Abstract
Introduction
The associations of maternal conditions, before or during pregnancy, with placental lesions have not been adequately studied in populations.
Methods
In the Boston Birth Cohort, we evaluated associations between three maternal medical conditions (hypertensive disorders [HDs], gestational/pre-gestational diabetes and obesity), and placental histological findings, using a standardized classification system proposed by the Amsterdam Placental Workshop Group. Placental pathology diagnoses and clinical data from 3,074 mothers with clinical indications who delivered singleton live births at the Boston Medical Center between October 1998 and November 2013 were evaluated. Associations between each maternal condition and maternal vascular malperfusion (MVM) of the placental bed and its standardized subgroups were examined using multivariate logistic and multinomial regressions.
Results
Women with HDs (chronic hypertension, eclampsia, preeclampsia, HELLP syndrome) had significantly increased odds of MVM lesions when compared to women with no HD (aOR 2.08 95% CI 1.74–2.50), after adjusting for demographics, substance use, diabetes and body mass index. No significant differences in frequencies or aORs were seen in women with and without diabetes, or across body mass index categories. Co-morbid condition patterns that included HDs were more likely to be associated with MVM than those without.
Discussion
Using a standardized classification system, we showed that MVM is strongly and specifically associated with maternal HDs, but not other maternal conditions. Additional studies are needed to confirm and validate our findings, and evaluate the role of maternal vascular lesions of the placental bed in relation to postnatal growth and development of the offspring and effect modifiers.
Introduction
Much evidence now supports the hypothesis that the placenta plays a central role in determining pregnancy outcomes, and maternal and fetal health [1–6]. Current research, arising from life course perspectives [7] and the fetal origins hypothesis [8], has extended the placenta’s impact to predict adult chronic diseases [9], and potentially transgenerational effects [10]. Key gaps in our knowledge, and the limited means of studying placental structure, function and development, have spurred initiatives to encourage use of innovative strategies and novel technologies to study the placenta, such as the Human Placental Project [11]. Nevertheless, traditional histological examination remains an integral part of clinical diagnosis, providing, not only identification of fetal disorders and gestational conditions at risk for recurrence, but also potentially valuable data for life course epidemiological studies.
Maternal medical conditions (e.g., hypertensive disorders [HDs], gestational/pre-gestational diabetes [GD/DM], and obesity) are common during pregnancy. HDs are among the most frequent and significant, involving between 6–8% of all pregnancies and accounting for almost 15% of maternal deaths (second only to thromboembolism)[12]. The US population-based prevalence of gestational diabetes is as high as 9.2%, depending on testing employed and population studied [13]. The growing problem of maternal pre-pregnancy overweight and obesity is reflected in the prevalence, among women aged 20–39 years, of 59.5% and 34.0%, respectively [14]. Despite the strong evidence that these conditions can adversely affect pregnancy outcomes, data are limited about whether and to what extent these conditions affect placenta structure and functions. These data would be important for future prognostication studies connected with adverse perinatal outcomes.
There are challenges to pursuing this line of research. Epidemiological research to date has employed a mélange of placental diagnoses, in part, due to lack of placental data in large, well-designed birth cohort studies. In addition, inconsistent coding and definitions applied to placental diagnoses make it challenging to compare and converge the findings across studies. To move the field forward, there is a particular need for epidemiological research that incorporates standardized placental diagnoses, ideally biologically based [15]. We anticipate that placental morphological findings may help us gain insight into possible biological pathways by which maternal medical conditions affect fetal (or long-term) health outcomes.
Therefore, our study’s objective was to describe the characteristics and frequencies of placental pathology findings in the Boston Birth Cohort (BBC)—a large contemporary, multi-ethnic, predominantly minority, cohort—using a “standardized, reproducible, and biologically based classification system” recently proposed by Redline, incorporating the 2014 Amsterdam Placental Workshop Group criteria [15,16]. We further examined the relationship between placental morphology and three major maternal medical conditions (HDs, GDM/DM, and obesity) in this unique population.
