependence, tolerance and abstinence symptoms, it can be, in the future, a good alternative as a substitute for high potency benzodiazepines and antidepressant drugs in PD patients who are resistant to the current treatments. However, it is important to stress that we are just in the first steps in the route to get a possible final approval of CBD for the treatment of PD. Several clinical trials using CBD alone or in combination with other cannabinoids are under development. The GW compound Epidolex®, that basically has CBD in its formula, is currently in phase 3 trial for the treatment of orphan pediatric epilepsy syndrome. Therefore, new studies conducted with a reasonable number of PD patients (phase 2 and phase 3 studies) are necessary to demonstrate the efficacy and the dose range of CBD for the treatment of this anxiety disorder.
ACKNOWLEDGEMENT
The authors declare that they have no conflicts of interest in the research.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Diagnostic and statistical manual of mental disorders. 4th ed. text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Roy-Byrne P.P., Craske M.G., Stein M.B. Panic disorder. Lancet. 2006;368(9540):1023–1032. doi: 10.1016/S0140-6736(06)69418-X. [http://dx.doi.org/10.1016/S0140-6736(06)69418-X]. [PMID: 16980119]. [DOI] [PubMed] [Google Scholar]
- 3.Klein D.F. Delineation of Two Drug-Responsive Anxiety Syndromes. Psychopharmacology (Berl.) 1964;5:397–408. doi: 10.1007/BF02193476. [http:// dx.doi.org/10.1007/BF02193476]. [PMID: 14194683]. [DOI] [PubMed] [Google Scholar]
- 4.Loga S., Loga-Zec S., Spremo M. Cannabis and psychiatric disorders. Psychiatr. Danub. 2010;22(2):296–297. [PMID: 20562767]. [PubMed] [Google Scholar]
- 5.Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [http://dx.doi.org/10.1126/science.1470919]. [PMID: 1470919]. [DOI] [PubMed] [Google Scholar]
- 6.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [http://dx. doi.org/10.1016/0006-2952(95)00109-D]. [PMID: 7605349]. [DOI] [PubMed] [Google Scholar]
- 7.Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimaraes FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. Royal Soc. Lond. Series B, Biol. Sci., 2012;367(1607) doi: 10.1098/rstb.2011.0389. 3364-3378. [http://dx.doi.org/10.1098/rstb.2011.0389] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas A., Baillie G.L., Phillips A.M., Razdan R.K., Ross R.A., Pertwee R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007;150(5):613–623. doi: 10.1038/sj.bjp.0707133. [http://dx.doi.org/ 10.1038/sj.bjp.0707133]. [PMID: 17245363]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. [http://dx.doi.org/10.1038/sj.bjp.0704327]. [PMID: 11606325]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos A.C., Guimarães F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl.) 2008;199(2):223–230. doi: 10.1007/s00213-008-1168-x. [http://dx.doi.org/10.1007/s00213-008-1168-x]. [PMID: 18446323]. [DOI] [PubMed] [Google Scholar]
- 11.Carrier E.J., Auchampach J.A., Hillard C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA. 2006;103(20):7895–7900. doi: 10.1073/pnas.0511232103. [http://dx.doi.org/10.1073/pnas.0511232103]. [PMID: 16672367]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo E.B., Burnett A., Hall B., Parker K.K. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 2005;30(8):1037–1043. doi: 10.1007/s11064-005-6978-1. [http://dx.doi.org/10.1007/s11064-005-6978-1]. [PMID: 16258853]. [DOI] [PubMed] [Google Scholar]
