Abstract
Background
Current guidelines recommend frequent surveillance colonoscopies after polyp removal, but there is a striking lack of evidence supporting the recommendations. This report outlines the rationale and design of two randomized trials, and one observational study investigating the optimal surveillance strategy following adenoma and serrated polyp removal.
Study design and endpoints
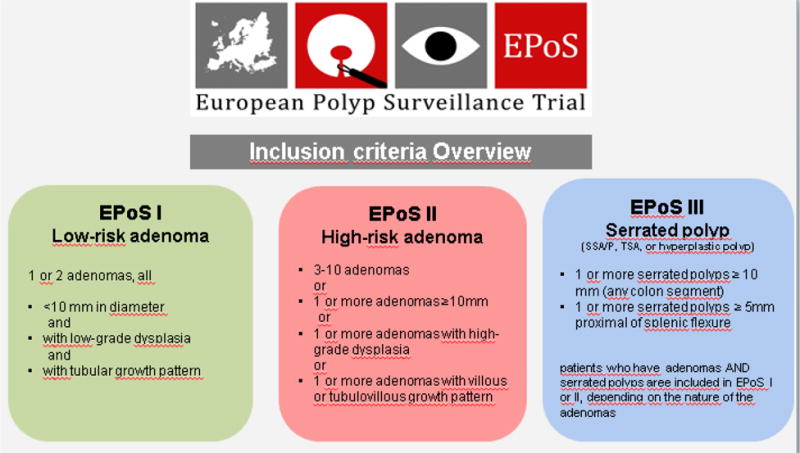
EPoS study I randomizes 13,766 patients with low-risk adenomas (1–2 tubular adenomas size <10mm with low-grade dysplasia) to surveillance after 5 and 10 years, or 10 years only. EPoS study II randomizes 13,704 patients with high-risk adenomas (3–10 adenomas; or adenoma ≥10mm in diameter, or adenoma with high-grade dysplasia or >25% villous features to surveillance after 3, 5 and 10 years, or 5 and 10 years only. EPoS study III offers surveillance after 5 and 10 years to patients with serrated polyps ≥10mm in diameter at any colorectal location, or serrated polyps ≥5mm in diameter proximal to the splenic flexure. All polyps are removed before patients enter the trials. The primary endpoint is colorectal cancer (CRC) incidence after 10 years. We assume a CRC risk of 1% for patients in EPoS I, and 2% for patients in EPoS II. Using a non-inferiority hypothesis with an equivalence interval of 0.5% for EPoS I and 0.7% for EPoS II, the trials are 90% powered to uncover differences larger than the equivalence intervals. For EPoS III, no power analyses have been performed.
Conclusions
The present trial aims to develop evidence-based strategies for polyp surveillance, thereby maximizing effectiveness and minimizing resources. Clinicaltrials.gov ID: NCT02319928
Introduction
Colorectal cancer (CRC) is a major global disease burden with more than 1.2 million people diagnosed each year and a high mortality rate (1). Most colorectal cancers derive from benign polyps through a series of genetic and epigenetic changes over a period of 10 to 15 years (2). Until recently, it was believed that only adenomas had malignant potential, but increasing evidence suggests that 15 to 30% of CRC cases develop from serrated polyps (3). The prevalence of adenomas and serrated polyps is high in the general population, as compared with the lifetime risk of CRC; 50% of healthy, asymptomatic 60 year olds have polyps, whilst the CRC lifetime risk is approximately 5% in Western countries (4). Thus, only a small fraction of all adenomas and serrated polyps progress to cancer.
To tackle the public health burden of CRC, most Western countries have introduced, or are considering introducing population-based screening programs for CRC. The primary aim of such programs is to reduce CRC incidence and mortality. The predominant screening tools for CRC are fecal occult blood testing (FOBT), fecal immunochemical testing (FIT), flexible sigmoidoscopy and colonoscopy (2). Because colonoscopy has the highest sensitivity, it is the gold standard for detection of polyps and CRC, and the examination of choice after a positive screening test other than colonoscopy, as well as for surveillance of individuals considered at high risk for CRC due to previous adenomas or cancer, or a family history of CRC.
An efficient screening intervention requires optimal surveillance intervals (5). However, today, there is a lack of scientific knowledge regarding the exact risk of patients with adenomas to develop CRC during follow up. Therefore, an evidence-based risk stratification algorithm cannot be established. Although some studies suggest a protective effect of colonoscopy among patients with adenomas, no studies have indeed convincingly demonstrated that post-polypectomy surveillance reduces CRC incidence or mortality (6,7). Recently, a large, nation-wide study in an environment with limited surveillance showed no excess risk of CRC after removal of low-risk adenomas, but a small excess risk after removal of high-risk adenomas (8).
