Abstract
Background
Sex specific comparative effectiveness of direct oral anticoagulants (DOAC) among patients with non-valvular atrial fibrillation (AF) is not known. Via this retrospective cohort study we assessed the sex specific, comparative effectiveness of DOACs [Rivaroxaban (RIVA) and Dabigatran (DABI)], compared to each other and to Warfarin among patients with AF.
Methods and Results
Elderly (age >=66 years) Medicare beneficiaries enrolled in Medicare Part D benefit plan from November 2011 to October 2013 with newly diagnosed AF formed the study cohort [65,734 (44.8%) men and 81,137 (55.2%) women]. Primary outcomes of inpatient admissions for ischemic strokes, and major bleeding were compared across the three drugs (RIVA: 20 mg daily, DABI: 150 mg two times a day or Warfarin) using three-way propensity matched samples. In men, RIVA use decreased stroke risk when compared to Warfarin use (HR: 0.69, 95% CI: 0.48 – 0.99, P = 0.048) and DABI use (HR: 0.66, 95% CI: 0.45 – 0.96, P = 0.029), and was associated with a similar risk of any major bleeding when compared to Warfarin and DABI. In women, though ischemic stroke risk was similar in the 3 anticoagulant groups, RIVA use significantly increased the risk for any major bleeding when compared with Warfarin (HR: 1.20, 95% CI: 1.03 – 1.42, P = 0.021) and DABI (HR: 1.27, 95% CI: 1.09 – 1.48, P = 0.011).
Conclusions
The reduced risk of ischemic stroke in patients taking RIVA, compared with DABI and Warfarin appears to be limited to men, while the higher risk of bleeding appears to be limited to women.
Keywords: atrial fibrillation, women, stroke
Subject Terms: Atrial Fibrillation, Women, Ischemic Stroke
Non-valvular atrial fibrillation (AF) affects 3 million adults in the United States (US).1, 2 By 2050, nearly 8 million US adults will have AF.1 AF confers a 3–5 fold increase in the risk of stroke.3, 4 For decades, Warfarin was the only oral anticoagulant available for stroke prophylaxis in patients with AF. In recent years, direct oral anticoagulants (DOAC) Dabigatran (DABI) and Rivaroxaban (RIVA) have been approved for stroke prophylaxis in this population.5, 6 Randomized trial (RCT) data support the efficacy of these DOACs7–11, and they are commonly used in clinical practice.
Women with AF suffer significantly higher stroke risk compared to their male counterparts, irrespective of their age, and comorbid disease profile.12, 13 As a result, female sex has been incorporated into the most widely accepted risk scoring algorithms to identify AF patients who will benefit from anticoagulation.14, 15 However, stroke risk remains elevated in women compared to men even after initiating and sustaining similar quality Warfarin therapy.16 Hence, there is a need to understand the effectiveness of DOACs in women with AF.
One shortcoming of directly extrapolating RCT data regarding DOACs into clinical management of women with AF is that women constituted much smaller numbers in both the DABI and RIVA trials7–9 compared to contemporary clinical practice. Also, variability in treatment adherence and patient follow-up are some of the challenges in the clinical management of patients that are not reflected in randomized trials.17, 18 Finally, previous clinical trials for DOACs do not allow for head to head comparisons of RIVA to DABI, and sex specific effectiveness of RIVA to DABI is not known.
In-order to bridge this literature gap, we used a nationally representative cohort of elderly Medicare beneficiaries with newly diagnosed AF to assess the sex specific comparative effectiveness of RIVA and DABI to each other and to Warfarin.
Methods
Using the Centers for Medicare and Medicaid Services (CMS) patient records, we linked data sources including: 1) Beneficiary Summary File Base and Chronic Conditions segments; 2) Inpatient (Part A) and Carrier (Part B) Standard Analytic Files for 2011 through 2013; 3) Pharmacy Drug Event (Part D) files for 2011–2013. The institutional review board of University of Iowa approved the study. This being a retrospective cohort analysis of claims data informed consent was not required.
We identified 213,705 Medicare beneficiaries who were enrolled in CMS Part D prescription drug coverage plan, were newly diagnosed with AF between November 1, 2011 and October 31, 2013, and initiated DABI 150 mg twice daily, RIVA 20 mg once daily, or Warfarin within 90 days after AF diagnosis. Patient selection and study cohort formation algorithms are detailed in Supplemental figure 1. New AF was defined based on previously published algorithms (i.e., one inpatient claim or two outpatient claims within 90 days with ICD-9-CM code 427.31 as primary or first secondary diagnosis).19, 20
Outcomes
The primary outcomes were inpatient admissions for acute ischemic stroke or major bleeding, as defined by Rothendler et al21 and Suh et al22 based on the primary ICD-9-CM diagnosis on inpatient SAF claims for acute care stays. The secondary outcomes were subdivisions of major bleeding, defined as intracranial hemorrhage (ICH, including hemorrhagic stroke), gastrointestinal hemorrhage (GIH), and other major non-GIH, based on previously published algorithms.23
Patient Characteristics