- 13.Fusar-Poli P., Crippa J.A., Bhattacharyya S., Borgwardt S.J., Allen P., Martin-Santos R., Seal M., Surguladze S.A. OCarrol, C.; Atakan, Z.; Zuardi, A.W.; McGuire, P.K. Distinct effects of delta9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch. Gen. Psychiatry. 2009;66(1):95–105. doi: 10.1001/archgenpsychiatry.2008.519. [http://dx.doi.org/10.1001/archgenpsychiatry.2008.519]. [PMID: 19124693]. [DOI] [PubMed] [Google Scholar]
- 14.Zuardi A.W., Cosme R.A., Graeff F.G., Guimarães F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. (Oxford) 1993;7(1) Suppl.:82–88. doi: 10.1177/026988119300700112. [PMID: 22290374]. [DOI] [PubMed] [Google Scholar]
- 15.Graeff F.G. Rev. Bras. Psiquiatr. 2003;25(Suppl. 2):42–45. doi: 10.1590/s1516-44462003000600010. [Serotonin, periaqueductal gray matter and panic disorder]. [Serotonin, periaqueductal gray matter and panic disorder]. [PMID: 14978586]. [DOI] [PubMed] [Google Scholar]
- 16.Graeff F.G., Del-Ben C.M. Neurobiology of panic disorder: from animal models to brain neuroimaging. Neurosci. Biobehav. Rev. 2008;32(7):1326–1335. doi: 10.1016/j.neubiorev.2008.05.017. [http://dx.doi.org/10.1016/j.neubiorev. 2008.05.017]. [PMID: 18573531]. [DOI] [PubMed] [Google Scholar]
- 17.McNair D.M., Frankenthaler L.M., Czerlinsky T., White T.W., Sasson S., Fisher S. Simulated public speaking as a model of clinical anxiety. Psychopharmacology (Berl.) 1982;77(1):7–10. doi: 10.1007/BF00436092. [http://dx.doi.org/10.1007/BF00436092]. [PMID: 6126900]. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann S.G., Dibartolo P.M. An instrument to assess self-statements during public speaking: scale development and preliminary psychometric properties. Behav. Ther. 2000;31(3):499–515. doi: 10.1016/s0005-7894(00)80027-1. [http://dx.doi.org/10.1016/S0005-7894(00)80027-1]. [PMID: 16763666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology, 2011;36(6):1219–1226. doi: 10.1038/npp.2011.6. [http://dx.doi.org/10.1038/npp.2011.6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard C, Blanchard R, Fellous JM, Guimaraes FS, Irwin W, Ledoux JE. Guimaraes, FS; Irwin, W; Ledoux, JE The brain decade in debate: III. Neurobiology of emotion. Braz. J. Med. Biol. Res.,, 2001;34(3):283–293. doi: 10.1590/s0100-879x2001000300001. [DOI] [PubMed] [Google Scholar]
- 21.Moreira F.A., Gobira P.H., Viana T.G., Vicente M.A., Zangrossi H., Graeff F.G. Modeling panic disorder in rodents. Cell Tissue Res. 2013;354(1):119–125. doi: 10.1007/s00441-013-1610-1. [http://dx.doi.org/10.1007/ s00441-013-1610-1]. [PMID: 23584609]. [DOI] [PubMed] [Google Scholar]
- 22.Uribe-Marino A, Francisco A, Castiblanco-Urbina MA, Twardowschy A, Salgado-Rohner CJ, Crippa JA. Salgado-Rohner, CJ; Crippa, JA Anti-aversive effects of cannabidiol on innate fear-induced behaviors evoked by an ethological model of panic attacks based on a prey vs the wild snake Epicrates cenchria crassus confrontation paradigm. Neuropsychopharmacology, 2012;37(2):412–421. doi: 10.1038/npp.2011.188. [http://dx.doi.org/10.1038/npp.2011.188] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares V. P.; Campos, A.C.; Bortoli, V.C.; Zangrossi, H., Jr; Guimarães, F.S.; Zuardi, A.W. Intra-dorsal periaqueductal gray administration of cannabidiol blocks panic-like response by activating 5-HT1A receptors. Behav. Brain Res. 2010;213(2):225–229. doi: 10.1016/j.bbr.2010.05.004. [http://dx.doi.org/10.1016/j.bbr.2010.05.004]. [PMID: 20457188]. [DOI] [PubMed] [Google Scholar]