Current guidelines recommend frequent surveillance colonoscopies for patients after polyp removal (Table 1) (9–11). However, due to the striking lack of large-scale clinical trials, there is uncertainty regarding the effectiveness and thus cost-effectiveness of these recommendations, which are largely based on expert opinion and low quality data. Guidelines have been developed by consensus groups, and are often influenced by a safety-first approach. This may result in too many colonoscopies for too many people, in line with the recent recognition of “Too-Much Medicine” (12). Guideline recommendations vary, and in fact all guidelines emphasize the need for large-scale clinical trials to close the knowledge gap in post-polypectomy surveillance (9–11).
Table 1.
Current guideline recommendations for polyp surveillance
| Low-risk adenoma patients | High-risk adenoma patients | Serrated polyp patients | |
|---|---|---|---|
| European Union Guidelines (9) | 10 years/none | 1–3 years | No recommendation |
| European Society for Gastrointestinal Endoscopy (11) | 10 years | 3 years | 3 or 10 years |
| United States Multi-Society Task Force (10) | 5 years | 3 years | 3–5 years |
Colonoscopy is a resource-demanding, invasive procedure and requires highly trained and skilled colonoscopists and assistants, and well-equipped centres. Patients may consider colonoscopy burdensome. Also, there is a small, but not negligible, risk of complications (mainly bleeding and perforation). Thus, colonoscopy should be reserved for individuals who are likely to benefit from it, and unnecessary procedures should be avoided. However, due to the increasing use of screening for CRC, more and more people are diagnosed with adenomas and referred for surveillance programmes. Therefore, the number of patients referred for polyp surveillance after colonoscopy is rapidly increasing in developed countries.
Surveillance colonoscopy is one of the main indications for colonoscopy, accounting for more than 20% of all colonoscopies performed in patients older than 55 years in the USA (13). In many countries, surveillance colonoscopies are placing a huge demand upon capacity for colonoscopy, utilizing large amounts of resources without evidence of benefit. Too much surveillance may jeopardize the effectiveness and cost-effectiveness of CRC screening programs. The gastroenterological scientific community should generate new and robust evidence for the utility of surveillance after polyp resection, and appropriate surveillance intervals (14).
This report outlines the rationale, design and methodology of a large-scale randomized controlled trial investigating the optimal surveillance interval after removal of low-risk and high-risk adenomas and also to elucidate the natural history of serrated polyps. The hypothesis is that current surveillance guidelines recommend colonoscopy too often. The primary study endpoint is CRC incidence after 10 years of follow-up.
Methods
Current evidence
On the basis of adenomas characteristics, patients will be stratified into different risk groups for subsequent development of CRC. Usually, patients are classified into low risk and high risk – although some guidelines define an intermediate risk group – based on polyp size and number, histologic type (villous or tubular growth pattern), and grade of dysplasia (low-grade or high-grade) (9–11). The low-risk group usually includes patients with 1–2 tubular adenomas smaller than 10 mm in diameter without high-grade dysplasia; and the high-risk group includes patients with 3 or more adenomas; or an adenoma larger than 9 mm in diameter, or an adenoma with high-grade dysplasia or > 25% villous growth pattern (so-called “advanced adenoma”).
Only two high-quality randomized clinical trials have compared different surveillance colonoscopy intervals (15,16). In the US study (15), after baseline colonoscopy with excision of adenomas, follow-up colonoscopy at 1 and 3 years was compared with colonoscopy at 3 years. There were only 3 outcome events (CRC) in the trial, and no differences in the rate of advanced adenomas were observed between the two groups. A surveillance interval of at least 3 years was recommended. These results are the main basis for current recommendation of at least 3 years intervals after removal of high-risk adenomas. A study from Denmark randomized individuals into multiple comparison groups by adenoma characteristics. The study was, however, too small to uncover clinically significant differences between the groups (16).
Trial Design
The EPoS trials constitute two parallel-group randomized controlled trials: EPoS I for patients with low-risk adenomas; EPoS II for patients with high-risk adenomas. EPoS III is an observational study for patients with serrated polyps.
Study endpoints
The primary endpoint in EPoS I, II and III is CRC incidence over 10 years. CRC incidence will be compared in the different arms in EPoS I and II, as well as across EPoS I and II, and compared to EPoS III.
The following secondary endpoints will also be compared in the different arms in EPoS I and II, across EPoS I and II, and compared to EPoS III
Cost-effectiveness
Yield of adenomas and serrated polyps (including subtypes) during follow-up
Major adverse events (perforations, bleedings) in surveillance colonoscopies
Subgroups are defined by variables which may be associated with the risk of CRC and adenoma yield during follow-up. These include patient age and sex, indication for colonoscopy (colonoscopy screening; colonoscopy after a positive CRC screening test (FIT, FOBT, sigmoidoscopy)); clinical symptoms or other indications) and the following polyp characteristics: polyp type (adenoma, serrated polyp), polyp size, adenoma growth pattern (tubular, tubulovillous or villous), grade of dysplasia (high grade versus low); multiplicity and location (proximal versus distal colon), and serrated polyp subtype. New molecular markers which may develop during the course of the study will be considered.
