Introduction
α1-acid glycoprotein (AGP), also known as orosomucoid, is a 41–43 kDa glycoprotein with 45% oligosaccharide component. The peptide moiety is a single chain of 183 (human) or 187 (rat) amino acids with two disulfide bridges (Ceciliani and Pocacqua, 2007). AGP, a positive acute phase protein (APP), is elevated 1–10 times during inflammation in humans and rats while the major plasma protein, albumin (a negative APP) is typically decreased. These changes in plasma proteins have led to drug binding characterization studies of AGP, which shows AGP preferentially binds basic central nervous system (CNS) drugs like imipramine and carbamazepine (Zhang et al., 2007; Zsila and Iwao, 2007). AGP has been reported to have anti-inflammatory, immunomodulatory and vascular protective roles suggesting a role as a biomarker of inflammatory diseases (Curry et al., 1989; Jorgensen et al., 1998; Pukhal’skii et al., 2000; Ceciliani et al., 2007). AGP acts on immune cells that are involved in the inflammatory process, such as decreasing thymocyte proliferation via macrophage derived IL-1 inhibitor (Bories et al., 1990), and modulating lymphocyte proliferation as well as regulate cytokine (IL-1, IL-2, IL-6, and TNF-α) production by leukocytes via mitogen activators (Shiyan and Bovin, 1997). Reducing reactive oxygen species production by neutrophils upon phorbol 12-myristate 13-acetate (PMA) stimulation (Stakauskas et al., 2005) and changing the shape of platelets via Rho/Rho-kinase signaling pathway (Gunnarsson et al., 2009) are also biological characteristics of AGP. In particular, AGP has been shown to protect against inflammation-induced tissue injury through the inhibition of neutrophil activation and reduced prostaglandin E2 generation (Matsumoto et al., 2007), as well as inhibition of TNF-α-induced apoptosis of hepatocytes (Van Molle et al., 1997).
As cytokines play important roles during inflammation, whether AGP can exert its biological functions on endothelial cells via regulation of cytokines is not well known. There is evidence for cross-talk between APPs and pro-inflammatory cytokines like IL-1, IL-6 and TNF-α, which are known to stimulate hepatocytes to increase the expression of AGP and other positive APPs during inflammation (Castell et al., 1989; Christian Pous, 1989; Baumann and Gauldie, 1990). Bories et al. showed that AGP can inhibit IL-1 activity by modulating an unknown macrophage derived factor (Bories et al., 1990). While AGP is primarily produced by hepatocytes, it is also produced by endothelial cells where it plays an important role in forming the glycocalyx (Curry et al., 1989; Sorensson et al., 1999). Some studies suggest that AGP is necessary to maintain capillary permeability, likely by increasing the polyanionic charge selectivity of the endothelial barrier as well as by interacting with components of the endothelial glycocalyx (Haraldsson and Rippe, 1987; Curry et al., 1989; Huxley et al., 1993).
Despite the evidence implicating inflammatory cytokines (e.g. TNF-α, IL-1β and IL-6) in disrupting the blood-brain barrier (BBB) (Mark and Miller, 1999; Paul et al., 2003; McColl et al., 2008), little is currently known about the response of brain microvascular endothelial cells to acute phase proteins such as AGP. The BBB is crucial in maintaining brain homeostasis and protecting the CNS from harmful solutes in the peripheral circulation. Tight junction (TJ) proteins and basement membrane proteins are two important components for maintaining the integrity of the BBB. TJ proteins are composed of transmembrane proteins (occludin and claudins) and intracellular zonula occludens (ZO-1, 2, 3) which bind cytoskeletal F-actin. Occludin is the major TJ integral membrane protein, which is highly expressed at brain endothelial cells where the structure and localization of occludin are critical in maintaining BBB functional integrity (i.e., low paracellular permeability) (Furuse et al., 1993; Lochhead et al., 2011). Among the 23 claudin family members, claudin-5 and claudin-2 are located at the BBB where they contribute to barrier “tightness” (Nitta et al., 2003). Tethered to occludin-based strands through ZO-1, junction adhesion molecules (JAM) concentrated at cell–cell adhesion sites facilitate TJ protein reassembly (Itoh et al., 2001). Inflammatory models have shown to disrupt both structural components (occludin and claudin-5) and function of the BBB (Mark and Miller, 1999; Ferrari et al., 2004; Brooks et al., 2005);
To date, the effects of AGP in regulating the expression of TJ proteins (ZO-1 and occludin) and changing the integrity of BBB have not been investigated. Whether AGP can stimulate brain endothelial cells to express anti- or pro-inflammatory cytokines remains largely unknown. As inflammatory cytokines have been shown to alter the BBB, we hypothesized that AGP changes BBB integrity by altering inflammatory cytokines and TJ protein expression. In this study, we used primary rat brain microvessel endothelial cells (RBMECs) to investigate the effects of AGP at the BBB.
Materials and methods
Isolating and Culturing of Primary Rat Brain Microvessel Endothelial Cells (RBMECs)
The RBMECs are a well characterized in vitro BBB model and this study has been approved by the Institutional Animal Care and Use Committee (IACUC), Texas Tech University Health Science Center, Amarillo, USA. Primary isolated RBMECs were collected from female Sprague-Dawley rats (200–250g) using a modified enzymatic and centrifugation steps as previously described (Abbott et al., 1992; Miller et al., 1997). RBMECs were seeded onto collagen coated, fibronectin-treated 75 cm2 tissue culture flasks 6-well culture plates or 12-well Transwell® plates (Corning Inc., NY). Culture medium for RBMECs consisted of: 45% minimum essential medium (MEM), 45% Ham’s F12 nutrient mix, 10 mM HEPES, 13 mM NaHCO3, 50 μg/ml gentamycin, 10% equine serum, 2.5 μg/ml amphotericin B, and 100 μg/ml heparin. 200 μg/ml endothelial cell growth supplement (Sigma; St. Louis, MO) was added to the medium for first 3 days. Cell cultures were grown in a humidified 37°C incubator w/5% CO2 and medium was replaced every other day until the monolayers reached confluency (about 11–14 days).
Alpha 1-acid Glycoprotein (AGP) Treatment of RBMECs
Confluent RBMECs monolayers were pre-treated with low serum (0.5%) medium for 6–24 hrs prior to AGP treatment. The acute phase protein, AGP from human plasma (Sigma; St. Louis, MO), was diluted into low serum (0.5%) medium prior to treatment of RBMECs. Confluent monolayers were treated with varying doses of AGP (50 μg/ml or 500 μg/ml) or culture medium alone (control) at various exposure times (1, 3, 6, 12, 24, 48, or 72 hrs) as indicated in the particular experiments; AGP treatment was added to apical side including the Transwell® plates (Corning; Lowell, MA).