- 24.Campos A.C., de Paula Soares V., Carvalho M.C., Ferreira F.R., Vicente M.A., Brandão M.L., Zuardi A.W., Zangrossi H., Jr, Guimarães F.S. Involvement of serotonin-mediated neurotransmission in the dorsal periaqueductal gray matter on cannabidiol chronic effects in panic-like responses in rats. Psychopharmacology (Berl.) 2013;226(1):13–24. doi: 10.1007/s00213-012-2878-7. [http://dx.doi.org/ 10.1007/s00213-012-2878-7]. [PMID: 23007604]. [DOI] [PubMed] [Google Scholar]
- 25.Dresler T., Guhn A., Tupak S.V., Ehlis A.C., Herrmann M.J., Fallgatter A.J., Deckert J., Domschke K. Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. J Neural Transm (Vienna) 2013;120(1):3–29. doi: 10.1007/s00702-012-0811-1. [http://dx. doi.org/10.1007/s00702-012-0811-1]. [PMID: 22692647]. [DOI] [PubMed] [Google Scholar]
- 26.Pannekoek JN, van der Werff SJ, Stein DJ, van der Wee NJ. NJ Advances in the neuroimaging of panic disorder. Human Psychopharmacol.Clin. Exper. 2013;28(6):608–611. doi: 10.1002/hup.2349. [http://dx.doi.org/ 10.1002/hup.2349] [DOI] [PubMed] [Google Scholar]
- 27.Pfleiderer B., Zinkirciran S., Arolt V., Heindel W., Deckert J., Domschke K. fMRI amygdala activation during a spontaneous panic attack in a patient with panic disorder. World J. Biol. Psychiatry. 2007;8(4):269–272. doi: 10.1080/15622970701216673. [http://dx.doi.org/10.1080/ 15622970701216673]. [PMID: 17853295]. [DOI] [PubMed] [Google Scholar]
- 28.Spiegelhalder K., Hornyak M., Kyle S.D., Paul D., Blechert J., Seifritz E., Hennig J., Tebartz van Elst L., Riemann D., Feige B. Cerebral correlates of heart rate variations during a spontaneous panic attack in the fMRI scanner. Neurocase. 2009;15(6):527–534. doi: 10.1080/13554790903066909. [http://dx.doi.org/10.1080/13554790903066909]. [PMID: 19657971]. [DOI] [PubMed] [Google Scholar]
- 29.Dresler T., Hahn T., Plichta M.M., Ernst L.H., Tupak S.V., Ehlis A.C., Warrings B., Deckert J., Fallgatter A.J. Neural correlates of spontaneous panic attacks. Journal of neural transmission (Vienna) 2011;118(2):263–269. doi: 10.1007/s00702-010-0540-2. [http://dx.doi.org/10.1007/s00702-010-0540-2]. [DOI] [PubMed] [Google Scholar]
- 30.Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R. L Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology, 2004;29(2):417–426. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- 31.Reiman E.M., Raichle M.E., Robins E., Mintun M.A., Fusselman M.J., Fox P.T., Price J.L., Hackman K.A. Neuroanatomical correlates of a lactate-induced anxiety attack. Arch. Gen. Psychiatry. 1989;46(6):493–500. doi: 10.1001/archpsyc.1989.01810060013003. [http://dx.doi.org/10. 1001/archpsyc.1989.01810060013003]. [PMID: 2786401]. [DOI] [PubMed] [Google Scholar]
- 32.Javanmard M., Shlik J., Kennedy S.H., Vaccarino F.J., Houle S., Bradwejn J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol. Psychiatry. 1999;45(7):872–882. doi: 10.1016/s0006-3223(98)00348-5. [http://dx.doi.org/10.1016/ S0006-3223(98)00348-5]. [PMID: 10202575]. [DOI] [PubMed] [Google Scholar]