Methods
Study design, setting and participants
As illustrated in the flow chart (Supplemental Figure), we evaluated a subset of the BBC that enrolled a total of 8,159 mothers at delivery at Boston Medical Center (BMC) between October 1998 and November 2013. Of these, 4,850 had no clinical indications for placental pathology examination and 220 had no available postpartum questionnaires. From the resulting placentas with pathological examination (n=3,089), 15 were excluded due to missing information on key covariates. The final sample for this analysis (n=3,074) consists of participants with placenta pathology reports, after exclusion for missing key covariate values.
The parent study is a case-control study examining environmental and genetic determinants of preterm birth [17]. The BBC consists of a multi-ethnic, predominantly minority urban population. Any woman who delivered a singleton live birth at BMC was eligible to participate in the study as a case (preterm birth < 37 weeks’ gestation or birth weight < 2,500 grams) or control (term birth ≥ 37 weeks’ gestation or birth weight ≥ 2,500 grams). Mothers with a multiple gestation, stillbirth, trauma-induced birth or newborn with major birth defect were excluded. All eligible women were approached postpartum. The participation rate was over 85%.
After obtaining informed consent, research staff used a standardized questionnaire to collect demographic information, medical and reproductive history and substance use. A standardized abstraction form recorded data from medical records review, including prenatal and intrapartum clinical care, pregnancy complications, birth outcomes, ultrasonographic findings, laboratory test results and placental pathology reports.
As described in our previous publication [18], placentas were obtained by the labor and delivery nurses at the time of delivery and sent to the hospital perinatal pathologist (Dr. Kasznica) to be processed and reviewed. During the course of the Boston Birth Cohort, a new hospital pathologist (Dr. Cerda) took over examination of placentas. Before this, for training purposes, a subset (n = 298) of the placental pathology slides was randomly selected and independently reviewed by the two placental pathologists, who compared readings and reached consensus about the reporting of the pathology findings.
The perinatal pathologists, who were not blinded to clinical information, examined all placentas (in accordance with College of American Pathologists guidelines [19]) when clinically indicated, per BBC protocol. Fresh placentas were placed in containers with 10% neutral buffered formalin. After fixing for at least 24–48 hours, dissection of the placental plate was performed by serially sectioning the placental disc every 2–3 cm for diagnostic interpretation. Routine sampling included a rolled section of membranes, and umbilical cord in cassette 1, and three transmural/full thickness sections of placental plate, including fetal and maternal surfaces, in cassettes 2–4. Grossly identified lesions were additionally sampled. Pathologic placental lesions were diagnosed according to commonly-used, recommended criteria [20–22].
Institutional Review Boards at BMC, the Massachusetts Department of Public Health, and Johns Hopkins Bloomberg School of Public Health approved the study.
Study outcomes and covariates
Placental pathology diagnoses were recoded by a perinatal pathologist (Dr. Bustamante Helfrich) into eight predominantly inflammatory and vascular categories, based upon the classification proposed by Redline [15,23]. To establish reliability, a second perinatal pathologist (Dr. Cerda) confirmed the placental coding. Categories included chorioamnionitis, chronic villitis, MVM, marginal (venous) abruption, umbilical cord obstruction, fetal vascular malperfusion, villous stromal-vascular abnormalities, and a miscellaneous group. Table 1 lists diagnoses coded into each category.
TABLE 1.