Patient characteristics were derived from Medicare enrollment data and inpatient and carrier claims. Age, sex, and race were identified from Medicare enrollment data. Comorbid diseases24, 25 defined by Eluxhauser et al26 were identified by ICD-9-CM diagnoses in inpatient and outpatient claims during the 12 months preceding AF diagnosis. Previous cerebrovascular events and prior bleeding episodes were also identified using previously published algorithms.21, 22 We also identified additional comorbidities of importance to AF outcomes, including: other dysrhythmias (ICD-9-CM codes 427.X, excluding 427.3), cardiomyopathy (ICD9 codes 425.X), cardiac conduction disorder (e.g., bundle branch block; ICD9 codes 426.X), and previous implantable cardiac device (e.g., pacemaker; ICD9 codes V45.0, V53.3). Stroke risk was assessed using the standard CHA2DS2-VASc scoring system.27 The HAS-BLED scoring algorithm, was used to assess bleeding risk.28 Finally, the comorbidity score defined by Gagne et al,29 was calculated to assess disease burden. This score is of proven value to improve death prediction in hospitalized patients.29
Statistical analysis
Separate male and female cohorts were constructed. Comparisons were made between 3 treatment groups: participants initiated on DABI 150 mg twice a day (DABI group), participants initiated on RIVA 20 mg daily (RIVA group) and participants who were initiated on Warfarin (Warfarin group). Demographic variables, co-morbid diseases, medication use, CHA2DS2-VASc score, HAS-BLED score and Gagne score were compared between the three treatment groups separately in men and women, using a Chi-square test or one-way analysis of variance as applicable. We then used the three-way propensity matching method described by Rassen et. al. 30 to create groups of patients receiving DABI, RIVA, or warfarin that were balanced with respect to patient covariates and also had clinical equipoise --that is, patients included in the matched samples were plausible candidates for all three anticoagulants under study. Propensity matching was conducted separately for men and women. Success of the matching algorithm was evaluated by comparing standardized differences in demographic variables, co-morbid diseases, medication use, CHA2DS2-VASc score, HAS-BLED score and the Gagne score between each drug in the matched samples. In accordance with Austin,31 we evaluated the success of the matching algorithm using standardized differences rather than p-values, as p-values depend on sample sizes and may therefore not adequately reflect meaningful differences. Standardized differences less than 10% (i.e., 0.10 times the standard deviation of the difference) suggest adequate balance.31 We then used the propensity matched samples to calculate event rates/patient year of follow-up for each outcome for the 3 anticoagulant groups in men and women separately. In addition, Kaplan-Meier curves for each anticoagulant were plotted for each study outcome in males and females. Log-rank test was performed to compare the curves for the 3 anticoagulants. Finally, we used multivariable Cox proportional hazards regression on the matched samples to further control for possible differences between treatment groups within individual sex groups. In these models, the dependent variables were time (in days) from anticoagulant initiation to a given event (e.g., admission for stroke or censoring), while candidate independent variables included patient demographics, comorbid conditions, concurrent medication use, and prior health services utilization as described previously. Censoring events included end of observation (December 31, 2013), cessation of the initial anticoagulant (defined as the date of the last fill plus days supplied), or death. Variables were selected for inclusion in Cox models based on relationship to the outcome, using a statistical criterion on 0.05. Covariates adjusted in the Cox models for each of the outcomes are detailed in supplemental methods. Models also included indicators for the type of anticoagulant used. [DABI vs Warfarin (reference), RIVA Vs Warfarin (reference) and RIVA vs DABI (reference)]. Since propensity matching created dependencies in the data we used robust standard errors for the Cox regression models. Results of the regression analyses were reported as hazard ratios (HR) and 95% confidence intervals (CI) for DABI vs Warfarin, RIVA Vs Warfarin, and RIVA vs DABI. Dataset creation and propensity matching were conducted using SAS; all other analysis was performed using STATA 11 software.
Results
The final study cohort included 21,979 patients in the DABI group, 23,177 in the RIVA group, and 101,715 in the Warfarin group. There were 65,734 men (44.7%) and among them 10,740 initiated DABI, 11,606 initiated RIVA, and 43,388 initiated Warfarin. There were 81,137 women (55.3%), of which 11,239 initiated DABI, 11,571 initiated RIVA, and 58,327 initiated Warfarin.
There were significant differences in baseline characteristics across the 3 anticoagulant groups in men and women (Supplemental Table 1) prior to propensity matching. After propensity matching (Table 1, supplemental table 2 and supplemental table 3), there were 22,854 total men in the matched sample (7,618 taking each drug), and 33,093 women (11,031 taking each drug). After propensity matching in men, there were no statistically significant differences in demographic characteristics, comorbid conditions, medications in prior 90 days and health care utilization between the 3 anticoagulant groups (Table 1). Moreover, all standardized differences between drug groups for men were substantially lower than 10%. In women, after propensity matching, statistically significant differences remained for some comorbid conditions (e.g., heart failure, diabetes, CHA2DS2-Vasc score). However, all standardized differences between the 3 anticoagulant groups were substantially lower than 10%, suggesting good covariate balance.
Table 1.