Joint Design Settings
Individuals between 40 and 74 years with no history of CRC or adenomas who have undergone colonoscopy with removal of one or more adenoma or serrated polyps at one of the study centres are eligible for recruitment. The indication for colonoscopy includes screening as well as clinical symptoms or other indications. At the baseline colonoscopy, the cecum must be intubated, adequate colonic cleansing achieved (Boston Bowel Preparation Score equal to or higher than 2 points in all colonic segments), and all polyps completely removed (judged by the endoscopist). Patients can be included within 6 months following baseline colonoscopy. For patients who require multiple procedures for complete polyp removal or other reasons, the last procedure counts as the date of baseline colonoscopy. All polyps removed (not biopsied) at each procedure will be recorded and added up to assess eligibility for the EPoS trials. The only exception is diminutive rectal polyps (5 mm or smaller); they do not need to be removed.
Surveillance colonoscopies
At surveillance colonoscopy, all detected polyps (except small diminutive rectal polyps, see above) will be registered and described, removed whenever possible and subjected to histopathology. All required data from endoscopy reports, quality-data used for quality monitoring and histopathologic results are registered in the central study database. Local pathology units serving the participating centres perform histopathological analysis and use WHO guidelines for classification and grading of polyps (17). Serrated polyps will be classified according to recent guidelines (17,18). Polyp specimen slides will be stored at the sites to facilitate re-assessment to accommodate for changing classification systems in the future.
Surveillance colonoscopies will be scheduled according to the time intervals defined in the protocol (see below). Surveillance colonoscopies will be performed within a 6-month time window from the protocol-defined due date. Unscheduled colonoscopies performed outside the 6 month window from (either before or after) the protocol-defined date will be recorded as a protocol deviation (results and reasons for deviation are recorded in the trial database). The trial centres will invite patients for scheduled surveillance colonoscopies. The database management team provides centres with lists of patients due for protocol-based surveillance colonoscopy.
Exclusion criteria
The following exclusion criteria apply to all EPoS trials
Lack of consent
Personal history of CRC or adenomas; personal history of serrated polyps ≥ 10 mm in diameter at any colorectal location or ≥ 5 mm if located proximal to the splenic flexure
More than 10 adenomas
Incomplete colonoscopy
Incomplete endoscopic excision of polyps
Non-retrieval of any polyp (for EPoS I); non-retrieval of any polyp > 9 mm in diameter for patients already eligible for EPoS II.
Genetic Hereditary cancer syndrome (familial adenomatous polyposis (FAP), attenuated FAP, Mut-YH associated polyposis; Lynch or Lynch-like syndrome), or serrated polyposis syndrome
Inflammatory bowel disease
History of surgical colon resection for any reason
Severe co-morbidity with reduced life expectancy (NYHA 3-4)
On-going cytotoxic treatment or radiotherapy for malignant disease
Long-lasting attention and nursing services (somatic or psychosocial, mental retardation)
Specific Design and Settings
EPoS I
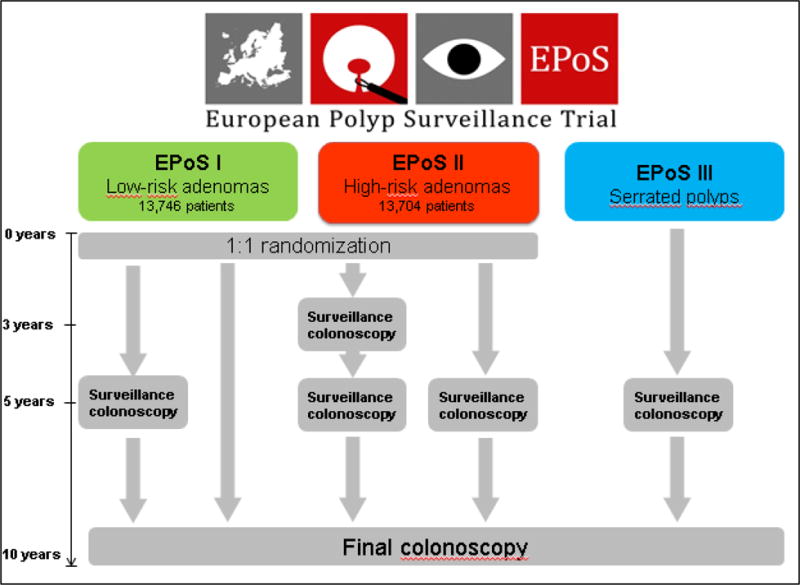
Eligible individuals are persons with low-risk adenomas removed at baseline colonoscopy (1–2 tubular adenomas size <10 mm with low-grade dysplasia). Synchronous serrated polyps may have been removed as well. Individuals will be randomized in a 1:1 ratio into one of two intervention groups:
Group 1: Surveillance colonoscopy at 5 and 10 years after baseline colonoscopy.