Paracellular Permeability of AGP Treated RBMEC Monolayer
After treatment and prior to the permeability study, primary RBMEC monolayers were assessed by transendothelial electrical resistance (TEER) measurement via EVOM resistance meter (World Precision Instruments, Sarasota, FL, USA). Those monolayers with resistance values > 100Ω•cm2 were used to assess the permeability effects of AGP on RBMEC monolayers. After treatment, RBMEC monolayers were incubated with assay buffer composed of (in mM) 122 NaCl, 3 KCl, 1.4 CaCl2, 1.2 MgSO4, 25 NaHCO3, 10 HEPES, 10 glucose, and 0.5 K2HPO4 for 30 min at 37°C. Permeability across RBMEC monolayers was determined by adding 5 μM sodium fluorescein (NaFl, 376 MW, a paracellular transport marker) in the assay buffer to the apical side (upper compartment of Transwell). Samples (100 μl) were removed from the basolateral side (lower chamber of Transwell) at 0, 60, and 120 min and replaced with fresh assay buffer. The concentration of NaFl was measured by Synergy Mx microplate reader (BioTek, Winooski, VT) at excitation: 485 nm and emission: 528 nm. Concentration of NaFl applied to the apical side was determined by removing samples (50 μl) at time zero. Permeability coefficients (PC) for NaFl were expressed using the equation below as previously described (Mark and Miller, 1999); where V is volume in receiver chamber (1.5 cm3), SA is surface area of cell monolayer (1.12 cm2), Cd is the concentration of marker in the donor chamber at time 0, and Cr is the concentration of marker in the receiver at sample time T.
Western Blot Analysis of Tight Junction Protein Expression in RBMECs
Following treatment, the RBMECs were solubilized in Cellytic buffer (Sigma) with 1% protease inhibitor cocktail (Research Products International Corp.). Protein concentration of cell lysates was quantified using the bicinchoninic acid (BCA) protein assay. RBMECs lysate samples (8 μg total protein) were loaded onto a 4–12% tris-glycine gel (Invitrogen) and protein expression was determined via standard Western blot protocol as previously described (Mark and Davis, 2002). Antibodies used are occludin (1:10,000; Invitrogen), ZO-1 (1:10,000; Invitrogen), or β-actin (1:10,000; Sigma) for primary and anti-mouse or anti-rabbit antibody (1:50,000; Invitrogen) for secondary. The protein bands were developed and visualized using the enzyme chemiluminescence method (Femto ECL; Thermo Science) and developed on X-ray film. Protein expression was semi-quantified by using Image J software (courtesy of N.I.H.) and normalized by β-actin expression and presented as % of control.
Real-time PCR (quantitative PCR)
mRNA was isolated from RBMECs following AGP treatment using an RNeasy Mini Kit (Qiagen). Synthesis of cDNA was performed by reverse transcription of the isolated mRNA using the iScript cDNA synthesis kit (Bio-rad). qPCR was performed on cDNA (corresponding to 35–50 ng RNA per reaction) and amplified through 35 cycles using SYBR Green Master Mix (Applied Biosystems (ABI)) and an ABI 7300 real-time PCR instrument. Specific primer sets were designed using IDT DNA website or published literature (see Table 1) and synthesized by Invitrogen (Carlsbad, CA). GAPDH was amplified as an internal standard and target cDNA was normalized by GAPDH cDNA. The effect of the AGP-treatment was demonstrated as a change (ratio) in mRNA expression for the respective target genes as calculated using the Pfaffl method and the equation below, where controls were RBMECs receiving media alone, treated were AGP-treated RBMECs, and reference is GAPDH gene.
Table 1.
Primer sets for Real-time PCR
| Target | Forward (5′ to 3′) | Reverse (5′ to 3′) | Size (bp) | Reference |
|---|---|---|---|---|
| Cytokines | ||||
| IL-10 | GCCAAGCCTTGTCAGAAATGA | TTTCTGGGCCATGGTTCTCT | 75 | (Gayle et al., 2004); NM_012854 |
| TNF-α | TGGCCAATGGCCATGGATCTCAA | ATGAAGTGGCAAATCGGCTGAC | 147 | IDT; NM_012675.2 |
| IL-1β | TGTCCTGTGTGATGAAAGACGG | TTGGGTATTGTTTGGGATCCA | 69 | IDT; NM_031512.2 |
| IL-6 | AGCCACTGCCTTCCCTACTT | GCCATTGCACAACTCTTTTCTC | 154 | (Lin et al., 2009); NM_012589.1 |
| TJ Proteins | ||||
| Occludin | GTTTACTGGCAGAACTCGAC | CCAGCATCTGTCTAGGTTTTC | 247 | IDT; NM031329.2 |
| ZO-1 | GGCTGTCTCAACTCCTGTAA | CGCCAGCTACAAATATTCCG | 299 | IDT; NM_001106266.1 |
| Claudin-2 | TATGTTGGTGCCAGCATTGT | ACTCCACCCACTACAGCCAC | 266 | (Abuazza et al., 2006); NM_001106846.2 |
| Claudin-5 | CGCTTGTGGCACTCTTTGT | ACTCCCGGACTACGATGTTG | 168 | (Wongdee et al., 2010); NM_031701.2 |
| JAM-1 | ACCCCGTGACAGCCTTTGATA | TCAGCTCCACATCATCCATGT | 105 | IDT; NM_053796.1 |
| GAPDH | TGACTCTACCCACGGCAAGTTCAA | ACGACATACTCAGCACCAGCATCA | 141 | IDT; NM_017008.3 |
IDT: Integrated DNA Technologies.
AP-1 family transcription factor assay
The effect of AGP on the DNA binding activity of two major AP-1 subunits, namely, cFos and cJun, were determined using an ELISA-based assay kit (TransAM® kit, from Active Motif; Carlsbad, CA). In brief, 5 μg of nuclear extract from control or AGP (50 or 500 μg/ml) treated RBMECs for 1–72 hrs were incubated in separate wells of a 96-well plate possessing an immobilized AP-1 consensus sequence [TPA responsive element (TRE) oligonucleotide 5′-TGAGTCA-3′], inactive AP-1 molecules were removed with wash buffer, and the active TRE-bound AP-1 was identified using primary antibodies directed against the c-Fos or c-Jun subunits in conjunction with horseradish peroxidase-conjugated secondary antibodies and a colorimetric substrate quantified by spectrophotometry (Synergy Mx, Bio Tek) at 450 nm with a reference wavelength of 655 nm. Results are presented as fold of the absorbance values of control.