- 33.Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S. S Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int. J. Neuropsychopharmacol., 2010;13(4):412–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- 34.Pissiota A., Frans O., Michelgård A., Appel L., Långström B., Flaten M.A., Fredrikson M. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. Eur. J. Neurosci. 2003;18(5):1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [http://dx.doi. org/10.1046/j.1460-9568.2003.02855.x]. [PMID: 12956731]. [DOI] [PubMed] [Google Scholar]
- 35.Kowal M.A., Hazekamp A., Colzato L.S., van Steenbergen H., Hommel B. Modulation of cognitive and emotional processing by cannabidiol: the role of the anterior cingulate cortex. Front. Hum. Neurosci. 2013;7:147. doi: 10.3389/fnhum.2013.00147. [http://dx.doi.org/10.3389/fnhum.2013. 00147]. [PMID: 23616760]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgwardt S.J., Allen P., Bhattacharyya S., Fusar-Poli P., Crippa J.A., Seal M.L., Fraccaro V., Atakan Z., Martin-Santos R. OCarroll, C.; Rubia, K.; McGuire, P.K. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol. Psychiatry. 2008;64(11):966–973. doi: 10.1016/j.biopsych.2008.05.011. [http://dx.doi. org/10.1016/j.biopsych.2008.05.011]. [PMID: 18589404]. [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharyya S., Fusar-Poli P., Borgwardt S., Martin-Santos R., Nosarti C. OCarroll, C.; Allen, P.; Seal, M.L.; Fletcher, P.C.; Crippa, J.A.; Giampietro, V.; Mechelli, A.; Atakan, Z.; McGuire, P. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch. Gen. Psychiatry. 2009;66(4):442–451. doi: 10.1001/archgenpsychiatry.2009.17. [http://dx.doi.org/10.1001/ archgenpsychiatry.2009.17]. [PMID: 19349314]. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T. T Opposite effects of delta-9- tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology, 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crippa J.A., Derenusson G.N., Ferrari T.B., Wichert-Ana L., Duran F.L., Martin-Santos R., Simões M.V., Bhattacharyya S., Fusar-Poli P., Atakan Z., Santos Filho A., Freitas-Ferrari M.C., McGuire P.K., Zuardi A.W., Busatto G.F., Hallak J.E. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J. Psychopharmacol. (Oxford) 2011;25(1):121–130. doi: 10.1177/0269881110379283. [http://dx.doi.org/10.1177/ 0269881110379283]. [PMID: 20829306]. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharyya S., Atakan Z., Martin-Santos R., Crippa J.A., McGuire P.K. Neural mechanisms for the cannabinoid modulation of cognition and affect in man: a critical review of neuroimaging studies. Curr. Pharm. Des. 2012;18(32):5045–5054. doi: 10.2174/138161212802884636. [http://dx. doi.org/10.2174/138161212802884636]. [PMID: 22716136]. [DOI] [PubMed] [Google Scholar]
- 41.Sakai Y., Kumano H., Nishikawa M., Sakano Y., Kaiya H., Imabayashi E., Ohnishi T., Matsuda H., Yasuda A., Sato A., Diksic M., Kuboki T. Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport. 2005;16(9):927–931. doi: 10.1097/00001756-200506210-00010. [http://dx.doi.org/10.1097/00001756-200506210-00010]. [PMID: 15931063]. [DOI] [PubMed] [Google Scholar]