Placental Diagnostic Categories and Corresponding Reported Diagnoses *
| Placental categories | Reported diagnoses** |
|---|---|
| Histologic chorioamnionitis | Acute chorioamnionitis; acute subchorionitis; acute funisitis; acute villitis***; acute intervillositis***; acute intervillositis*** |
| Chronic villitis | Chronic villitis; lymphoplasmacytic villitis |
| Maternal vascular malperfusion | Acute atherosis; decidual vasculopathy; decidual vascular thrombosis; fibrinoid changes/necrosis in decidual vessels; retroplacental hematoma; hypermature chorionic villi; intervillous thrombus; increased perivillous fibrin; accelerated villous maturation; infarction |
| Marginal (venous) abruption | Circummarginate/circumvallate placenta; marginal hematoma; marginal abruption with membranous hemosiderin; subchorionic hematoma |
| Umbilical cord (UC) obstruction | UC knot; UC thrombosis |
| Fetal vascular malperfusion | Avascular villi; stem villus thrombus; hemorrhagic endovasculitis; villous sclerosis; fetal vascular thrombosis; chorionic plate vascular thrombosis |
| Villous stromal-vascular abnormalities | Chorangioma/chorangiosis; hypomature villi (delayed maturation); increased Hofbauer cells |
| Miscellaneous findings | Meconium-laden macrophages; maternal floor infarction; unusual placental shape; UC insertion abnormalities |
Three maternal medical conditions were chosen as major risk factors based upon their established associations with pregnancy and fetal outcomes [24–31]. History of HD and/or diabetes was obtained from the medical record. HD included chronic hypertension, preeclampsia, eclampsia and HELLP syndrome. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, which either pre-existed gestation or occurred before 20 weeks of gestation [32]. Preeclampsia was defined as hypertension of new-onset during pregnancy and proteinuria ≥300 mg protein in 24 hours [32]. Eclampsia was the occurrence of seizures in a woman with preeclampsia that could not be attributed to other causes [32]. The constellation of hemolysis, elevated liver enzymes and low platelets developing during pregnancy defined HELLP syndrome [32]. Diabetes was defined by both GDM and DM. Maternal pre-pregnancy BMI (kg/m2) was calculated from self-reported weight and height, and based on World Health Organization categorization: underweight (<18.5), normal (18.5–24.9), overweight (25–29.9) and obese (≥30) [33].
Self-reported alcohol consumption at any time during pregnancy was analyzed as binary data. Cigarette use was self-reported and categorized as “never smoker” for those who did not smoke during pregnancy and for 3 months prior, “quit during pregnancy” for those who smoked but quit during the first trimester, and “continued during pregnancy” for those who smoked continuously throughout pregnancy. For frequency estimation, gestational age at birth (obtained from the medical record) was categorized as preterm when <37 weeks and as term when ≥37 weeks. Full term and post-term gestations were combined due to the small number of post-term births in the sample. Infant birth weight, from the medical record, was categorized as normal when ≥ 2,500 grams and low birth weight when < 2,500 grams.
Statistical Analysis
Descriptive analyses reported the frequencies and percentages of the eight major categories of pathology diagnoses. Chi-square test was used to compare the frequencies between maternal medical conditions (HD, GDM/DM, overweight/obesity) in the various pathologic placental categories and MVM subgroups. Multiple logistic regression was performed to assess the associations between each of the three maternal medical conditions and histological MVM. If one of the maternal medical conditions was significantly (p<0.05) associated with MVM, stratified analyses would follow to examine the association by gestational week category at birth. The relationship between maternal medical conditions and sub-groups of MVM was examined using multinomial logistic regression. Adjustment was made for maternal age, race/ethnicity, education, marital status, parity, gestational age, smoking and alcohol use, and other medical conditions (depending on the covariate examined). Using a multivariate logistic regression model we further examined the subtypes of HDs: chronic hypertension, preeclampsia only, pre-eclampsia plus chronic hypertension, pre-eclampsia plus HELLP syndrome. Sensitivity analyses were done to check if the results were robust to additional adjustment of covariances. Data analyses were performed with Stata 14.1 software (StatCorp LP, College Station, TX).
Results
Consistent with the study design, prenatal characteristics and birth outcomes of participants with placental examination and those without are somewhat different (Supplemental Table 1).
Clinical characteristics
Characteristics of the 3,074 mother-newborn pairs are presented in Supplemental Table 2.