Characteristics of study patients taking dabigatran (150 mg twice daily), rivaroxaban (20 mg once daily), or warfarin (after matching)
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Dabigatran 150 mg twice daily | Rivaroxaban 20 mg once daily | Warfarin | p-value | Dabigatran 150 mg twice daily | Rivaroxaban 20 mg once daily | Warfarin | p-value | |
| Number of Patients | 7,618 | 7,618 | 7,618 | 11,031 | 11,031 | 11,031 | ||
| Demographics | ||||||||
| Mean Age (SD) | 75.9 (6.1) | 75.1 (6.2) | 74.8 (6.1) | 0.097 | 76.8 | 76.8 | 76.8 | 0.184 |
| Number (%) Age 85 or older | 626 (8.2) | 655 (8.6) | 615 (8.1) | 0.479 | 1522 (13.8) | 1565 (14.2) | 1598 (14.5) | 0.338 |
| Race | ||||||||
| White (%) | 6,947 (91.2%) | 6,973 (91.5%) | 6,985 (91.7%) | 0.810 | 9755 (88.4) | 9824 (89.1) | 9906 (89.8) | 0.026 |
| Black (%) | 205 (2.7%) | 205 (2.7%) | 189 (2.5%) | 465 (4.2) | 452 (4.1) | 419 (3.8) | ||
| Hispanic (%) | 251 (3.3%) | 249 (3.3%) | 235 (3.1%) | 478 (4.3) | 478 (4.3) | 424 (3.8) | ||
| Other (%) | 215 (2.8%) | 191 (2.5%) | 208 (2.7%) | 333 (3.0) | 277 (2.5) | 282 (2.6) | ||
| Comorbid Illnesses | ||||||||
| Heart Failure | 1,895 (24.9%) | 1,885 (24.7%) | 1,884 (24.7%) | 0.974 | 2666 (24.2%) | 2630 (23.8%) | 2474 (22.4%) | 0.005 |
| Cardiomyopathy | 663 (8.7%) | 699 (9.2%) | 672 (8.8%) | 0.567 | 560 (5.1%) | 606 (5.5%) | 588 (5.3%) | 0.378 |
| Other Dysrhythmia | 2,481 (32.6%) | 2,531 (33.2%) | 2,499 (32.8%) | 0.683 | 3632 (32.9%) | 3698 (33.5%) | 3560 (32.3%) | 0.147 |
| Implantable Cardiac Device | 488 (6.4%) | 530 (6.9%) | 523 (6.9%) | 0.347 | 408 (3.7%) | 453 (4.1%) | 444 (4.0%) | 0.263 |
| Peripheral Vascular Disease | 1,524 (20.0%) | 1,509 (19.8%) | 1,490 (19.6%) | 0.787 | 1986 (18.0%) | 2027 (18.4%) | 1893 (17.2%) | 0.054 |
| Hypertension | 6,286 (82.5%) | 6,289 (82.5%) | 6,314 (82.9%) | 0.805 | 9439 (85.6%) | 9450 (85.6%) | 9425 (85.4%) | 0.893 |
| Diabetes | 2,679 (35.1%) | 2,623 (34.4%) | 2,597 (34.1%) | 0.390 | 3450 (31.3%) | 3403 (30.9%) | 3256 (29.5%) | 0.011 |
| Renal Disease | 800 (10.5%) | 834 (10.9%) | 832 (10.9%) | 0.609 | 892 (8.1%) | 868 (7.9%) | 885 (8.0%) | 0.834 |
| Liver Disease | 325 (4.3%) | 292 (3.8%) | 300 (3.9%) | 0.364 | 447 (4.0%) | 452 (4.1%) | 397 (3.6%) | 0.114 |
| Previous Stroke or TIA | 838 (11.0%) | 845 (11.1%) | 862 (11.3%) | 0.817 | 1446 (13.1%) | 1409 (12.8%) | 1293 (12.7%) | 0.105 |
| Previous Major Bleeding | ||||||||
| Intracranial | 32 (0.42%) | 38 (0.50%) | 29 (0.38%) | 0.528 | 53 (0.48%) | 50 (0.45%) | 51 (0.46%) | 0.955 |
| Gastro-Intestinal | 1,765 (23.2%) | 1,771 (23.3%) | 1,809 (23.8%) | 0.659 | 3073 (27.9%) | 3152 (28.6%) | 2903 (26.3%) | 0.601 |
| Comorbidity Scores | ||||||||
| Gagne Score | 3.1 | 3.1 | 3.1 | 0.644 | 3.0 | 3.0 | 2.9 | 0.076 |
| CHADS2 Score | 2.3 | 2.3 | 2.3 | 0.781 | 2.4 | 2.4 | 2.4 | 0.917 |
| CHADS2-Vasc Score | 3.8 | 3.8 | 3.8 | 0.683 | 4.8 | 4.8 | 4.8 | 0.711 |
| HAS-BLED Score | 1.7 | 1.7 | 1.7 | 0.089 | 1.6 | 1.6 | 1.6 | 0.091 |
| Medications in prior 90 days | ||||||||
| Statin | 3,573 (46.9%) | 3,550 (46.6%) | 3,624 (47.6%) | 0.470 | 4708 (42.7%) | 4636 (42.0%) | 4521 (40.9%) | 0.136 |
| Prescription Antiplatelet- | 418 (5.5%) | 426 (5.6%) | 420 (5.5%) | 0.957 | 496 (4.5%) | 482 (4.4%) | 464 (4.2%) | 0.574 |
| Proton Pump Inhibitors | 1,405 (18.4%) | 1,360 (17.9%) | 1,360 (17.9%) | 0.549 | 2434 (22.1%) | 2464 (22.3%) | 2335 (21.2%) | 0.089 |
| NSAID | 846 (11.1%) | 824 (10.8%) | 765 (10.0%) | 0.089 | 1695 (15.4%) | 1623 (14.7%) | 1603 (14.5%) | 0.188 |
| Prior Health Services Utilization | ||||||||
| Mean Inpatient Hospital Days | 2.3 | 2.3 | 2.5 | 0.117 | 2.7 | 2.7 | 2.7 | 0.891 |
| Number Prescriptions | 8.5 | 8.3 | 8.4 | 0.771 | 9.6 | 9.6 | 9.4 | 0.327 |
| Previous stay in Extended Care or Skilled Nursing Facility | 138 (1.8%) | 147 (1.9%) | 169 (2.2%) | 0.180 | 354 (3.2%) | 352 (3.2%) | 334 (3.0%) | 0.697 |
Outcomes
Sex specific rates of each outcome expressed as number of events and as rates/patient year of follow-up are provided in Table 2 for the propensity matched cohorts. As expected, stroke rates were higher among women than men. There were 185 strokes experienced by men and 356 experienced by women. There were 533 major bleeding events (ICH, GIH, and non-GIH) experienced by men and 897 experienced by women, of which more than 80% were GIH.
Table 2.