AGP Stability Throughout the Treatment
Culture supernatants were collected at different time points (0, 6, 12, 24, 48, or 72 hrs) from the same well of RBMECs monolayer treated with 50 μg/ml or 500 μg/ml human AGP, to determine stability of AGP in the treatment medium over 72 hrs. Proteins in supernatants were separated by electrophoresis on 10% acrylamide/bis gels and subsequently stained with fluorescent Pro-Q Emerald 300 dye (Molecular Probe) as described (Steinberg et al., 2001). This dye binds to periodate-oxidized carbohydrate groups, forming a green fluorescent signal on glycoproteins. Briefly, after protein fixation in 50% methanol, glycans of gel-separated proteins were oxidized to aldehydes by incubation in 1% periodic acid/3% acetic acid. After washing in 3% acetic acid, gels were incubated in Pro-Q Emerald 300 dye solution. Glycosylated proteins were visualized by 300 nm UV transillumination in Gel Doc 2000 gel documentation system (Bio-Rad). AGP levels were determined by using Image J software (courtesy of N.I.H.) and data are presented as fold of level at time zero (0 hr). This method has high sensitivity for AGP (linear range tested: 12.5–100 ng/lane) and it has been used for quantification of glycoproteins (Ogata et al., 2005).
Measurement of Cytokines Released from RBMECs
Cytokine levels in cell culture supernatant from AGP-treated RBMECs were measured using Quantikine® ELISA kits (R&D systems; Minneapolis, MN) for IL-1β and TNF-α, Platinum ELISA kits (eBioscience; San Diego, CA) for IL-6. Supernatant samples were centrifuged (5000 × g, 5 min) and kept frozen until performing the ELISA. Sensitivity for cytokines (IL-1β, TNF-α, and IL-6) are: 31.2 pg/ml, 6.25 pg/ml, and 15.5 pg/ml; respectively.
Briefly, the specific standards and experimental samples were incubated with cytokine-antibody coated ELISA plate. The HRP-conjugated antibody was then added and incubated to form the antibody-antigen-antibody complex. After washing, the substrate was added and incubated prior to stopping the enzymatic reaction. Absorbance was measured by a microplate reader (BioTek Synergy Mx) at 450 nm with 540 nm correction. Cytokine concentrations were calculated based on the respective standard curve.
Statistical Analysis
SigmaStat™ statistical software program was used for statistical analyses. Comparisons of data were made using one- or two-way ANOVA followed by Student Newman Keuls post-hoc analysis. p-values < 0.05 were considered statistically significant.
Results
It should be noted that throughout these studies, two concentrations were used, 50 μg/ml that is high normal serum level of AGP and 500 μg/ml, which represents the 10-fold increase seen during inflammation.
AGP Concentration Maintained Throughout 72 hr
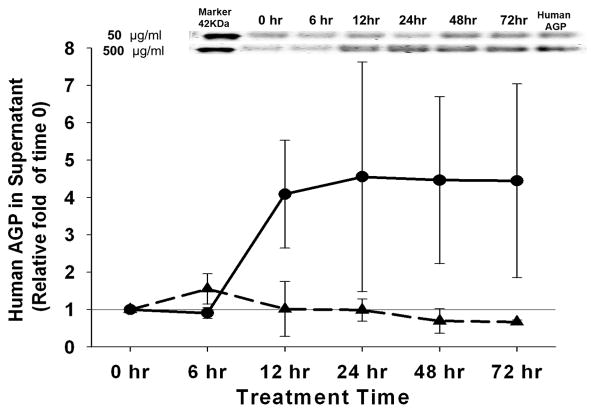
Stability of the AGP in culture conditions was determined by collecting culture supernatants at different time points (0, 6, 12, 24, 48, or 72 hrs) from the same well of RBMECs monolayer treated with 50 μg/ml or 500 μg/ml human AGP. AGP levels were detected by Pro-Q Emerald 300 glycoprotein stain. No significant decrease of AGP level was detected in the treatment supernatant at either concentration throughout the 72 hr treatment time (Figure 1).
Figure 1. Human AGP Concentration in the Supernatant of RBMECs Treatment.
Emerald 300 glycoprotein staining was performed on supernatant collected from RBMECs treated with α-1-acid glycoprotein (AGP) for 6, 12, 24, 48 and 72 hrs. AGP levels in the supernatant of RBMECs treated with 50 μg/ml (long dash line) and 500 μg/ml (solid line) are presented as fold of the human AGP band density at time zero (0 hr). Representative blots are shown above the plot; n=4.
Effect of AGP on Paracellular Permeability of RBMEC Monolayer
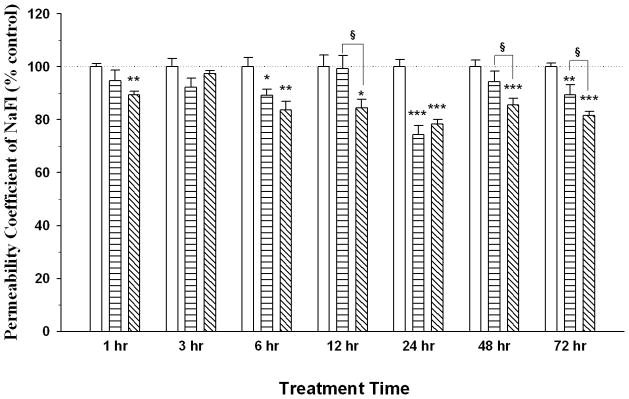
Following AGP treatment on RBMEC monolayers, AGP showed a dose-dependent effect in paracellular permeability of RBMECs. As early as 1 hr, high concentration AGP (500 μg/ml) showed an immediate decrease in permeability which returned to control level by 3 hrs before a second decrease occurred at 6 hrs and lasted until 72 hrs. In contrast, low concentration AGP (50 μg/ml) showed a significant decrease in permeability at 6, 24 and 72 hrs. Interestingly, both concentrations showed the most significant decrease (> 20%) at 24 hrs when compared to control (Figure 2). No permeability change was observed for either concentration at 3 hrs. Furthermore, the high dose of AGP decreased permeability at later times (12, 48 and 72 hrs) when compared to the low dose AGP- treatment. We also measured TEER values (range from 101 to 142 Ωcm2) of post-treatment monolayers, which mirrored the change of permeability coefficient (PC) values (data not shown). Together, these results suggest that AGP has a direct effect in enhancing the integrity of RBMEC monolayers.
Figure 2. Permeability Study in AGP Treated RBMEC Monolayers.
Sodium fluorescein (NaFl) permeability study was performed on RBMECs monolayers treated with α-1-acid glycoprotein (AGP) for 1, 3, 6, 12, 24, 48 and 72 hrs. Permeability coefficient (PC) values for AGP treatment at 50 μg/ml (horizontal bars) and 500 μg/ml (diagonal line) are presented as % of control monolayers (open bars). Absolute PC values for controls are 9.2 ± 3.5× 10−4 cm/min. Data represents mean +/− SEM with statistical analysis using one-way ANOVA followed by Student Newman–Keuls post-hoc analysis. * indicates p-value <0.05; ** indicates p-value <0.01; *** indicates p-value <0.001 when compared to corresponding control; § indicates p-value <0.05 when compared to 50 μg/ml AGP at respective time points; n = 4–8.