Group 2: Surveillance colonoscopy at 10 years after baseline colonoscopy.
The primary and secondary endpoints will be assessed at the 10 year surveillance colonoscopy (Figure 3).
Figure 3.
EPoS trial flowchart for initial surveillance interval
EPoS II
Eligible individuals are persons with high-risk adenomas removed at baseline colonoscopy (three to ten adenomas; or one or more adenoma with size ≥10 mm in diameter, or one or more adenoma with high-grade dysplasia or > 25% villous growth pattern). Synchronous serrated polyps may have been removed as well. Individuals will be randomized in a 1:1 ratio into one of two intervention groups:
Group 1: Surveillance colonoscopy 3 and 5 years after baseline colonoscopy
Group 2: Surveillance colonoscopy 5 years after baseline colonoscopy
Individuals in both groups 1 and group 2 will be subjected to a final surveillance after 10 years (Figure 3). The primary and secondary endpoints will be assessed at the 10 year surveillance colonoscopy. An interim analysis will be performed after the 5-year colonoscopy.
In both EPoS I and EPoS II, surveillance intervals after the first surveillance colonoscopy will take into account findings at the first surveillance colonoscopy. Surveillance intervals after first surveillance colonoscopy are shown in Figure 4, panels A and B.
Figure 4.
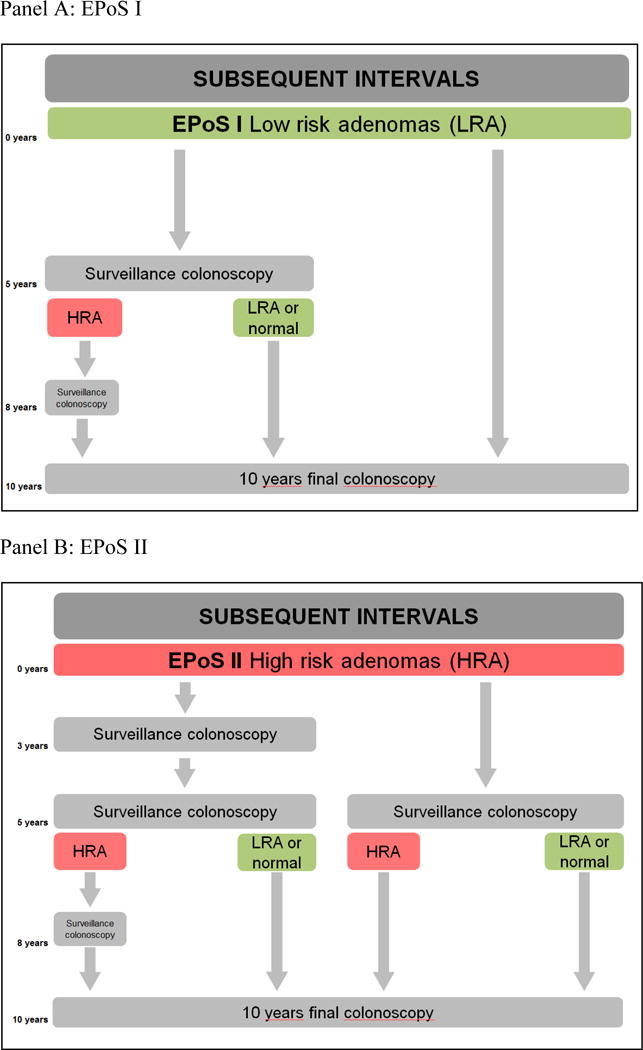
EPoS trial flowchart of subsequent surveillance intervals
EPoS III
Individuals are eligible if they have one or more serrated polyps (including hyperplastic polyp, sessile serrated adenomas/polyps (without and with dysplasia), and traditional serrated adenomas) ≥ 10 mm in diameter at any colorectal location, or one or more serrated polyp ≥ 5 mm if located proximal to the splenic flexure removed at baseline colonoscopy. Patients with both serrated polyps and adenomas are not eligible for EPoS III but can be included in EPoS I or II according to the characteristics of their adenomas. Colonoscopy will be repeated at 5 and 10 years in all individuals. The primary and secondary endpoints will be assessed at the 10 year surveillance colonoscopy. Findings at surveillance will be compared with those obtained in the EPoS I and II trials.