Gender specific outcomes in propensity matched samples reported as rates/ 100 patient year of follow-up (95% CI) and (number of events)
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Dabigatran 150 mg twice daily | Rivaroxaban 20 mg once daily | Warfarin | Dabigatran 150 mg twice daily | Rivaroxaban 20 mg once daily | Warfarin | |
| Number of patients | 7,618 | 7,618 | 7,618 | 11,031 | 11,031 | 11,031 |
| Stroke | 1.53 (1.31 – 1.81)(69) | 0.97 (0.66 – 1.24)(48) | 1.42 (1.29 – 1.77)(68) | 1.44 (1.21 – 2.01) (107) | 1.67 (1.20 – 2.11) (119) | 1.75 (1.41 – 2.59) (130) |
| Any hemorrhage | 3.16 (2.61 – 3.69)(142) | 3.86 (3.66 – 4.48)(189) | 4.23 (3.81 – 4.78) (202) | 3.82 (3.10 – 4.27) (282) | 5.18 (4.70 – 5.99) (327) | 3.90 (3.01 – 4.36) (288) |
| GI hemorrhage (GIH) | 2.73 (2.15 – 3.27) (123) | 3.30 (3.09 – 4.01) (162) | 3.24 (2.83 – 3.82) (155) | 3.49 (2.88 – 3.89) (258) | 4.55 (3.98 – 5.24) (288) | 2.89 (2.13 – 3.61) (214) |
| Intra-cranial Hemorrhage (ICH) | 0.13 (0.03 – (0.29)(6) | 0.26 (0.08 – 0.58) (13) | 0.44 (0.33 – 0.91) (21) | 0.21 (0.01 – 0.33) (16) | 0.38 (0.10 – 0.96) (24) | 0.54 (0.37 – 1.01) (40) |
| Other Non-GI hemorrhage | 0.49 (0.10 – 0.77) (22) | 0.55 (0.15 – 0.83) (27) | 1.02 (0.87 – 1.67) (49) | 0.35 (0.06 – 0.51) (26) | 0.66 (0.53 – 0.81) (42) | 1.07 (0.90 – 1.77) (80) |
Table 3 shows the hazard of each outcome in patients taking DABI (relative to Warfarin), RIVA (relative to Warfarin), and RIVA (relative to DABI), separately for men and women, based on multivariable Cox regression on propensity matched samples. Figures 1A, 1B, 2A, and 2B show the associated survival curves (with embedded graphs showing log-transformed survival rates to provide visual separation between curves).
Table 3.
Risk adjusted relative hazard of each outcome for men and women taking rivaroxaban vs warfarin, dabigatran vs warfarin, and rivaroxaban vs dabigatran, in separate propensity-matched samples for men and women
| Men | P value | Women | P value | |
|---|---|---|---|---|
| Stroke | ||||
| Rivaroxaban Vs Warfarin | 0.69 (0.48 – 0.99) | 0.048 | 0.98 (0.76 – 1.25) | 0.881 |
| Dabigatran Vs Warfarin | 1.05 (0.75 – 1.47) | 0.788 | 0.81 (0.62 – 1.04) | 0.098 |
| Rivaroxaban Vs Dabigatran | 0.66 (0.45 – 0.96) | 0.029 | 1.20 (0.93 – 1.61) | 0.271 |
| Any Major Bleeding | ||||
| Rivaroxaban Vs Warfarin | 0.91 (0.75 – 1.11) | 0.344 | 1.20 (1.03 – 1.42) | 0.021 |
| Dabigatran Vs Warfarin | 0.73 (0.59 – 0.90) | 0.004 | 0.97 (0.82 – 1.14) | 0.716 |
| Rivaroxaban Vs Dabigatran | 1.24 (0.99 – 1.55) | 0.051 | 1.27 (1.09 – 1.48) | 0.011 |
| GI hemorrhage (GIH) | ||||
| Rivaroxaban Vs Warfarin | 1.02 (0.82 – 1.27) | 0.854 | 1.43 (1.20 – 1.71) | < 0.001 |
| Dabigatran Vs Warfarin | 0.82 (0.65 – 1.04) | 0.100 | 1.20 (0.98 – 1.44) | 0.099 |
| Rivaroxaban Vs Dabigatran | 1.24 (0.98 – 1.59) | 0.083 | 1.19 (1.01 – 1.44) | 0.039 |
| Intra-cranial hemorrhage (ICH) | ||||
| Rivaroxaban Vs Warfarin | 0.59 (0.29 – 1.17) | 0.133 | 0.66 (0.40 – 1.11) | 0.110 |
| Dabigatran Vs Warfarin | 0.29 (0.12 – 0.75) | 0.010 | 0.40 (0.22 – 0.71) | 0.002 |
| Rivaroxaban Vs Dabigatran | 2.00 (0.76 – 5.31) | 0.162 | 1.65 (0.88 – 3.14) | 0.121 |
| Other Non-GI hemorrhage (non-GIH) | ||||
| Rivaroxaban Vs Warfarin | 0.53 (0.33 – 0.84) | 0.007 | 0.56 (0.38 – 0.81) | 0.002 |
| Dabigatran Vs Warfarin | 0.47 (0.28 – 0.78) | 0.003 | 0.32 (0.20 – 0.50) | < 0.001 |
| Rivaroxaban Vs Dabigatran | 1.11 (0.63 – 1.94) | 0.725 | 1.78 (1.13 – 2.87) | 0.023 |
Figure 1.