AGP Changes mRNA Expression of Pro-inflammatory Cytokines and Tight Junction Proteins
IL-1β mRNA expression was maximally elevated at 6 hrs (3-fold) following 50 μg/ml AGP treatment, with a strong expression seen at the higher concentration of AGP (500 μg/ml) at 12 hrs and 24 hrs (12-fold and 5-fold, respectively; Table 2).
Table 2.
Real-time PCR (qPCR) Data
| Treatment | 50 μg/ml AGP | 500 μg/ml AGP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Target Gene | 3 hrs | 6 hrs | 12 hrs | 24 hrs | 48 hrs | 3 hrs | 6 hrs | 12 hrs | 24 hrs | 48 hrs |
| Cytokines | ||||||||||
| IL-10 | 1.17 ± 0.1 | 1.34 ± 0.3 | 0.72 ± 0.1 | 1.03 ± 0.2 | 0.97 ± 0.0 | 0.47 ± 0.0 | 1.68 § ± 0.0 | 1.89 † ± 0.2 | 1.02 ± 0.1 | 0.49 * ± 0.1 |
| TNF-α | 0.25 ± 0.1 | 1.99 † ± 0.1 | 1.22 ± 0.2 | 0.74 ± 0.1 | 1.18 ± 0.3 | 0.40 ± 0.0 | 1.71 § ± 0.1 | 1.69 § ± 0.1 | 0.97 ± 0.3 | 0.97 ± 0.0 |
| IL-1β | 0.84 ± 0.1 | 3.42 § ± 1.1 | 0.93 ± 0.1 | 0.29 ± 0.1 | 2.02 ± 0.6 | 2.27 ±0.9 | 1.81 ± 0.7 | 12.36 † ± 0.4 | 4.55 † ± 0.3 | 1.46 ± 0.2 |
| IL-6 | 0.96 ± 0.0 | 2.46 ± 0.1 | 3.06 ± 0.1 | 2.45 ± 0.5 | 2.63 ± 0.4 | 1.49 ±0.6 | 30.45 † ± 4.4 | 8.56 § ± 1.0 | 26.32 † ± 2.9 | 20.09 § ± 3.7 |
| TJ Proteins | ||||||||||
| Occludin | 1.43 ± 0.4 | 0.90 ± 0.0 | 1.60 § ± 0.2 | 1.01 ± 0.2 | 0.64 ± 0.2 | 0.66 ± 0.1 | 0.82 ± 0.1 | 0.58 * ± 0.1 | 0.47 † ± 0.2 | 0.36 † ± 0.1 |
| ZO-1 | 2.19* ±0.7 | 0.66 * ± 0.1 | 1.22 ± 0.1 | 0.75 ± 0.2 | 0.73 ± 0.1 | 3.4 § ± 0.9 | 0.97 ± 0.1 | 0.90 ± 0.2 | 0.38 † ± 0.1 | 0.39 † ± 0.1 |
| Claudin-2 | 1.45 ± 0.1 | 0.48 † ± 0.2 | 1.08 ± 0.1 | 0.24 † ± 0.1 | 0.29 † ± 0.0 | 0.73 ± 0.0 | 0.76 ± 0.1 | 1.09 ± 0.0 | 0.48 † ± 0.0 | 0.22 † ± 0.0 |
| Claudin-5 | 1.20 ± 0.1 | 1.01 ± 0.1 | 1.34 ± 0.4 | 1.89 ± 0.4 | 5.87 † ± 0.7 | 0.54 ± 0.1 | 1.36 ± 0.1 | 0.54 * ± 0.1 | 0.85 ± 0.2 | 2.42 § ± 0.3 |
| JAM-1 | 1.08 ± 0.2 | 0.64 ± 0.1 | 1.60 ± 0.2 | 0.28 † ± 0.1 | 0.38 § ± 0.2 | 0.65 ± 0.3 | 0.45 § ± 0.1 | 1.44 * ± 0.2 | 0.47 § ± 0.1 | 0.32 † ± 0.1 |
Results have been calculated using the Pfaffl method to produce a ratio (treatment: control) that represents mean ± SEM for the effect of the treatment on mRNA expression for the respective target gene with GAPDH as the reference gene.
indicates p-value <0.05;
indicates p-value <0.01;
indicates p-value <0.001, compared with respective control; ratio means larger than two or less than 0.5 are bolded; n= 3–6.
AGP treatment (500 μg/ml) of RBMECs resulted in a significant increase of IL-6 mRNA expression at 6 hrs (30-fold), 24 hrs (26-fold) and 48 hrs (20-fold) despite no protein detected in the culture supernatant. TNF-α and IL-10 mRNA expression showed mild increases at 6 hrs and 12 hrs following 500 μg/ml AGP treatment, supporting the undetectable levels of protein in the supernatant. Taken together, these results suggest that inflammatory levels of AGP stimulate mRNA expression of IL-1β and IL-6.
In addition to cytokine mRNA expression, we also examined changes in mRNA expression of key TJ proteins (see Table 2). After 24 and 48 hrs of AGP treatment, occludin, ZO-1, claudin-2 and JAM-1 mRNA expression was decreased by 50% or more with both AGP concentrations (50 and 500 μg/ml). A significant increase in ZO-1 mRNA was observed with both AGP concentrations only at the early time point of 3 hrs. In contrast, claudin-5 mRNA expression was increased two fold or more with both AGP concentrations at a later time point of 48 hrs. Together, these results indicate that AGP regulates TJ mRNA expression with diverse time profiles.
AGP Induced Changes of TJ Protein Expression in a Dose- and Time-dependent Manner
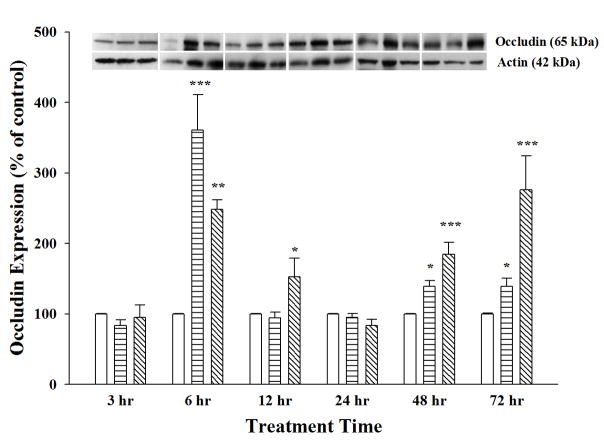
In order to follow up the alterations of TJ mRNA expression, TJ protein expression was examined in RBMECs after treatment with AGP (50 μg/ml or 500 μg/ml) for 3, 6, 12, 24, 48 or 72 hrs and compared to control (Figures 3–4). Occludin protein expression showed a biphasic dose-dependent response upon AGP treatment as seen in Figure 3. A significant increase in occludin expression was seen with 500 μg/ml AGP treatment after 6 hrs and 12 hrs treatment (2.8- and 1.5-fold), which returned to control level at 24 hrs before increasing again at later time point of 48 hrs and 72 hrs. The time profile for these changes was different with the lower dose of AGP (50 μg/ml) where the occludin expression sharply increased at 6 hrs (3.5 fold), returned to control by 12 hrs before a mild increase again at 48 hrs and 72 hrs.