Randomization and database management
Eligible individuals will be invited to participate in the study and provide written informed consent prior to randomisation. A central randomization and data management center has been established for the trial. Randomization into the relevant trial is performed using a central electronic web-based randomisation system provided by Frontier Science and Technology Research Foundation Inc. using a stratified permuted block algorithm (19). Individuals included in the randomized trials (EPoS I and II) are randomly assigned to a corresponding treatment arm in a 1:1 ratio, depending on their polyp characteristics, stratified by country, age (age blocks 40–55; 56–65; and 66–74 years, respectively), and sex. Trial centres log into the electronic randomization and data entry system through a dedicated, secured virtual private network (VPN) system provided by the data management team (Frontier Science). The data management office team (Frontier Science Scotland) is responsible for implementing quality control systems for the data collected. This includes checks on data values at the time of data entry (or, if data is transferred in batches, at the time that the data is uploaded into the central database). More complex central checks will also be conducted, and any relevant discrepancy queries are sent to the participating centres.
Ethics and Oversight
The study is approved by the ethical committees at the participating centres. An independent Data Safety and Monitoring Board (DSMB) will review endpoint data. The members of the DSMB are appointed by the Study Investigators and must not otherwise be involved in the study. The DSMB will always have at least one member with biostatistical experience. The activities of the DSMB will be coordinated by the data management office. The study is registered at clinicaltrials.gov (NCT02319928)
Statistical analysis plan
Participants will be followed for the primary endpoint (incidence of CRC), until death, diagnosis of CRC, or end of follow-up, whichever happens first. The primary analysis will estimate the difference in 10-year risk of CRC between the study groups under the intention-to-treat (ITT) principle. We will conduct a one-tailed test of equivalence and will estimate a 95% confidence interval. For each endpoint, we will also estimate Kaplan-Meier survival curves and the average hazard ratio by fitting a Cox model. If the distribution of any baseline characteristics is found to be imbalanced between the arms, we will conduct a sensitivity analysis in which they are included as covariates in the model.
We will also perform adherence-adjusted analyses to estimate the causal effect that would have been observed if all individuals in the study groups had adhered to the study protocol, i.e., the per-protocol effect (20,21). We will use two analytic approaches to estimate the per-protocol effect: instrumental variable estimation and inverse probability weighting. For comparability, we will translate the estimates from both approaches into a common metric: adjusted survival curves, where survival means free of CRC. To implement instrumental variable estimation, we will use the indicator for treatment arm as the instrument (22). To implement inverse probability weighted estimation, we will estimate the weights as a function of variables that jointly predict adherence and the endpoint (23,24). These variables include age, sex and baseline findings. Other variables, such as physical activity, family history of colorectal cancer, smoking status, use of aspirin, NSAIDs, and hormone replacement therapy will be included if available from public registries at the study sites.
Power calculations
In the usual-care control groups, we assume a 1% risk of CRC at 10 years of follow-up for patients with low risk adenomas at baseline, and 2% for patients with high-risk adenomas at baseline. These numbers are based on data from cancer registries (13), and in accordance with CRC risks observed in the flexible sigmoidoscopy screening trials after comparable follow-up (22, 25–27). The study has 90% power to detect an absolute difference using a non-inferiority hypothesis with an equivalence interval of 0.5% for patients with low risk adenomas and 0.7% for patients with high-risk adenomas, with a one-sided alpha of 0.05. To achieve this, we need to include a total of 13,766 individuals in the EPoS I and 13,704 in the EPoS II trial. Table 2 outlines sample size scenarios for 80% and 90% power for EPoS I and II, respectively. For patients with serrated polyps, there are no reliable surveillance data available in the literature. Thus, EPoS III is a one-arm observational study, with no power analyses performed.
Table 2.
EPoS power*
| 10 year CRC incidence (proportion) | |||||
|---|---|---|---|---|---|
| Power | P1 proportion | P2 proportion | Group size | Total sample size | |
| EPoSI | 80% | 0.01 | 0.015 | 4897 | 9794 |
| EPoSI | 90% | 0.01 | 0.015 | 6783 | 13566 |
| EPoS II | 80% | 0.02 | 0.027 | 4947 | 9894 |
| EPoS II | 90% | 0.02 | 0.027 | 6852 | 13704 |
P1 is the control group (shorter surveillance intervals), P2 is the intervention group (longer surveillance intervals).
Sample size needed to exclude a difference between the control group and the intervention group of 0.5% or more for EPoS I and 0.7% or more for EPoS II
Considerations and justification
Removal of precursor lesions is assumed to prevent development of invasive cancer. Therefore, it is implausible that less intense colonoscopic surveillance following adenoma removal would entail a lower cumulative incidence of CRC than more frequent surveillance. Such an outcome would, however, arise if the preventive effect of more frequent surveillance (with removal of incident adenomas) is outweighed by overdiagnosis of invasive cancer. A similar, or even identical, cumulative incidence of colorectal cancer following more or less frequent surveillance is theoretically possible, notably if the average progression time from a detectable precursor lesion (adenoma) to invasive cancer are longer than the different surveillance intervals applied in the EPoS trials. However, trials designed to document equivalence would require unrealistically large sample sizes and are therefore unlikely to ever be undertaken.
We considered as the most plausible outcome of the EPoS trials a slightly, perhaps only marginally, higher cumulative incidence with less frequent as compared to more frequent surveillance. As a corollary, both EPoS I and EPoS II are designed as non-inferiority randomized trials.