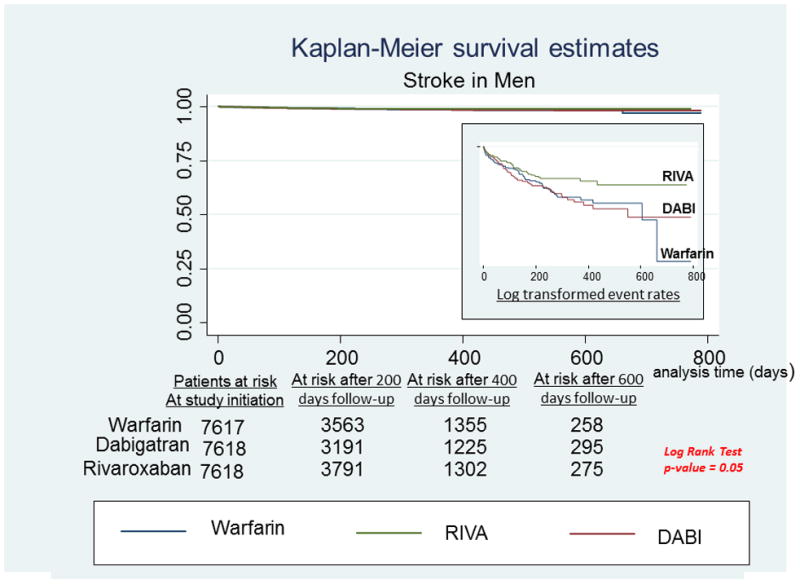
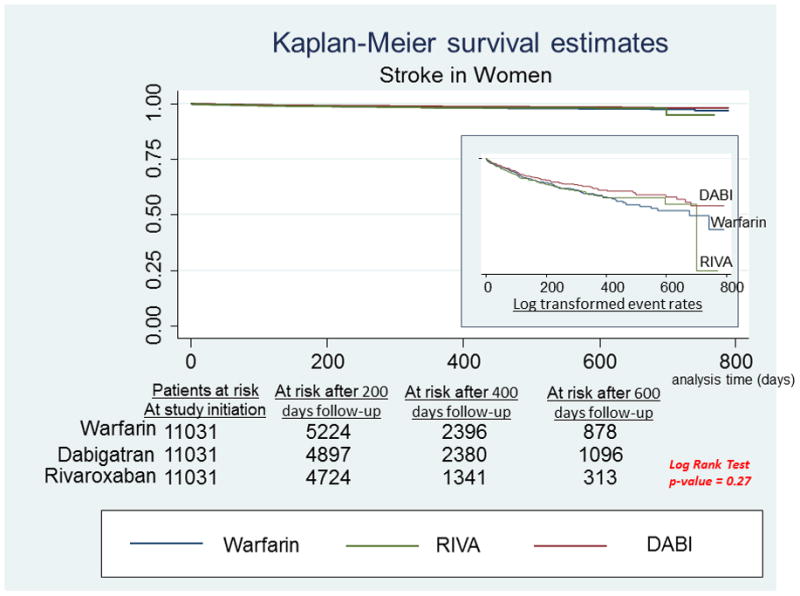
A, Stroke in men. Survival curves for stroke comparing the 3 anticoagulants in men with newly diagnosed atrial fibrillation. On the right hand side corner is the curve separation figure, which are based on log-transformed survival rates. B, Stroke in women. Survival curves for stroke comparing the 3 anticoagulants in women with newly diagnosed atrial fibrillation. On the right-hand side corner is the curve separation figure, which are based on log-transformed survival rates
Figure 2.
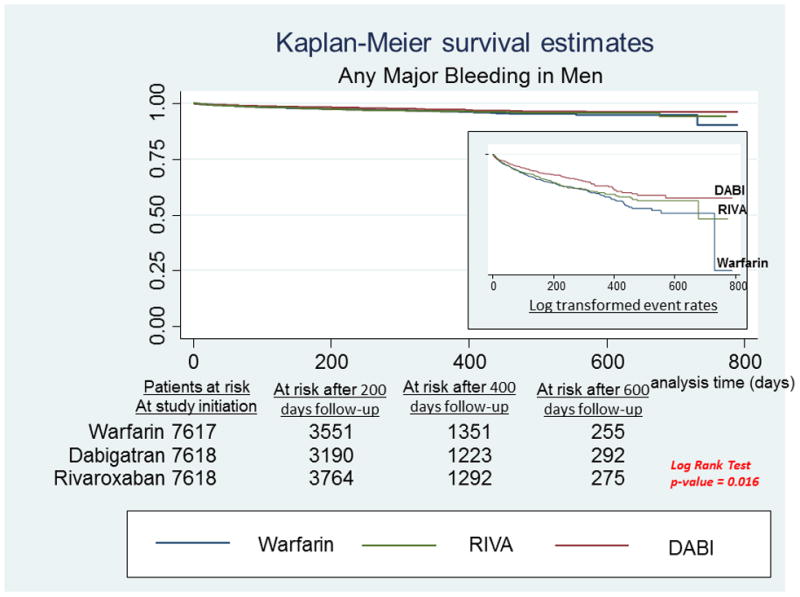
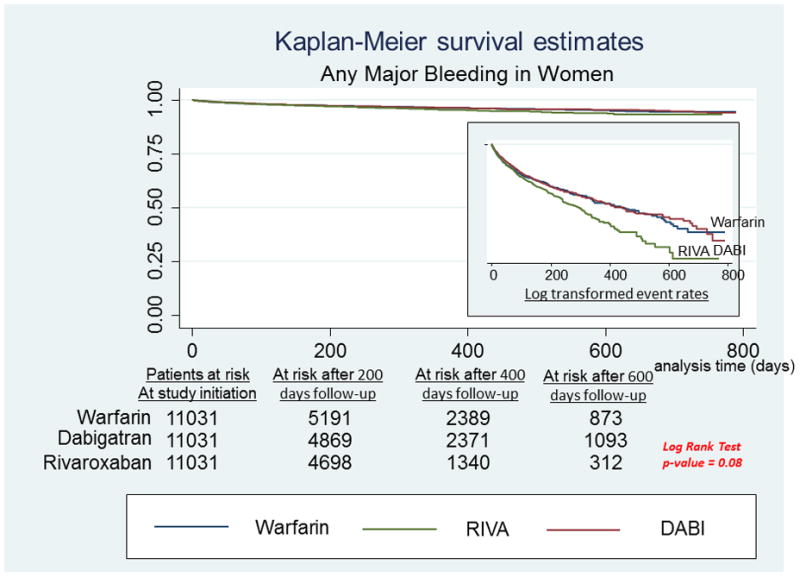
A, Any major bleeding in men. Survival curves for any major bleeding comparing the 3 anticoagulants in men with newly diagnosed atrial fibrillation. On the right-hand side corner is the curve separation figure, which are based on log-transformed survival rates. B, Any major bleeding in women. Survival curves for any major bleeding comparing the 3 anticoagulants in women with newly diagnosed atrial fibrillation. On the right-hand side corner is the curve separation figure, which are based on log-transformed survival rates
Among men, RIVA use was associated with significantly reduced risk of stroke compared to DABI use [HR: 0.66, 95% CI: 0.45 – 0.96, P = 0.029] and Warfarin use [HR: 0.69, 95% CI: 0.48 – 0.99, P = 0.048] (Figure 1A). There were no significant differences between treatment groups in relative risk of stroke in women (Figure 1B).