Figure 3. Biphasic Expression of Occludin Protein.
Occludin protein expression was determined on cell lysates collected from RBMECs treated with α-1-acid glycoprotein (AGP) for 3, 6, 12, 24, 48 and 72 hrs, using Western blot analysis. AGP treatment at 50 μg/ml (horizontal bars) and 500 μg/ml (diagonal line) were compared to control monolayers (open bars). Occludin protein expression was normalized by β-actin expression with representative blots shown above the bar graph. Statistical analysis was done using one-way ANOVA with Student Neuman–Keuls post-hoc analysis. * indicates p-value <0.05; ** indicates p-value <0.01; *** indicates p-value <0.001 when compared with respective control; n = 4–9. Two-way ANOVA results indicate that occludin protein levels are significantly different following 50 μg/ml AGP treatment versus 500μg/ml treatment at 6, 12 and 72 hrs.
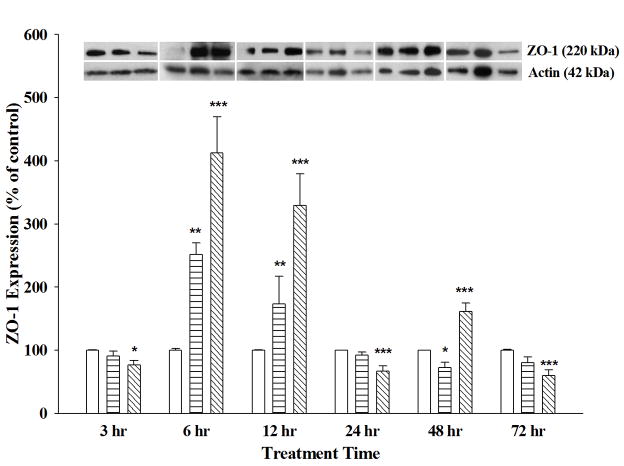
Figure 4. ZO-1 Protein Expression Changes in AGP-treated RBMECs.
ZO-1 protein expression was determined on cell lysates collected from RBMECs treated with α-1-acid glycoprotein (AGP) for 6, 12, 24, 48 and 72 hrs, using Western blot analysis. AGP treatment at 50 μg/ml (horizontal bars) and 500 μg/ml (diagonal line) were compared to controls (open bars). ZO-1 protein expression was normalized by β-actin expression with representative blots shown above the bar graph. Statistical analysis used One-way ANOVA with Student Neuman Keuls post-hoc. * indicates p-value <0.05; ** indicates p-value <0.01; *** indicates p-value <0.001 compared to respective control; n = 3–7. Two-way ANOVA results indicate that ZO-1 protein levels are significantly different following 50 μg/ml AGP treatment versus 500μg/ml treatment at 6 and 12 hrs.
In contrast to occludin, ZO-1 protein expression demonstrated a different time profile upon AGP treatment. High dose of AGP decreased ZO-1 protein levels at 3 hrs and again at 24 and 72 hrs. RBMECs showed a significant increase in ZO-1 protein expression at early time points (6 and 12 hrs) with both AGP concentrations and a later increase at 48 hrs but only with high dose of AGP (Figure 4). Together, these results show AGP-induced changes in ZO-1 and occludin expression occur in a time- and dose-dependent manner. That inversely correlates with permeability changes seen with the RBMEC.
Transcription Factor AP-1 Involvement of AGP Effects in RBMECs
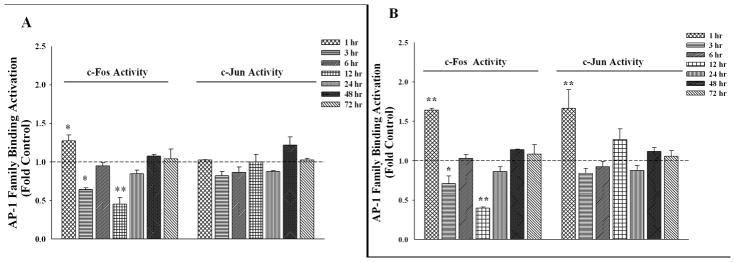
In order to identify a cell signaling candidate or transcription factor that may be involved in these AGP-mediated effects, we performed a DNA/TF protein microarray (Panomics/Affymetrix, data not shown) where AP-1 was seen to be dramatically decreased at 6 hrs treatment with a high-dose AGP (500 μg/ml). This was followed up by an ELISA based transcription factor assay (Active Motif) using nuclear extract of AGP treated RBMECs (See Figure 5). c-Jun DNA-binding activity was unchanged when compared with control after treatment with normal level of AGP (50 μg/ml; 1–72 hrs). Interestingly, c-Jun DNA-binding activity was significantly increased only at 1 hr after high-concentration AGP treatment (Figure 5, panel B-right). In contrast, both concentrations of AGP activated c-Fos at 1 hr while an inhibition of c-Fos DNA-binding activity was seen at 3 hrs and 12 hrs. Overall, the high-concentration AGP showed a greater impact on c-Fos activity changes than the lower-concentration treatment.
Figure 5. AGP Effects on AP-1 Family DNA Binding Activity in RBMECs.
c-Fos and c-Jun DNA binding activity was examined using nuclear extract collected from RBMECs treated with α-1-acid glycoprotein (AGP); 50 μg/ml (A) or 500μg/ml (B) for 1, 3, 6, 12, 24, 48, and 72 hrs (See materials and methods). AGP stimulates/inhibits nuclear c-Fos (left side) and c-Jun (right side) activity toward their consensus sequence (TRE) in a time- and concentration-dependent manner. Both 50 μg/ml (A) and 500μg/ml (B) AGP increased c-Fos activity at 1 hr while inhibition of c-Fos was seen at 3 and 12 hrs. An increase of c-Jun activity was observed following high-concentration AGP treatment at 1 hr while the low-concentration AGP did not change c-Jun activity during the treatment period tested. * indicates p-value <0.05; ** indicates p-value <0.01; *** indicates p-value <0.001, compared with respective control; n = 2–4.
As AP-1 activity negatively regulates ZO-1 gene expression (Chen et al., 2008), both activated c-Fos and c-Jun at 1 hr correlates with the decreased ZO-1 protein at 3 hrs following high-concentration AGP treatment. Furthermore, the inhibition of c-Fos activity at later time points (3 and 12 hrs) may lead to the increase in ZO-1 protein levels seen at 6 and 12 hrs following AGP treatment with both concentrations.