- 42.Del-Ben C.M. Graeff, FG Panic disorder: is the PAG involved? Neural Plast. 2009 doi: 10.1155/2009/108135. [http://dx.doi.org/10.1155/2009/108135]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nashold B.S., Jr, Wilson W.P., Slaughter D.G. Sensations evoked by stimulation in the midbrain of man. J. Neurosurg. 1969;30(1):14–24. doi: 10.3171/jns.1969.30.1.0014. [http://dx.doi.org/10.3171/jns.1969.30.1.0014]. [PMID: 4885810]. [DOI] [PubMed] [Google Scholar]
- 44.Twardowschy A., Castiblanco-Urbina M.A., Uribe-Mariño A., Biagioni A.F., Salgado-Rohner C.J., Crippa J.A., Coimbra N.C. The role of 5-HT1A receptors in the anti-aversive effects of cannabidiol on panic attack-like behaviors evoked in the presence of the wild snake Epicrates cenchria crassus (Reptilia, Boidae). J. Psychopharmacol. (Oxford) 2013;27(12):1149–1159. doi: 10.1177/0269881113493363. [http://dx. doi.org/10.1177/0269881113493363]. [PMID: 23926240]. [DOI] [PubMed] [Google Scholar]
- 45.Graeff F.G., Zangrossi H., Jr The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent. Nerv. Syst. Agents Med. Chem. 2010;10(3):207–217. doi: 10.2174/1871524911006030207. [http://dx.doi.org/10.2174/1871524911006030207]. [PMID: 20528764]. [DOI] [PubMed] [Google Scholar]
- 46.Strauss C.V., Vicente M.A., Zangrossi H. Jr Activation of 5-HT1A receptors in the rat basolateral amygdala induces both anxiolytic and antipanic-like effects. Behav. Brain Res. 2013;246:103–110. doi: 10.1016/j.bbr.2013.03.005. [http://dx.doi.org/10.1016/j.bbr.2013.03.005]. [PMID: 23499701]. [DOI] [PubMed] [Google Scholar]
- 47.de Bortoli V.C., Yamashita P.S., Zangrossi H., Jr 5-HT1A and 5-HT2A receptor control of a panic-like defensive response in the rat dorsomedial hypothalamic nucleus. Journal of Psychophar-macology. 2013;27(12):1116–1123. doi: 10.1177/0269881113492900. [DOI] [PubMed] [Google Scholar]
- 48.Neumeister A., Bain E., Nugent A.C., Carson R.E., Bonne O., Luckenbaugh D.A., Eckelman W., Herscovitch P., Charney D.S., Drevets W.C. Reduced serotonin type 1A receptor binding in panic disorder. J. Neurosci. 2004;24(3):589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [http://dx. doi.org/10.1523/JNEUROSCI.4921-03.2004]. [PMID: 14736842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaya C., Salum G.A., Moorjani P., Seganfredo A.C., Heldt E., Leistner-Segal S., Smoller J.W., Manfro G.G. Panic disorder and serotonergic genes (SLC6A4, HTR1A and HTR2A): Association and interaction with childhood trauma and parenting. Neurosci. Lett. 2010;485(1):11–15. doi: 10.1016/j.neulet.2010.08.042. [http://dx.doi.org/10.1016/j.neulet.2010. 08.042]. [PMID: 20817074]. [DOI] [PubMed] [Google Scholar]
- 50.Espejo-Porras F., Fernández-Ruiz J., Pertwee R.G., Mechoulam R., García C. Motor effects of the non-psychotropic phyto- cannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacology. 2013;75:155–163. doi: 10.1016/j.neuropharm.2013.07.024. [http://dx.doi.org/10. 1016/j.neuropharm.2013.07.024]. [PMID: 23924692]. [DOI] [PubMed] [Google Scholar]
- 51.Gomes F.V., Resstel L.B., Guimarães F.S. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology (Berl.) 2011;213(2-3):465–473. doi: 10.1007/s00213-010-2036-z. [http://dx.doi.org/10.1007/ s00213-010-2036-z]. [PMID: 20945065]. [DOI] [PubMed] [Google Scholar]