In men, there were no significant differences between the two DOAC groups in any of the bleeding outcomes (Table 3, Figure 2A). There were also no significant differences between treatment groups in GIH. Men who initiated DABI had lower risk of any major bleeding [HR: 0.73, 95% CI: 0.59 – 0.90] and lower risk of ICH [HR: 0.29, 95% CI 0.12 – 0.75] compared to men who initiated Warfarin. Both DOAC groups had a lower risk of other non-GIH compared to Warfarin users [HR: 0.53, 95% CI: 0.33 – 0.84 and HR: 0.47, 95% CI 0.28 – 0.78] for men taking RIVA vs warfarin and DABI vs warfarin, respectively.
Several differences across drugs with respect to bleeding outcomes were noted in women, with women RIVA users having the highest relative bleeding risks (Figure 2B). RIVA use was associated with an increased risk of any major bleeding compared with both Warfarin [HR: 1.20, 95% CI: 1.03 – 1.42] and DABI [HR: 1.27, 95% CI: 1.09 – 1.48]. A significant increase in GIH risk was also observed for RIVA compared to Warfarin [HR=1.43, 95% CI: 1.20–1.71] and DABI [HR: 1.19, 95% CI: 1.01 – 1.44]. There was no significant difference between DABI and Warfarin users in risk of any major bleeding or GIH. For ICH, a significant risk reduction compared with Warfarin was observed for women taking DABI [HR: 0.40, 95% CI 0.22 – 0.71] but not for women taking RIVA. Finally, women taking either RIVA [HR: 0.56, 95% CI: 0.38 – 0.81] or DABI [HR: 0.32, 95% CI 0.20 – 0.50] had a significantly lower risk of other non-GIH compared to Warfarin users, while the risk of other non-GIH was greater for women taking RIVA than DABI [HR: 1.78, 95% CI 1.13 – 2.87].
Discussion
In this nationally representative analysis of Medicare claims data from the United States; we report sex specific comparative effectiveness of oral anticoagulants in patients with newly diagnosed AF. In men, RIVA use decreased stroke risk when compared to DABI use and Warfarin use, and was associated with similar risk of major bleeding. In women, although stroke risk was similar in the 3 anticoagulant groups, risk of major bleeding was higher with RIVA use.
In a sub-group analysis of the ROCKET-AF9 trial that compared sex specific effectiveness of RIVA Vs warfarin, the risk of stroke and major bleeding were similar with RIVA compared to Warfarin in both men and women9. In contrast, we observed RIVA to be more effective than Warfarin for stroke prevention in men, and to be similarly effective to Warfarin in women. Also, risk of major bleeding was higher in women (but not in men) with RIVA use. Though the discrepancies between our findings and those of ROCKET AF are hard to explain, the baseline CHADS2 score in ROCKET-AF was 3.48 ± 0.94 whereas the baseline CHADS2 scores in our men and women were 2.3 ± 0.97 and 2.4 ± 1.1 respectively. The lower baseline stroke risk in our study participants may explain the superiority of RIVA over warfarin for stroke prevention in men, noted in our study that was not reported in ROCKET-AF. Also, nearly 20% of ROCKET AF participants used 15 mg of RIVA which could have decreased efficacy of RIVA in ROCKET AF study, while all our study participants used 20 mg RIVA. This could explain the better stroke prevention observed in men and increased risk of major bleeding noted in women in our study. In the survival curves comparing stroke related hospitalizations in men (Figure 1A), most strokes appears to have happened in the first 200 days of follow-up among RIVA users. After 200 days, stroke rates seem to have decreased for the remaining duration of follow-up. Although hard to explain based on our data, and that this is possibly an observation due to chance, it may be worthwhile for future studies to assess if there is an association between the duration of RIVA use and the effectiveness of stroke protection it confers. In contrary this observation was not seen in women (Figure 1B). Previous studies using observational data report conflicting results32,33, 34, 35 with some supporting a superiority of RIVA to Warfarin for stroke prevention and bleeding risk34, 35, and some reporting similar efficacy of the 2 anticoagulants.33 However, no prior study using observational data has assessed sex specific effectiveness of these 2 anticoagulants.
In a sex specific sub-group analysis of RE-LY, 7 DABI 150 mg twice daily was superior to Warfarin for stroke prevention in both men and women, while sex specific bleeding outcomes were not reported. The baseline stroke risk of RELY trail participants (CHADS2 score of 2.1 ± 1.1) were similar to our men (2.3 ± 0.97) and women (2.4 ± 1.1) using DABI. In spite we noted similar effectiveness of DABI to warfarin with stroke protection in men and women whereas RELY reported superiority of DABI to warfarin for stroke prevention in men and women. Observational data has both supported23, 36, 38 and contradicted37, 39, 40 the primary analysis of the RELY trial. However, sex specific outcomes were not reported in these observational studies. A Canadian study41, using a propensity matched analysis involving 31,786 women and 31,324 men with AF from administrative data, compared sex specific effectiveness of DABI (110 mg and 150 mg) to Warfarin. The study concluded that DABI use was associated with similar stroke risk compared to Warfarin in both sexes, but was protective against major bleeding only in men. The results of this Canadian observational study are in concordance with our findings, in spite of the fact that all our study participants used DABI 150 mg twice daily. In our study, DABI and Warfarin were similarly effective for stroke prevention in both genders, while DABI decreased risk of major bleeding in men but not in women.