Discussion
This study shows that the positive acute phase protein, alpha-1-acid glycoprotein (AGP) enhances BBB functional integrity in a dose-dependent manner by increasing TJ protein (occludin and ZO-1) expression, to increase cell-to-cell contact, which results in reduced paracellular permeability (“barrier tightness”) of RBMEC monolayers. In contrast to the effects seen with pro-inflammatory cytokines, where BBB structure and functional changes lead to a disrupted or leaky barrier, AGP appears to have direct effects in the brain endothelium to presumably correct these changes and enhance both BBB structure and function. Previous reports have proposed that AGP modulates permeability microvascular endothelial cells by increasing the negative charge of the surface glycocalyx layer (Curry et al., 1989; Yuan et al., 2010a; Yuan et al., 2010b). Recently, Yuan et al. showed that high dose (1000 μg/ml) of AGP increased the permeability of positively charged ribonuclease (13.7 kDa) across bEnd3 (mouse cerebral endothelial) cell monolayers while decreasing the permeability of negatively charged α-lactalbumin (14.2 kDa) (Yuan et al., 2010a). They also found that 100 μg/ml AGP in a Ringer-BSA perfusate had similar effects on the in situ permeability of charged molecules across pial microvessels with no change in permeability for neutral charged, large dextran (10kDa and 40kDa) molecules (Yuan et al., 2010b). In this study, we show that AGP decreased RBMEC paracellular permeability of a small paracellular marker NaFl (MW 376 Da); most likely by reinforcing the physical barrier of the BBB via TJ regulation. The discrepancies between AGP effects on the endothelium and its integrity to charged and uncharged molecules are likely due to the size of molecular marker and/or the cellular mechanisms affected by AGP. Interestingly, Curry and Yuan both used high concentrations (100–1000 μg/ml) of this acute phase glycoprotein and large size molecules (10 kDa – 40 kDa); while in our experimental paradigm, we used a small size marker (376 Da) and physiologically relevant concentrations of AGP representing high normal (50 μg/ml) as well as an inflammatory relevant concentration (500 μg/ml).
Both in vitro and in vivo studies have shown permeability changes in brain capillary endothelium have an inverse correlation with TJ protein expression and localization, in particular occludin and ZO-1 proteins (Staddon et al., 1995; Staddon and Rubin, 1996; Mark and Davis, 2002; Jiao et al., 2011). In the current study, alterations in ZO-1 protein expression were seen with both low- and high-concentration AGP treatment in a dose- and time-dependent manner. In contrast, occludin protein showed biphasic increases at early and late phases with both concentrations of AGP treatment. In either case, the higher dose of AGP treatment on primary RBMECs changed TJ protein expression to a larger extent than with the lower dose, indicating that changes in TJ proteins are more dramatic during inflammatory conditions than under normal conditions. Occludin is a critical TJ protein connecting adjacent endothelial cells to form the physical barrier of the BBB (Abbott et al., 2010). In the present study, a biphasic response in occludin protein expression inversely correlated with the decreased paracellular permeability where occludin expression was increased at early time points (6 and 12 hrs) as well as at later time points (48 and 72 hrs), both of which time points paracellular permeability was decreased. The increase of ZO-1, an important TJ accessory protein that links the occludin molecule to the cytoskeleton, supports its role in tightening the barrier at 6, 12 and 48 hrs. It was not surprising that either of these TJ proteins were not increased at 3 hrs as we observed no change in paracellular permeability at this time. However, it is unclear why there was no significant increase in either TJ protein concomitant with the decreased permeability observed at 24 hrs. This might be affected by other cellular mechanisms not examined in the current study, such as changes in kinase or phosphatase activity, which could affect phosphorylation of TJ proteins and impact localization of these proteins. In fact, changes in phosphorylation state of certain amino acid residues have been associated with change in function of proteins (Staddon et al., 1995). In the case of occludin, increased tyrosine phosphorylation has been shown to target the protein for degradation while increased serine/threonine phosphorylation has been correlated with increased membrane-bound occludin resulting in decreased paracellular permeability (reviewed by Cummins, 2012). In addition to phosphorylation of TJ proteins, other potential mechanisms that could underlie the decreased permeability we have seen at 24 hrs may be through increased cAMP and/or intracellular Ca2+ levels, both of which have been shown to tighten the brain endothelial barrier (reviewed by Deli et al., 2005). These cellular mechanisms will require further investigation in our experimental paradigm. While we have been unsuccessful in quantifying protein expression of other TJ proteins (claudin-2 and claudin-5) and adhesion molecule (JAM-1) proteins, we have seen changes mRNA expression of these TJ proteins following AGP treatment, these changes may also contribute to functional changes observed with AGP in RBMECs monolayers.
As an intracellular signaling molecule, ZO-1 is required for the formation of adherens junctions as well as acts to regulate cingulin mediated myosin cytoskeleton changes (Cordenonsi et al., 1999; Ikenouchi et al., 2007). The increased level of ZO-1 protein observed at 6 and 12 hrs may be acting to regulate either adherens junctions or secondary TJ accessory proteins to enhance the functional tightness of the barrier. In this study, we showed that the high concentration AGP treatment increased TJ protein expression and decreased permeability to a larger extent than the treatment with the low concentration. This suggests that during inflammation the increased circulating AGP works to enhance BBB integrity. Overall, our findings herein indicate that AGP acts directly on brain endothelial cells by changing expression of TJ proteins in a concerted beneficial manner that is both time- and dose-dependent. Therefore, our results suggest a protective role by AGP during normal conditions and more so during an inflammatory state, which may be necessary to offset the effects of pro-inflammatory cytokines that disrupt the BBB integrity and decreased expression of TJ proteins.
While the physiological level of AGP in Sprague-Dawley (SD) rat has been reported to be 200–300 μg/ml (Schmid, 1975), we have detected levels of 25–30 μg/ml in naïve SD animals. We found that the average AGP serum levels rise 10- to 30-fold (300 – 900 μg/ml) in the female SD rats with an inflammatory-related chronic liver fibrosis. These values correspond to the two AGP concentrations (50 and 500 μg/ml) used for the treatment on the RBMEC monolayers. In our in vitro study, there was no significant degradation in AGP levels during the 72 hr treatment of RBMECs, as indicated by quantification of AGP in supernatant using Pro-Q® Emerald 300 glycoprotein stain (Figure 1). Not surprising, decreased levels of circulating AGP with a terminal half-life of 19.3 ± 1.5 hr was reported in an in vivo study of administered human AGP in male Holtzman rats (Keyler et al., 1987). While the source of increased AGP in the present study with the high-dose AGP-treated RBMEC is unclear (Figure1), it may be a stimulatory reaction by RBMECs to secret AGP.