There are several reasons why trials designed to test this hypothesis are considered ethically justifiable. Firstly, colonoscopy carries risks, chiefly caused by bleeding or perforation of the colon. Although such complications are rare, notably in high quality settings, they are severe and sometimes life threatening. Hence, complications might substantially counteract the possible survival benefit of more intense surveillance which, in absolute terms (number of lives saved per colonoscopy), is small anyway. Secondly, screening to detect precursor lesions and invasive cancer may entail overdiagnosis and overtreatment of lesions that would never have progressed to a lethal stage during the individual’s remaining lifetime. The probability of overdiagnosis is likely to be positively correlated with the number of surveillance colonoscopies. Although the extent of overdiagnosis is poorly quantified for colonoscopic screening, it is substantial for other, more established, screening tools such as Pap-smear for cervical cancer, mammography for breast cancer and prostate-specific antigen (PSA) for prostate cancer. Thirdly, colonoscopy is costly and resource demanding and places a heavy burden upon patients. As a corollary, resources saved by less frequent surveillance of adenoma patients might convey greater public health benefit if used for other purposes in the health care system. Fourthly, the level of inferiority in absolute difference considered acceptable should not be the same in EPoS I as in EPoS II. Instead, a slightly higher absolute difference (inferiority) in cumulative incidence of colorectal cancer would be justifiable in EPoS II. This argument is based on the fact that in EPoS II, subjects randomized to less intense surveillance are spared one or probably two colonoscopies during a 5-year period compared with currently applied guidelines. In contrast, subjects enrolled in the EPoS I trial would be spared only one colonoscopy over a 10-year period.
Based on these arguments and acknowledging that any defined level of inferiority can be considered arbitrary, we have accepted an equivalence level of 0.5% in absolute colorectal cancer incidence in EPoS I, and of 0.7% in EPoS II. This implies that the null hypothesis of non-inferiority would be rejected only if the upper limit of the 95% confidence interval for the absolute difference in cumulative incidence of colorectal cancer after ten years of follow-up exceeds 0.5% in EPoS I and 0.7% in EPoS II.
Discussion
The EPoS trials are the first large-scale randomized trials to address surveillance intervals following polypectomy. While national and international guidelines for polyp surveillance in patients who have undergone removal of adenomas or serrated polyps are in place, they are based upon consensus opinion as opposed to high-quality evidence. Although guidance for clinicians is valuable also in areas where high-quality evidence is lacking, it is nonetheless vital to perform large clinical trials to fill the knowledge gap and thus subsequently revise and update guidelines using the evidence developed.
Trial endpoints
The primary goal of surveillance after polypectomy is to reduce future CRC risk in patients with previously removed adenomas or serrated polyps. Thus, CRC incidence is the obvious endpoint for a trial of comparative effectiveness of different surveillance strategies. CRC mortality is also a very important outcome and relevant endpoint in CRC surveillance. However, as the goal is prevention of the disease rather than reduction of mortality by early detection, we believe that incidence is the better of these two important endpoints. Using incidence, however, requires a design which permits comparison of groups without the risk of overdiagnosis and lead-time bias. Overdiagnosis of CRC can occur because CRCs grow slowly and do not harm the patients in their remaining lifetime, or (if CRCs grow rapidly) the patient is older and does not live long enough to get harmed. The EPoS trial design permits such a comparison because all patients are offered surveillance at the end of the trial after 10 years (see below).
Based on data from cancer registries, wWe expect that in the standard care arm, 1 in 100 patients in EPoS I (1%), and 2 in 100 patients in EPoS II (2%) will develop CRC during the 10-year follow-up period. This is in accordance with the absolute risks of CRC observed in the flexible sigmoidoscopy screening trials after a comparable follow-up period (22, 25–27) as well as in pooled analyses (28). Due to the low absolute risk for CRC, studies evaluating screening effects often focus on surrogate endpoints such as adenoma or advanced adenoma yield, or a combination of advanced adenomas and CRC. However, due to the great uncertainties regarding transition rates and transition and times from adenomas to cancer, studies using surrogate endpoints are problematic (29,30). Because the optimal frequency of surveillance colonoscopies after polyp removal concerns hundreds of thousands of individuals each year, a high quality trial using a strong endpoint such as CRC incidence is urgently needed.
In addition to the challenge of large numbers, a study of incidence may be affected by lead time bias if disease is detected earlier among asymptomatic individuals (e.g. through screening or surveillance colonoscopy) as compared to detection in patients who become symptomatic (30). This bias also occurs when different surveillance strategies entail the last follow-up colonoscopy at different time intervals for the measurement of CRC incidence. To eliminate such bias, all patients enrolled to the EPoS trials undergo their last surveillance colonoscopy 10 years after inclusion.