Placental characteristics
Three categories of placental lesions were predominantly found among participants (Figure 1): MVM (39.9%), villous stromal-vascular abnormalities (37.1%) and chorioamnionitis (26.6%). Lesions of MVM were significantly more frequent among women with HD (52.3%) than without (36.4%) p<0.01 (Table 2). Women with HD had more than two times higher odds of MVM compared to women with no HD (aOR 2.08 95% CI 1.74-2.50), after adjusting for demographics, substance use, GDM/DM and BMI. No significant differences in frequencies or aORs were seen in women with and without GDM/DM, or between known BMI categories. Stratified analysis demonstrated the association between HD and MVM was significant among early preterm (<34 gestational weeks) and late preterm (34–36 gestational weeks) births (aOR 3.88 95%CI 2.70–5.58 and aOR 2.61 95%CI 1.85–3.67, respectively), but was not significant among births which occurred at, or later than, 37 gestational weeks. (Supplemental Table 3) Frequencies of diagnostic subgroups of MVM (Supplemental Table 4) among the total sample (N=3074) were 10.1% infarct, 6.1% intervillous thrombus/intraplacental hematoma (IT/IH), 2.7% retroplacental hematoma (RHA), and 2.3% decidual vasculopathy (DV). Combined infarct and IT/IH occurred in 12.2% of placentas overall. Frequencies of the MVM subgroups were significantly higher among women with HD compared to those without (Figure 2A) for infarct (15.7% vs. 8.5%), DV (6.2% vs. 1.2%) and infarct + DV (5.7% vs. 0.5%). Figure 2B and Supplemental Table 5 show results from an adjusted model of multinomial logistic regression in which women with HD had significantly increased odds of certain MVM diagnostic subgroups compared to women without HD: infarct only (aOR 2.96 95% CI 2.23–3.93), infarct and IT/IH (aOR 1.46 95% CI 1.08–1.98), DV only (aOR 5.58 95% CI 3.33–9.37) and DV and infarct (aOR 14.75 95% CI 7.29–29.85). No significant differences were found between women with GDM/DM or abnormal BMI and those without.
FIGURE 1.
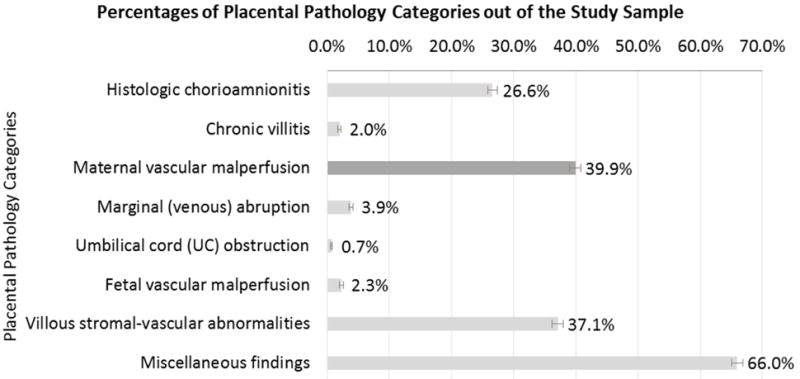
Note: N=3O74
TABLE 2.
Frequencies of Maternal Medical Conditions and Associations with Maternal Vascular Malperfusion
| Medical Conditions | MVM | Model 1a Unadjusted |
Model 2a Adjustedb |
|---|---|---|---|
| n/N (%) | OR (95% CI) | aOR (95% CI) | |
| Total | 1,228/3,074 (39.9) | ||
|
| |||
| Hypertensive Disorderc | |||
| No | 867/2,384 (36.4) | ref | ref |
| Yes | 361/690 (52.3) | 1.92** (1.62–2.28) | 2.08** (1.74–2.50) |
|
| |||
| Diabetes | |||
| No | 1,075/2,710 (39.7) | ref | ref |
| GDM/DM | 153/364 (42.0) | 1.10 (0.88–1.38) | 1.00 (0.79–1.26) |
|
| |||
| BMI categoriesd | |||
| Underweight (<18.5) | 46/129 (35.7) | 0.84 (0.57–1.22) | 0.89 (0.60–1.31) |
| Normal (18.5–24.9) | 527/1,322 (39.9) | ref | ref |
| Overweight (25–29.9) | 327/779 (42.0) | 1.09 (0.91–1.31) | 1.05 (0.87–1.26) |
| Obese (≥30) | 261/625 (41.8) | 1.08 (0.89–1.31) | 0.98 (0.79–1.20) |
Abbreviations: n, number of placenta with MVM; N, number of sample for each exposure level; OR, odds ratio; aOR, adjusted odds ratio; 95%CI, 95% confidence interval; ref, reference; GDM/DM, gestational diabetes/pre-gestational diabetes; BMI, body mass index.
Significant associations (p-value<0.05) denoted with boldface type.
p<0.05
p<0.01
Models 1 and 2 were performed using univariate and multivariate logistic regression, respectively.
Model 2 is adjusted for maternal age, race/ethnicity, education, marital status, parity, gestational age, smoking, alcohol use and other chronic conditions (if diabetes, adjusted for hypertensive disorder and BMI categories; if hypertensive disorder, adjusted for diabetes and BMI categories; if obesity by BMI categories, adjusted for diabetes and hypertensive disorder).