Our study suggests the possibility of a higher bleeding risk in women with AF treated with DOACs; an observation noted in other clinical settings as well. The meta-analysis by Alotaibi et. al. reported a 21% higher relative risk of bleeding in women treated with DOACs for venous thromboembolism compared to men.42 Women, by virtue of their lean body weight; especially our elderly Medicare population, have decreased creatinine clearance compared to men and hence may attain higher serum levels of DOACs predisposing them to bleed more. Further, differences in sex hormones between genders may influence variability in hemostasis and vascular reactivity; 43 although it should be noted that in our study, all female subjects were post-menopausal. It is also possible that gender gaps in access to care may contribute to bleeding differences between men and women. Evidence suggesting sub-optimal access to care in women with AF exists. Bhave et. al.44, using Medicare data from 2010 – 2011, reported that women are less likely than men to be prescribed an oral anticoagulant, and are often denied other evidence based AF therapies including catheter based ablations.
With regards to the site of bleeding, though women in our study bled more with DOACs and men did not, the bleeding was predominantly GI and not ICH for both comparisons (RIVA vs Warfarin and DABI vs Warfarin). This is in concordance with the literature behind all DOACS and they increase risk of GIH and not ICH when compared to Warfarin.45
Direct, randomized head to head comparisons between RIVA and DABI have not been conducted. Indirect comparisons in the form of network meta-analyses are available.46, 47 One suggests superiority of DABI over RIVA, 46 while the other suggests similar stroke prevention for the 2 DOACs.47 Both support a similar bleeding risk for RIVA and DABI. In a direct comparison by Graham et. al.48, involving 118,891 patients with AF from an administrative claims database, RIVA (20 mg daily) performed similar to DABI (150 mg two times a day) for stroke prevention, while RIVA use was associated with a significantly higher risk of major bleeding compared to DABI use. However, gender specific comparisons are lacking in these reports.
Limitations
Although the strengths of our study include the large sample size of patients, the use of propensity matching to address possible confounding, and inclusion of patients with only new onset AF who initiated standard dose DOACs thereby minimizing variability in exposure definition, there are several limitations to note. First, there is always the possibility of residual confounding in analysis of observational data. While we did achieve successful balance in patient characteristics across the three drugs compared, it is still possible that unmeasured confounders could have biased our results. Second, our study included only patients 66 years of age and older; the results therefore may not be extrapolated to younger patients (although we note that the age range in the Medicare data is consistent with patients in the RE-LY and ROCKET-AF trials). Third, inclusion of Medicare part D (prescription benefit plan) enrollees only could impact generalizability if such patients are systematically different than Medicare beneficiaries who do no enroll in a prescription benefit plan. Moreover, beneficiaries with prescription drug coverage may also have greater ability to adhere to prescription medications compared to patients without prescription coverage. Further, considering that our study sample was an administrative dataset, we lacked granular details such as AF burden, international normalized ratio, and time in therapeutic range in warfarin users. Finally, our patients had a relatively short duration of follow-up (median of 14 months); hence risk assessments may be considered to be short-term.
Conclusions
Sex differences are possible in the effectiveness of DOACs. Women tended to bleed more with DOACs compared to Warfarin, while the risk of bleeding in men was similar for DOACs and Warfarin. RIVA may be more effective for stroke prevention compared to DABI and Warfarin in men, but all three drugs appear to provide similar stroke prevention in women. Considering the observational nature of our analysis further validation is needed to replicate these findings and to understand the mechanism behind sex-specific effects. Our study results may help clinicians tailor their choice of anticoagulants in men and women.
Supplementary Material
What is known
Direct oral anti-coagulants (DOACS) have similar efficacy to warfarin for stroke prevention among patients with atrial fibrillation
DOACS are associated with similar overall bleeding rates when compared to warfarin, although some studies suggest higher rates of gastrointestinal hemorrhage and lower rates of intracranial hemorrhage with DOACs.
What the study adds
Women tended to bleed more with DOACs compared to Warfarin, while the risk of bleeding in men was similar for DOACs and Warfarin.
Rivaroxaban may be more effective for stroke prevention compared to DABI and Warfarin in men, but all three drugs appear to provide similar stroke prevention in women.
Acknowledgments
Sources of Funding: This study is supported by funding from the Agency for Healthcare Research and Quality (AHRQ; R01 HS023104), and by the Health Services Research and Development Service (HSR&D) of the Department of Veterans Affairs.
Footnotes
Disclosures: The authors do not have any conflicts of interest or financial relationships related to the content of this manuscript. All authors had access to the data and participated in the design and writing of the manuscript.