Non-inferiority margins
The underlying hypothesis in the EPoS non-inferiority trials is that less frequent surveillance will not result in higher CRC risk with the threshold of non-inferiority defined by the non-inferiority margin. Given the estimated cumulative CRC risks following standard care, we have defined a threshold of 0.5% for EPoS I and 0.7% in Epos II. As in any randomized trial, defining margins for superiority or non-inferiority should be based on what can be expected from the new intervention, what appears clinically relevant to detect, and what numbers of enrolled patients are feasible in relationship to the number needed to reach statistical significance. After extensive discussion, we reached consensus that the margins defined are clinically relevant and scientifically and ethically sound and feasible to test.
Stage migration
Stage migration is a well-known phenomenon in oncology and can occur when more sensitive diagnostic tools are implemented (32). Previously undetectable metastases may be visualized, for example, with high-resolution MRI or Positron Emission Tomography (PET). Patients previously staged favourably may become categorized in higher, more advanced stages. Such stage migration does not reflect real changes in disease stage, but will create spurious temporal trends in stage specific survival even in the absence of real improvements (32).
As a consequence of increased diagnostic sensitivity, the detection rates of adenomas and serrated polyps have increased during recent years. Hence, a mechanism similar to stage migration may affect the CRC risk in contemporary groups of polyp patients that were rarely detected and removed 10 or 15 years ago. However, current risk estimates which categorize patients according to number of polyps, are derived from studies for which patients were recruited 10, 15 or even 20 years ago. Hence, risk of CRC following adenoma removal may be lower among contemporary patients. To retain adequate statistical power according to the EPoS estimates, we envision recruitment numbers higher than considered the minimum required according to the power calculation outlined above.
Comparison between EPoS trials
In addition to the main comparisons, we also plan to compare the CRC incidence rates between EPoS I, II and III. This is important because current classification of high-risk versus low-risk adenomas based on polyp size, number, and features of growth and dysplasia may not optimally distinguish between high and low risk lesions (33). Hence, EPoS may provide evidence allowing a classification that better predicts risk of CRC. Furthermore, the comparative risk for patients with serrated polyps as compared to adenoma patients is currently unclear. However, EPoS III will allow a comparison of CRC risk in different categories of polyps without lead time bias. Further, we will be able to compare the risk of CRC in patients with concomitant serrated polyps and adenomas who are enrolled in EPoS trials I and II, respectively, with other subgroups.
Conclusions
The EPoS trials are the first large-scale, long-term randomized trials to address the topic of optimal surveillance intervals after polyp removal. We hope that these trials will bring new knowledge to the field which will change clinical practice towards more evidence-based and cost-effective surveillance recommendations.
EPoS trial investigators
EPoS scientific board
Rodrigo Jover, Michael Bretthauer, Evelien Dekker, Øyvind Holme, Michal F. Kaminski, Magnus Løberg, Ann G. Zauber, Miguel A. Hernán, Iris Lansdorp-Vogelaar, Eleanor McFadden, Antoni Castells, Maria Pellisé, Jaroslaw Regula, Enrique Quintero, Carlo Senore, Mette Kalager, Mario Dinis-Ribeiro, Louise Emilsson, Geir Hoff and Hans-Olov Adami.
EPoS investigators
Joaquín Cubiella, Pedro Zapater, Luis Bujanda, Isabel Portillo, Montserrat Andreu, Angel Ferrández, Vicent Hernández, Adolfo Suárez, Angeles Pizarro, Akiko Ono, Marta Ponce, Juan A. Casellas, María Chaparro, Paulina Wieszczy, Marek Bugajski, Wladek Januszewicz, Nereo Segnan, Cesare Hassan, Monique van Leerdam, Manon C. W. Spaander, Joep E.G. IJspeert, Gerrit Meijer, Ervin Toth, Andreas Pischel, Stefan Willmarsson, Thomas de Lange, Lene Larssen, Gunnar Qvigstad, Tom Christian Martinsen, Katrine Romstad, Marie Ek Olsen, Magne Henriksen, Christer Tønnesen, Kjetil Karlsen, Lars Aabakken, Jan Magnus Kvamme, Lars Mikal Aasen, Reidulf Stray-Pedersen, Volker Moritz, Ole Darre Næss, Colin Rees, Teresa Pinto Pais.
EPoS data safety and monitoring board
Ulrike Haug, Heiko Pohl, Thomas Rösch.
Figure 2.