- 52.Fogaca MV, Reis FM, Campos AC, Guimaraes FS. FS Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: involvement of 5HT1A receptors and previous stressful experience. Euro. Neuropsychopharmacol., 2014;24(3):410–419. doi: 10.1016/j.euroneuro.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Rock E.M., Bolognini D., Limebeer C.L., Cascio M.G., Anavi-Goffer S., Fletcher P.J., Mechoulam R., Pertwee R.G., Parker L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012;165(8):2620–2634. doi: 10.1111/j.1476-5381.2011.01621.x. [http://dx. doi.org/10.1111/j.1476-5381.2011.01621.x]. [PMID: 21827451]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsidoni V., Anagnostou I., Panagis G. Cannabidiol inhibits the reward-facilitating effect of morphine: involvement of 5-HT1A receptors in the dorsal raphe nucleus. Addict. Biol. 2013;18(2):286–296. doi: 10.1111/j.1369-1600.2012.00483.x. [http://dx.doi.org/10.1111/j.1369-1600.2012.00483.x]. [PMID: 22862835]. [DOI] [PubMed] [Google Scholar]
- 55.Mato S., Vidal R., Castro E., Díaz A., Pazos A., Valdizán E.M. Long-term fluoxetine treatment modulates cannabinoid type 1 receptor-mediated inhibition of adenylyl cyclase in the rat prefrontal cortex through 5-hydroxytryptamine 1A receptor-dependent mechanisms. Mol. Pharmacol. 2010;77(3):424–434. doi: 10.1124/mol.109.060079. [http://dx.doi.org/10.1124/mol.109.060079]. [PMID: 19995940]. [DOI] [PubMed] [Google Scholar]
- 56.Campos AC, Ortega Z, Palazuelos J, Fogaca MV, Aguiar DC, Diaz-Alonso J. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. Intl. J. Neuropsychopharmacol., 2013;16(6):1407–1419. doi: 10.1017/S1461145712001502. [http://dx.doi.org/10.1017/S1461145712001502] [DOI] [PubMed] [Google Scholar]
- 57.Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., De Filippis D., Cipriano M., Carratù M.R., Iuvone T., Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6(12):e28668. doi: 10.1371/journal.pone.0028668. [http://dx.doi.org/10.1371/journal.pone. 0028668]. [PMID: 22163051]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long L.E., Chesworth R., Huang X.F., Wong A., Spiro A., McGregor I.S., Arnold J.C., Karl T. Distinct neurobehavioural effects of cannabidiol in transmembrane domain neuregulin 1 mutant mice. PLoS One. 2012;7(4):e34129. doi: 10.1371/journal.pone.0034129. [http://dx.doi.org/10. 1371/journal.pone.0034129]. [PMID: 22509273]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valvassori S.S., Bavaresco D.V., Scaini G., Varela R.B., Streck E.L., Chagas M.H., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Acute and chronic administration of cannabidiol increases mitochondrial complex and creatine kinase activity in the rat brain. Rev. Bras. Psiquiatr. 2013;35(4):380–386. doi: 10.1590/1516-4446-2012-0886. [http://dx. doi.org/10.1590/1516-4446-2012-0886]. [PMID: 24402213]. [DOI] [PubMed] [Google Scholar]
- 60.Hsiao Y.T., Yi P.L., Li C.L., Chang F.C. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. Neuropharmacology. 2012;62(1):373–384. doi: 10.1016/j.neuropharm.2011.08.013. [http://dx.doi.org/10.1016/ j.neuropharm.2011.08.013]. [PMID: 21867717]. [DOI] [PubMed] [Google Scholar]
- 61.Lemos J.I., Resstel L.B., Guimarães F.S. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav. Brain Res. 2010;207(1):105–111. doi: 10.1016/j.bbr.2009.09.045. [http://dx.doi.org/10.1016/j.bbr.2009.09.045]. [PMID: 19800921]. [DOI] [PubMed] [Google Scholar]