References
- 1.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–295. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
- 7.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Randomized Evaluation of Long-Term Anticoagulation Therapy I. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875–1876. doi: 10.1056/NEJMc1007378. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, MaHaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM ROCKET AF Investigators. Rivaroxaban versus Warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 10.Deitelzweig S, Amin A, Jing Y, Makenbaeva D, Wiederkehr D, Lin J, Graham J. Medical costs in the US of clinical events associated with oral anticoagulant (OAC) use compared to warfarin among non-valvular atrial fibrillation patients <75 and >=75 years of age, based on the ARISTOTLE, RE-LY, and ROCKET-AF trials. J Med Econ. 2013;16:1163–1168. doi: 10.3111/13696998.2013.826664. [DOI] [PubMed] [Google Scholar]
- 11.Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New Oral Anticoagulants Increase Risk for Gastrointestinal Bleeding: A Systematic Review and Meta-analysis. Gastroenterology. 2013;145:105–112. doi: 10.1053/j.gastro.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012;307:1952–1958. doi: 10.1001/jama.2012.3490. [DOI] [PubMed] [Google Scholar]
- 13.Fang MC, Singer DE, Chang Y, Hylek EM, Henault LE, Jensvold NG, Go AS. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 16.Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Gender differences in stroke risk of atrial fibrillation patients on oral anticoagulant treatment. Thromb Haemost. 2009;101:938–942. [PubMed] [Google Scholar]
- 17.Steinberg BA, Holmes DN, Piccini JP, Ansell J, Chang P, Fonarow GC, Gersh B, Mahaffey KW, Kowey PR, Ezekowitz MD, Singer DE, Thomas L, Peterson ED, Hylek EM Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2:e000535. doi: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler TW, Chuang A, Huynh TD, Witt RG, Branch J, Pon T, White R. A retrospective descriptive analysis of patient adherence to dabigatran at a large academic medical center. J Manag Care Spec Pharm. 2014;20:1028–1034. doi: 10.18553/jmcp.2014.20.10.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–1273. doi: 10.1016/j.hrthm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrombotic therapy in Medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 21.Rothendler JA, Rose AJ, Reisman JI, Berlowitz DR, Kazis LE. Choices in the use of ICD-9 codes to identify stroke risk factors can affect the apparent population-level risk factor prevalence and distribution of CHADS2 scores. Am J Cardiovasc Disease. 2012;2:184–191. [PMC free article] [PubMed] [Google Scholar]
- 22.Suh DC, Nelson WW, Choi JC, Choi I. Risk of hemorrhage and treatment costs associated with warfarin drug interactions in patients with atrial fibrillation. Clin Ther. 2012;34:1569–1582. doi: 10.1016/j.clinthera.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 24.Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–1273. doi: 10.1016/j.hrthm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrombotic therapy in Medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Chen JY, Zhang AD, Lu HY, Guo J, Want FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol. 2013;10:258–266. doi: 10.3969/j.issn.1671-5411.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisters R, Lane DA, Niewlaat R, deVos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 29.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. doi: 10.1016/j.jclinepi.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rassen JA, Shelat AA, Franklin JM, Glynn RJ, Solomon DH, Schneeweiss S. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013;24:401–409. doi: 10.1097/EDE.0b013e318289dedf. [DOI] [PubMed] [Google Scholar]
- 31.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilote L, Humphries KH. Incorporating sex and gender in cardiovascular research: the time has come. Can J Cardiol. 2014;30:699–702. doi: 10.1016/j.cjca.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Coleman CI, Antz M, Bowrin K, Evers T, Simard EP, Bonnemeier H, Cappato R. Real-world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT-US study. Curr Med Res Opin. 2016;20:1–7. doi: 10.1080/03007995.2016.1237937. [DOI] [PubMed] [Google Scholar]
- 34.Yao X, Abraham NS, Sangaralingham LR, Bellolio MF4, McBane RD5, Shah ND6, Noseworthy PA. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2016;5:e003725. doi: 10.1161/JAHA.116.003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan YH, Kuo CT, Yeh YH, Chang SH, Wu LS, Lee HF, Tu HT, See LC. Thromboembolic, Bleeding, and Mortality Risks of Rivaroxaban and Dabigatran in Asians With Nonvalvular Atrial Fibrillation. J Am Coll Cardiol. 2016;68:1389–1401. doi: 10.1016/j.jacc.2016.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Villines T, Schnee J, Fraeman K, Siu K, Reynolds MW, Collins J, Schwartzman E. A comparison of the safety and effectiveness of dabigatran and warfarin in non-valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost. 2015;114:1290–1298. doi: 10.1160/TH15-06-0453. [DOI] [PubMed] [Google Scholar]
- 37.Maura G, Blotière P-O, Bouillon K, Billionnet C, Ricordeau P, Alla F, Zureik M. Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132:1252–1260. doi: 10.1161/CIRCULATIONAHA.115.015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Effectiveness and safety of dabigatran and warfarin in real-world US patients with non-valvular atrial fibrillation: a retrospective cohort study. J Am Heart Assoc. 2015;4:e001798. doi: 10.1161/JAHA.115.001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsen TB, Rasmussen LH, Skjøth F, Due KM, Callréus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–2273. doi: 10.1016/j.jacc.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Avgil-Tsadok M, Jackevicius C, Essebag V, Eisenberg MJ, Rahme E, Behlouli H, Pilote L. Dabigatran use in elderly patients with atrial fibrillation. Thromb Haemost. 2016;115:152–160. doi: 10.1160/TH15-03-0247. [DOI] [PubMed] [Google Scholar]
- 41.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Pilote L. Sex Differences in Dabigatran Use, Safety, And Effectiveness In a Population-Based Cohort of Patients With Atrial Fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:593–599. doi: 10.1161/CIRCOUTCOMES.114.001398. [DOI] [PubMed] [Google Scholar]
- 42.Alotaibi GS, Almodaimegh H, McMurtry MS, Wu C. Do women bleed more than men when prescribed novel oral anticoagulants for venous thromboembolism? A sex-based meta-analysis. Thromb Res. 2013;132:185–189. doi: 10.1016/j.thromres.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Schwertz DW, Penckofer S. Sex differences and the effects of sex hormones on hemostasis and vascular reactivity. Heart Lung. 2001;30:401–426. doi: 10.1067/mhl.2001.118764. [DOI] [PubMed] [Google Scholar]
- 44.Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–1412. doi: 10.1016/j.hrthm.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and Harms of Direct Oral Anticoagulants in the Elderly for Stroke Prevention in Atrial Fibrillation and Secondary Prevention of Venous Thromboembolism: Systematic Review and Meta-Analysis. Circulation. 2015;132:194–204. doi: 10.1161/CIRCULATIONAHA.114.013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harenberg J, Marx S, Diener HC, Lip GY, Marder VJ, Wehling M, Weiss C. Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network meta-analysis. Int Angiol. 2012;31:330–339. [PubMed] [Google Scholar]
- 47.Schneeweiss S, Gagne JJ, Patrick AR, Choudhry NK, Avorn J. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:480–486. doi: 10.1161/CIRCOUTCOMES.112.965988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, Wei Y, Liao J, Goulding MR, Mott K, Chillarige Y, MaCurdy TE, Worrall C, Kelman JA. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Beneficiaries Treated With Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA Intern Med. 2016;176:1662–1671. doi: 10.1001/jamainternmed.2016.5954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