EPoS trials inclusion criteria
References
- 1.GLOBOCAN Database. International Agency for Research on Cancer. World Health Organization; 2008. http://globocan.iarc.fr/ [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 3.Ijspeert JE, Vermeulen L, Meijer GA, Dekker E. Serrated neoplasia-role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol. 2015;12:401–9. doi: 10.1038/nrgastro.2015.73. [DOI] [PubMed] [Google Scholar]
- 4.Bretthauer M. Evidence for colorectal cancer screening. Best Practice & Research Clinical Gastroenterology. 2010;24:417–425. doi: 10.1016/j.bpg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Bretthauer M, Kalager M. Colonoscopy as a triage screening test. N Engl J Med. 2012;366:759–60. doi: 10.1056/NEJMe1114639. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Chang-Claude J, Rickert A, et al. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case control study. J Clin Oncol. 2012;30:2969–76. doi: 10.1200/JCO.2011.41.3377. [DOI] [PubMed] [Google Scholar]
- 7.Cottet V, Jooste V, Fournel I, et al. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61:1180–6. doi: 10.1136/gutjnl-2011-300295. [DOI] [PubMed] [Google Scholar]
- 8.Løberg M, Kalager M, Holme Ø, et al. Long-term risk of colorectal cancer death after adenoma removal. N Engl J Med. 2014;37:799–807. doi: 10.1056/NEJMoa1315870. [DOI] [PubMed] [Google Scholar]
- 9.Atkin WS, Valori R, Kuipers EJ, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition–Colonoscopic surveillance following adenoma removal. Endoscopy. 2012 Sep;44(Suppl 3):SE151–SE163. doi: 10.1055/s-0032-1309821. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 12.Moynihan R, Glasziou P, Woloshin S, Schwartz S, Santa J, Godlee F. Winding back the harms of too much medicine. BMJ. 2013;346 doi: 10.1136/bmj.f1271. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–83. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Jover R. Surveillance after colonic neoplasia: to die of success. Endoscopy. 2013;45:511–2. doi: 10.1055/s-0033-1344154. [DOI] [PubMed] [Google Scholar]
- 15.Winawer SJ, Zauber AG, O’Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 16.Kronborg O, Jørgensen OD, Fenger C, et al. Three randomized long-term surveillance trials in patients with sporadic colorectal adenomas. Scand J Gastroenterol. 2006;41:737–43. doi: 10.1080/00365520500442666. [DOI] [PubMed] [Google Scholar]
- 17.Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO classification of tumours of the digestive system. 4th IARC Lyon; 2010. [Google Scholar]
- 18.Rex DK, Ahnen DJ, Baron JA. Serrated Lesions of the Colorectum: Review and Recommendations From an Expert Panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelen M. The randomisation and stratification of patients to clinical trials. J Chron Dis. 1974;27:365–367. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 20.Hernán MA, Hernández-Díaz S, Robins JM. Randomized trials analyzed like observational studies. Annals Intern Medicine. 2013;159:560–562. doi: 10.7326/0003-4819-159-8-201310150-00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernán MA, Hernández-Díaz S. Beyond the intention to treat in comparative effectiveness research. Clinical Trials. 2012;9:48–55. doi: 10.1177/1740774511420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holme Ø, Løberg M, Kalager M, Bretthauer M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: A randomized clinical trial. JAMA. 2014;312:1–10. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toh S, Hernández-Díaz S, Logan R, Robins JM, Hernán MA. Estimating absolute risks in the presence of nonadherence: an application to a follow-up study with baseline randomization. Epidemiology. 2010;21:528–39. doi: 10.1097/EDE.0b013e3181df1b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toh S, Hernán MA. Causal inference from longitudinal studies with baseline randomization. Intern J Biostat. 2008;4:22. doi: 10.2202/1557-4679.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicenter randomized trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 26.Schoen RE, Pinsky PF, Weissfeld JL, et al. PLCO Project Team Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segnan N, Armaroli P, Bonelli L, et al. SCORE Working Group Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. J Natl Cancer Inst. 2011;103:1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 28.Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009:136832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin B. Potential Pitfalls in the Use of Surrogate Endpoints in Colorectal Adenoma Chemoprevention. J Natl Ca Inst. 2003;95:697–8. doi: 10.1093/jnci/95.10.697. [DOI] [PubMed] [Google Scholar]
- 30.Robertson DJ, Kaminski MF, Bretthauer M. Effectiveness, training and quality assurance of colonoscopy screening for colorectal cancer. Gut. 2015;64:982–90. doi: 10.1136/gutjnl-2014-308076. [DOI] [PubMed] [Google Scholar]
- 31.Bretthauer M, Kalager M, Adami HO. Do’s and don’ts in evaluation of endoscopic screening for gastrointestinal cancer. Endoscopy e-first. 2015 doi: 10.1055/s-0034-1393094. DOI http://dx.doi.org/10.1055/s-0034-1393094. [DOI] [PubMed]
- 32.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–8. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 33.van Heijningen E-MB, Lansdorp–vogelaar I, Kuipers EJ, et al. Features of Adenoma and Colonoscopy Associated With Recurrent Colorectal Neoplasia Based on a Large Community-Based Study. Gastroenterology. 2013;144:1410–1418. doi: 10.1053/j.gastro.2013.03.002. [DOI] [PubMed] [Google Scholar]