Hypertensive Disorder includes chronic hypertension, preeclampsia, eclampsia, and HELLP syndrome.
Total sample for BMI Model 1 and Model 2 is 2,855.
FIGURE 2.

Abbreviations: MVM = maternal vascular malperfusion, RHA = retroplacental hematoma/abruption; IT/IH = intervillous thrombus/intraplacental hematoma; DV = decidual vasculopathy.
Patterns of comorbid conditions that included HDs were more likely to be associated with MVM than those without (see Table 3), except when all three conditions coexisted. As shown in Table 4, subtype patterns of HDs significantly associated with MVM included preeclampsia (aOR 2.51 95% CI 2.00–3.16), preeclampsia and chronic hypertension (aOR 2.00 95% CI 1.38–2.92) and preeclampsia and HELLP syndrome (aOR 4.90 95% CI 2.22–10.83). Results from the sensitivity analyses further adjusting for infant sex and delivery mode were consistent with the above major findings.
TABLE 3.
Associations between Co-morbid Maternal Condition Patterns and Maternal Vascular Malperfusiona (N=3074)
| Co-morbid Maternal Medical Conditions | MVM | ||
|---|---|---|---|
|
| |||
| n/N | % | aOR (95% CI) | |
|
|
|||
| Number of Maternal Conditions | |||
| 0 | 610/1676 | 36.4 | ref |
| 1 | 428/981 | 43.6 | 1.41** (1.19–1.66) |
| 2 to 3 | 190/417 | 45.6 | 1.55** (1.24–1.95) |
|
| |||
| Pattern of Medical Conditions | |||
| None | 610/1676 | 36.4 | ref |
| Hypertensive Disorder Only b | 207/366 | 56.6 | 2.46** (1.94–3.12) |
| GDM/DM Only | 52/132 | 39.4 | 1.17 (0.81–1.69) |
| Obesity Only | 169/483 | 35.0 | 0.97 (0.78–1.20) |
| Hypertensive Disorder + Obesity | 89/185 | 48.1 | 1.78** (1.30–2.45) |
| GDM/DM + Obesity | 36/93 | 38.7 | 1.1 (0.71–1.70) |
| GDM/DM + Hypertensive Disorder | 31/56 | 55.4 | 2.37** (1.38–4.10) |
| GDM/DM + Hypertensive Disorder + Obesity | 34/83 | 41.0 | 1.33 (0.84–2.10) |
Abbreviations: MVM, maternal vascular malperfusion; n, number of placenta with MVM; N, number of sample for each exposure level; aOR, adjusted odds ratio; 95%CI, 95% confidence interval; ref, reference; GDM/DM, gestational diabetes/diabetes mellitus.
Significant values denoted with boldface type.
p<0.05
p<0.01
Multivariate logistic regression model adjusted for maternal age, race/ethnicity, education, marital status, parity, gestational age, smoking, alcohol use and chronic condition risk factors (if diabetes, adjusted for hypertensive disorder and Body Mass Index (BMI) categories; if hypertensive disorder, adjusted for diabetes and BMI categories; if obesity by BMI categories, adjusted for diabetes and hypertensive disorder).
Hypertensive disorder includes chronic hypertension, preeclampsia, eclampsia, and HELLP syndrome.
TABLE 4.
Association between Hypertensive Disorder Subtypes and Maternal Vascular Malperfusiona
| Hypertensive Disorder | n/N (%) | aOR | (95% CI) |
|---|---|---|---|
| Patterns of sub-types (N=3O74) | |||
| None | 867/2384 (36.4) | ref | |
| Chronic Hypertension | 39/113 (34.5) | 0.94 | (0.62–1.41) |
| Pre-eclampsia | 221/387 (57.1) | 2.51** | (2.00–3.16) |
| Pre-eclampsia + Chronic Hypertension | 64/127 (50.4) | 2.00** | (1.38–2.92) |
| Pre-eclampsia + HELLP syndrome | 22/31 (71.0) | 4.90** | (2.22–10.83) |
| Other | 15/32 (45.9) | 1.61 | (0.79–3.27) |
Abbreviation; n, number of placenta with MVM; N, number of sample for each exposure level; aOR, adjusted odds ratio; 95%CI, 95% confidence interval; ref, reference.