Proinflammatory cytokines like IL-6 and IL-1β have been studied for their regulatory effects of AGP production in the liver. In this study, we examined the potential for cross-talk, between cytokines and acute phase proteins, by measuring cytokine expression in AGP-treated RBMECs. While the high concentration of AGP stimulated a dramatic increase of IL-6 mRNA expression in RBMECs, the protein level of IL-6 in cell culture supernatant at all-time points tested was below detection, using Quantikine® ELISA kits (R&D systems; Minneapolis, MN). Therefore, AGP may inhibit the translation and/or accelerate degradation process of IL-6 mRNA, which leads to the undetectable protein in culture supernatant of RBMEC monolayer at the time points tested (3–72 hr). Although mRNA changes were detected, protein levels of IL-1β and TNF-α were also not detected using the same methodology. Sensitivity of the ELISA kits for cytokines (IL-1β, TNF-α, and IL-6) are: 31.2 pg/ml, 6.25 pg/ml, and 15.5 pg/ml; respectively. Interestingly, Boutten et al. reported that AGP potentiates LPS-induced secretion of IL-1β, IL-6 and TNF-α by human monocytes and macrophages while AGP alone showed little or no effect on the production of the three cytokines (Boutten et al., 1992). The different AGP effects to cytokine production may be due to different responses from the various cell types or the necessity for a co-stimulatory factor like LPS. To our knowledge, this is the first report that AGP directly stimulates proinflammatory cytokine expression in brain endothelial cells at the RNA levels.
In order to gain an understanding of how AGP exerts its effects on the brain microvasculature, we also looked at transcription factor (TF) and DNA binding activity. Our results showed that AGP had a direct effect in DNA binding activity of transcription factor activator protein (AP)-1. Jun (c-Jun, JunB, JunD) and Fos (c-Fos, FosB, Fra1, Fra2) proteins are primary components of the AP-1 transcription factor family (Karin et al., 1997). Specifically, c-Jun proteins can form AP-1 homodimers or heterodimers with c-Fos. These dimers can directly bind to their target promoters at specific DNA elements such as TGAGTCA (classical AP-1 site) to regulate target gene expression (Glover and Harrison, 1995). The heterodimer (c-Jun: c-Fos) has a higher binding affinity to the DNA consensus sequence compared to the homodimer (c-Jun: c-Jun) and the AP-1 DNA binding activity is regulated by phosphorylation of c-Fos and c-Jun. Overall, the high dose of AGP, which correlates to an inflammatory state, showed a larger change in c-Fos activity than the lower dose of AGP, i.e. the normal physiological level. AP-1 regulates many proinflammatory cytokine genes (TNF-α, IL-1 and IL-6 etc.) and plays a pivotal role during chronic inflammation (Ip and Davis, 1998). This is supported by the findings in another in vitro study, which showed that AGP possesses pro- and anti-inflammatory activities by stimulating or inhibiting TNF-α and IL-10 production via LPS stimulated peripheral blood mononuclear leukocytes (Pukhal’skii et al., 2000). In the present study, RBMECs had an acute response on c-Fos activation after 1hr at both AGP doses, while only the high concentration showed activation of both c-Fos and c-Jun. This c-Fos/c-Jun activation most likely leads to formation of high-affinity heterodimers, with increased DNA-binding activity, results in the decreased expression of ZO-1 protein seen at 3 hrs. The significant decrease in c-Fos activity seen at 3 hrs inversely correlated with the increase in ZO-1 mRNA observed at 3 hrs and subsequent protein expression at 6 and 12 hrs. These results are supported by Chen et al., who reported AP-1 activation was shown to decrease ZO-1 expression (mRNA and protein), in Caco-2 intestinal epithelial cells, via transcriptional repression of the ZO-1-promoter (Chen et al., 2008). Together, these results show that pro-inflammatory AGP has a direct effect on AP-1 binding activity and downstream transcription regulation of TJ proteins in the brain microvessel endothelium. Whether these changes in AP-1 occur due to its phosphorylation, requires further investigation.
In conclusion, this study has shown that AGP has direct effects on permeability, TJ protein expression, and transcriptional factor regulation in our in vitro BBB model, i.e. primary RBMECs. In particular, we show AGP enhances brain endothelial function by reinforcement of the monolayer integrity via increased TJ protein expression. Furthermore, our results suggest that the changes in TJ protein expression may, in part, occur via regulation of AP-1 DNA binding activity on the primary brain endothelial cells. Further studies will help in understanding the intracellular signaling pathways and how AGP exerts its protective role on the BBB.
Research highlights.
AGP decreased 20% paracellular permeability across RBMEC monolayers.
RBMECs showed a biphasic response in occludin expression following AGP treatment.
AGP changed ZO-1 expression of RBMECs in a dose- and time-dependent manner.
AGP significantly changed transcription factor AP-1 DNA binding activity.
Acknowledgments
This study was supported by the National Institute of Health - R15HL092561. We cordially thank Jill Roberts, Ph.D. and Pooja Naik, M.S. for their work with the RBMECs isolation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992;103 (Pt 1):23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990;7:147–159. [PubMed] [Google Scholar]
- Bories PN, Kodari E, Feger J, Rouzeau JD, Agneray J, Durand G. A macrophage-derived factor induced by alpha 1-acid glycoprotein that inhibits IL-1 comitogenic activity. Immunol Lett. 1990;26:105–110. doi: 10.1016/0165-2478(90)90184-r. [DOI] [PubMed] [Google Scholar]
- Boutten A, Dehoux M, Deschenes M, Rouzeau JD, Bories PN, Durand G. Alpha 1-acid glycoprotein potentiates lipopolysaccharide-induced secretion of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha by human monocytes and alveolar and peritoneal macrophages. Eur J Immunol. 1992;22:2687–2695. doi: 10.1002/eji.1830221032. [DOI] [PubMed] [Google Scholar]
- Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289:H738–743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Ceciliani F, Pocacqua V. The acute phase protein alpha1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8:91–108. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- Ceciliani F, Pocacqua V, Miranda-Ribera A, Bronzo V, Lecchi C, Sartorelli P. alpha(1)-Acid glycoprotein modulates apoptosis in bovine monocytes. Vet Immunol Immunopathol. 2007;116:145–152. doi: 10.1016/j.vetimm.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Chen J, Xiao L, Rao JN, Zou T, Liu L, Bellavance E, Gorospe M, Wang JY. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell. 2008;19:3701–3712. doi: 10.1091/mbc.E08-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian Pous J-PG, Damais Chantal, Raichvarg Denis, Chauvelot-Moachon Laurence. Effect of Recombinant Human Interleukin-1â and Tumor Necrosis Factor á On Liver Cytochrome P-450 and Serumá-1-Acid Glycoprotein Concentrations in the Rat. Drug metabolism and disposition. 1989;18:467–470. [PubMed] [Google Scholar]