- 62.Guimarães V.M., Zuardi A.W., Del Bel E.A., Guimarães F.S. Cannabidiol increases Fos expression in the nucleus accumbens but not in the dorsal striatum. Life Sci. 2004;75(5):633–638. doi: 10.1016/j.lfs.2004.01.015. [http:// dx.doi.org/10.1016/j.lfs.2004.01.015]. [PMID: 15158372]. [DOI] [PubMed] [Google Scholar]
- 63.Murillo-Rodríguez E., Millán-Aldaco D., Palomero-Rivero M., Mechoulam R., Drucker-Colín R. Cannabidiol, a constituent of Cannabis sativa, modulates sleep in rats. FEBS Lett. 2006;580(18):4337–4345. doi: 10.1016/j.febslet.2006.04.102. [http://dx.doi.org/10.1016/j.febslet.2006.04. 102]. [PMID: 16844117]. [DOI] [PubMed] [Google Scholar]
- 64.Sagredo O., Ramos J.A., Decio A., Mechoulam R., Fernández-Ruiz J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur. J. Neurosci. 2007;26(4):843–851. doi: 10.1111/j.1460-9568.2007.05717.x. [http://dx. doi.org/10.1111/j.1460-9568.2007.05717.x]. [PMID: 17672854]. [DOI] [PubMed] [Google Scholar]
- 65.ElBatsh M.M., Assareh N., Marsden C.A., Kendall D.A. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology (Berl.) 2012;221(2):239–247. doi: 10.1007/s00213-011-2566-z. [http:// dx.doi.org/10.1007/s00213-011-2566-z]. [PMID: 22083592]. [DOI] [PubMed] [Google Scholar]
- 66.Do Monte F.H., Souza R.R., Bitencourt R.M., Kroon J.A., Takahashi R.N. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav. Brain Res. 2013;250:23–27. doi: 10.1016/j.bbr.2013.04.045. [http://dx.doi.org/10.1016/j.bbr.2013.04.045]. [PMID: 23643693]. [DOI] [PubMed] [Google Scholar]
- 67.Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., De Filippis D., Cipriano M., Carratù M.R., Iuvone T., Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6(12):e28668. doi: 10.1371/journal.pone.0028668. [http://dx.doi.org/10.1371/journal.pone. 0028668]. [PMID: 22163051]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mijangos-Moreno S., Poot-Aké A., Arankowsky-Sandoval G., Murillo-Rodríguez E. Intrahypothalamic injection of cannabidiol increases the extracellular levels of adenosine in nucleus accumbens in rats. Neurosci. Res. 2014;84:60–63. doi: 10.1016/j.neures.2014.04.006. [http://dx. doi.org/10.1016/j.neures.2014.04.006]. [PMID: 24800644]. [DOI] [PubMed] [Google Scholar]
- 69.Maione S., Piscitelli F., Gatta L., Vita D., De Petrocellis L., Palazzo E., de Novellis V., Di Marzo V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br. J. Pharmacol. 2011;162(3):584–596. doi: 10.1111/j.1476-5381.2010.01063.x. [http://dx.doi.org/10.1111/ j.1476-5381.2010.01063.x]. [PMID: 20942863]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murillo-Rodríguez E., Millán-Aldaco D., Palomero-Rivero M., Mechoulam R., Drucker-Colín R. The nonpsychoactive Cannabis constituent cannabidiol is a wake-inducing agent. Behav. Neurosci. 2008;122(6):1378–1382. doi: 10.1037/a0013278. [http://dx.doi.org/10.1037/a0013278]. [PMID: 19045957]. [DOI] [PubMed] [Google Scholar]
- 71.Katsidoni V., Anagnostou I., Panagis G. Cannabidiol inhibits the reward-facilitating effect of morphine: involvement of 5-HT1A receptors in the dorsal raphe nucleus. Addict. Biol. 2013;18(2):286–296. doi: 10.1111/j.1369-1600.2012.00483.x. [http://dx.doi.org/10.1111/j.1369-1600.2012.00483.x]. [PMID: 22862835]. [DOI] [PubMed] [Google Scholar]