Significant values denoted with boldface type.
p<0.05
p<0.01
Multivariate logistic regression model adjusted for maternal age, race/ethnicity, education, marital status, parity, gestational age, smoking, alcohol use, diabetes and BMI.
Discussion
This is the first large-scale placental pathology study in a contemporary, predominantly US minority, urban-dwelling birth cohort with medical indications. We found that MVM was the most common placental pathology finding. The frequency of placental MVM lesions in our study was 39.9%—comparable to the 40.0% frequency of histopathologic ischemic changes found in a study of clinically selected singleton placentas by Beebe et al [34]. Similar frequencies were found despite the differences in racial and ethnic distribution between the two studies’ groups (26% Black and 8% Hispanic in Beebe et al. versus 54% Black and 23% Hispanic in our study).
We examined three major maternal medical conditions (HDs, DM/GDM, prepregnancy obesity) in relation to MVM. Compared to women without MVM in our sample, women with MVM (particularly infarcts and DV, alone and combined) had over two times higher odds of HDs, but not higher odds of GDM/DM or obesity. Our findings are consistent with previous epidemiological research on relationships between maternal HDs and placental lesions of decidual vasculopathy (DV), infarctions, ischemic changes, increased perivillous fibrin and chronic deciduitis [35].
We found a significant difference in the strength of association between MVM and HD subtypes. Chronic hypertension by itself was not associated with MVM. However, pre-eclampsia with or without chronic hypertension or HELLP syndrome was strongly and consistently associated with MVM. Although our findings may seem inconsistent with clinical experience, we are unaware of any previous study that specifically examined associations of the clinical subgroups defined by chronic hypertension and preeclampsia with MVM. Our findings raise the possibility that MVM is likely specific to preeclampsia, which should be confirmed by future studies.
Indeed, previous studies, primarily focused on preeclampsia, have consistently demonstrated association with placental MVM lesions. In a study of women with preeclampsia in Chile, Ogge et al. [36] found the prevalence of lesions consistent with maternal underperfusion was significantly greater in women with preeclampsia (n=910) than in controls (43.3% vs. 15.9%, unadjusted OR 4.0 95% CI 3.5–4.7). In a case-control study of women with preeclampsia (n=158) and normotensive controls (n=156), Moldenhauer et al. [37] found increased frequency and severity of placental lesions in women with preeclampsia. Lesions of uteroplacental malperfusion showed significantly elevated odds among preeclamptic women: decidual arteriolopathy (OR 23.8 95% CI 10.0–57.0) and central infarction (OR 5.9 95% CI 3.1–11.1). However, another case-control study [35] examined the differences in pathologic placental findings between the different clinical types of HDs during pregnancy (chronic hypertension, gestational hypertension, preeclampsia/eclampsia). Cases with HD (n=206) had a higher incidence of malperfusion lesions as compared to controls (p≤0.001), but no statistically significant difference in the incidence of placental lesions in the different types of HD (p> 0.05). Thus, more studies are needed to differentiate the role of chronic hypertension vs. pre-eclampsia in relation to MVM.
MVM due to defective deep placentation is of immense clinical importance, as evidenced by its association with many of the major obstetrical syndromes [38,39]. Abnormal placentation, characterized by incomplete physiologic transformation of the spiral arteries in the decidua and myometrium during pregnancy, has been associated with preeclampsia, preeclampsia with IUGR, IUGR without hypertension, preterm labor, preterm premature rupture of membranes, placental abruption, and second trimester abortion [38]. Our results support the current postulate that placental vascular dysfunction promotes systemic hypertension and subsequent placental damage. Using early-onset preeclampsia as an example, Roberts and Post [40] have summarized the process. Initiated by unknown root cause(s), defective deep placentation produces placental ischemia. The hypoxic placenta releases increased amounts of anti-angiogenic factors, soluble fms-like tyrosine kinase-1 (sFLT-1) and soluble endoglin (sENG), into the maternal circulation, thereby reducing levels of the angiogenic VEGF and placental growth factor (PlGF). Ensuing systemic endothelial dysfunction produces hypertension. Hypertension-induced placental injury augments preexisting damage caused by the unremodeled spiral arteries. Complete or partial retention of the arterial muscular walls produces high-speed blood flow rates that may damage chorionic villi, and lead to hypoxia/re-perfusion injury and oxidative stress. The ensuing placental damage is that of DV: fibrinoid necrosis with or without occlusive atherosis or thrombi. When the malperfusion is segmental/complete, infarction occurs [15]. This process supports the biological plausibility of the epidemiologic evidence from our study linking MVM to maternal HDs.