- Cordenonsi M, D’Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol. 1999;147:1569–1582. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32:242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry FE, Rutledge JC, Lenz JF. Modulation of microvessel wall charge by plasma glycoprotein orosomucoid. Am J Physiol. 1989;257:H1354–1359. doi: 10.1152/ajpheart.1989.257.5.H1354. [DOI] [PubMed] [Google Scholar]
- Deli MA, Abraham CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, Perry VH, Anthony DC, Pitossi FJ. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Pathol. 2004;165:1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JN, Harrison SC. Crystal structure of the heterodimeric bZIP transcription factor c-Fos-c-Jun bound to DNA. Nature. 1995;373:257–261. doi: 10.1038/373257a0. [DOI] [PubMed] [Google Scholar]
- Gunnarsson P, Levander L, Pahlsson P, Grenegard M. alpha(1)-acid glycoprotein (AGP)-induced platelet shape change involves the Rho/Rho kinase signalling pathway. Thromb Haemost. 2009;102:694–703. doi: 10.1160/TH09-03-0156. [DOI] [PubMed] [Google Scholar]
- Haraldsson B, Rippe B. Orosomucoid as one of the serum components contributing to normal capillary permselectivity in rat skeletal muscle. Acta Physiol Scand. 1987;129:127–135. doi: 10.1111/j.1748-1716.1987.tb08047.x. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Curry FE, Powers MR, Thipakorn B. Differential action of plasma and albumin on transcapillary exchange of anionic solute. Am J Physiol. 1993;264:H1428–1437. doi: 10.1152/ajpheart.1993.264.5.H1428. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Umeda K, Tsukita S, Furuse M. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44:130–139. doi: 10.1007/s12031-011-9496-4. [DOI] [PubMed] [Google Scholar]
- Jorgensen HG, Elliott MA, Priest R, Smith KD. Modulation of sialyl Lewis X dependent binding to E-selectin by glycoforms of alpha-1-acid glycoprotein expressed in rheumatoid arthritis. Biomed Chromatogr. 1998;12:343–349. doi: 10.1002/(SICI)1099-0801(199811/12)12:6<343::AID-BMC760>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Pentel PR, Haughey DB. Pharmacokinetics and toxicity of high-dose human alpha 1-acid glycoprotein infusion in the rat. J Pharm Sci. 1987;76:101–104. doi: 10.1002/jps.2600760203. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2011;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KS, Miller DW. Increased permeability of primary cultured brain microvessel endothelial cell monolayers following TNF-alpha exposure. Life Sci. 1999;64:1941–1953. doi: 10.1016/s0024-3205(99)00139-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nishi K, Kikuchi M, Kadowaki D, Tokutomi Y, Tokutomi N, Suenaga A, Otagiri M. Alpha1-acid glycoprotein suppresses rat acute inflammatory paw edema through the inhibition of neutrophils activation and prostaglandin E2 generation. Biol Pharm Bull. 2007;30:1226–1230. doi: 10.1248/bpb.30.1226. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DW, Batrakova EV, Waltner TO, Alakhov V, Kabanov AV. Interactions of pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjug Chem. 1997;8:649–657. doi: 10.1021/bc970118d. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata Y, Charlesworth MC, Muddiman DC. Evaluation of protein depletion methods for the analysis of total-, phospho- and glycoproteins in lumbar cerebrospinal fluid. J Proteome Res. 2005;4:837–845. doi: 10.1021/pr049750o. [DOI] [PubMed] [Google Scholar]
- Paul R, Koedel U, Winkler F, Kieseier BC, Fontana A, Kopf M, Hartung HP, Pfister HW. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain. 2003;126:1873–1882. doi: 10.1093/brain/awg171. [DOI] [PubMed] [Google Scholar]
- Pukhal’skii AL, Shmarina GV, Kalashnikova EA, Shiyan SD, Kokarovtseva SN, Pukhal’skaya DA, Bovin NV. Effect of semisynthetic analog of alpha(1)-acid glycoprotein on immunomodulatory and antiinflammatory activity of natural glycoprotein. Bull Exp Biol Med. 2000;129:480–483. doi: 10.1007/BF02439809. [DOI] [PubMed] [Google Scholar]
- Schmid K. Alpha 1-acid glycoprotein. In: Putnam FW, editor. The Plasma Proteins. Structure, function and genetic control. Academic Press; New York: 1975. pp. 184–226. [Google Scholar]
- Shiyan SD, Bovin NV. Carbohydrate composition and immunomodulatory activity of different glycoforms of alpha1-acid glycoprotein. Glycoconj J. 1997;14:631–638. doi: 10.1023/a:1018544711767. [DOI] [PubMed] [Google Scholar]
- Sorensson J, Matejka GL, Ohlson M, Haraldsson B. Human endothelial cells produce orosomucoid, an important component of the capillary barrier. Am J Physiol. 1999;276:H530–534. doi: 10.1152/ajpheart.1999.276.2.H530. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108 (Pt 2):609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Rubin LL. Cell adhesion, cell junctions and the blood-brain barrier. Curr Opin Neurobiol. 1996;6:622–627. doi: 10.1016/s0959-4388(96)80094-8. [DOI] [PubMed] [Google Scholar]
- Stakauskas R, Leibold W, Pieskus J, Mironova L, Schuberth HJ. Alpha-1-acid glycoprotein inhibits phorbol ester-induced but not Fc-receptor-induced generation of reactive oxygen species in bovine peripheral blood neutrophils. J Vet Med A Physiol Pathol Clin Med. 2005;52:213–218. doi: 10.1111/j.1439-0442.2005.00717.x. [DOI] [PubMed] [Google Scholar]
- Steinberg TH, Pretty On Top K, Berggren KN, Kemper C, Jones L, Diwu Z, Haugland RP, Patton WF. Rapid and simple single nanogram detection of glycoproteins in polyacrylamide gels and on electroblots. Proteomics. 2001;1:841–855. doi: 10.1002/1615-9861(200107)1:7<841::AID-PROT841>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Van Molle W, Libert C, Fiers W, Brouckaert P. Alpha 1-acid glycoprotein and alpha 1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J Immunol. 1997;159:3555–3564. [PubMed] [Google Scholar]
- Yuan W, Li G, Gil ES, Lowe TL, Fu BM. Effect of surface charge of immortalized mouse cerebral endothelial cell monolayer on transport of charged solutes. Ann Biomed Eng. 2010a;38:1463–1472. doi: 10.1007/s10439-010-9920-x. [DOI] [PubMed] [Google Scholar]
- Yuan W, Li G, Zeng M, Fu BM. Modulation of the blood-brain barrier permeability by plasma glycoprotein orosomucoid. Microvasc Res. 2010b;80:148–157. doi: 10.1016/j.mvr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Zhang F, Du Y, Ye B, Li P. Study on the interaction between the chiral drug of propranolol and alpha1-acid glycoprotein by fluorescence spectrophotometry. J Photochem Photobiol B. 2007;86:246–251. doi: 10.1016/j.jphotobiol.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zsila F, Iwao Y. The drug binding site of human alpha1-acid glycoprotein: insight from induced circular dichroism and electronic absorption spectra. Biochim Biophys Acta. 2007;1770:797–809. doi: 10.1016/j.bbagen.2007.01.009. [DOI] [PubMed] [Google Scholar]