Less is known about placental features in overweight/obese women, especially in those without GDM [41]. However, some evidence (not controlled for gestational age) suggests that obese women have increased relative risk of pathologic placental features, specifically placental infarctions and lesions of MVM [42]. Limitations of previous studies include samples that are generally small or derived from older cohorts. Moreover, they have produced conflicting results. For example, in a study by Huang et al. [42], maternal obesity was associated with increased risk of maternal vascular and villous lesions, and fetal acute inflammation. Yet, in a smaller case-control study [43], the association with obesity was limited to maternal inflammatory lesions. Our study did not find a significant association between maternal obesity and MVM. More studies are needed to explore other placental lesions such as inflammation.
Gestational and pre-gestational diabetes mellitus (GDM/DM) are associated with increased placental weight and volume, villous immaturity, increased measures of angiogenesis and maternal vascular lesions, including fibrinoid necrosis and chorangiosis [44]. Our study did not find a significant association between maternal obesity and MVM, nor a significant association between maternal GDM/DM and MVM adjusting for covariates. More studies are needed to confirm these findings and explore other placental lesions such as inflammation.
Our study has several strengths. First, our investigation was conducted with a large birth cohort, whereas prior studies are mostly small in sample size. Second, the large sample size allows for more robust determination of associations between placental findings and maternal medical conditions. Third, since perinatal pathologists performed all placental examinations and diagnostic coding, and separate perinatal pathologists confirmed diagnoses and coding, there is strong internal validity. Fourth, the use of a standardized classification system allows for more accurate comparisons with findings from future studies.
We acknowledge many limitations of our study. The correlation of placental pathologic findings with clinical disease is limited by the lack of complete specificity of morphologic features for any given maternal disorder. The absence of definitive immunohistochemical or molecular diagnostic testing for specific placental lesions precludes validation. Additionally, the selective nature of placental examinations in a hospital setting for women with clinical indications introduces the possibility of selection bias. This is an important consideration as most pathologic placental lesions may also be identified in clinically normal pregnancies. Furthermore, our study did not include a control cohort from an unselected sample of women, thereby introducing possible ascertainment bias in favor of women with clinical indications for placental review. There is, also, legitimate concern that pathologists performing placental examinations were not blinded to clinical history. Consequently, observer bias is likely, as clinical data may influence diagnostic interpretation. Sampling limitation is inherent in all study designs employing placental histopathology. In particular, certain lesions, such as decidual vasculopathy and infarction, may be localized and difficult to appreciate on gross examination. We mitigated this limitation by having all placental examinations and tissue sampling performed by a perinatal pathologist; grossly examining the placental disc at 2–3 cm section intervals; obtaining transmural sections of the placenta along with sections of any grossly identified lesions. While our study employed standard definitions for the different clinical phenotypes of maternal medical conditions, some further details, such as duration and severity of disease, treatment and disease control status etc., which may potentially affect development of placental lesions, were not assessed. This may limit somewhat the applicability of our findings. Finally, considering the potential differences in pathogenesis of GDM and DM, it would have been desirable to separate the two disorders in the analyses. However, our sample size was not large enough to perform such an analysis with significant power.
In summary, utilizing a standardized classification system, we showed a robust association between HDs (especially, preeclampsia) during pregnancy with MVM. Our findings, if further confirmed, provide a target for future mechanistic studies and a potential biomarker for studying fetal and child health outcomes. Despite many limitations associated with use of placental pathology data derived from clinical indications, our study’s findings raise the possibility that placenta pathology, as part of electronic medical records, may serve as a valuable data source to better understand maternal, placental and fetal factors in life course epidemiology research.
Supplementary Material
HIGHLIGHTS.
Placental pathology findings assessed in a birth cohort (N=3074)
Using standardized classification system for placental evaluation
Maternal vascular malperfusion lesions are associated with maternal hypertensive disorders but not diabetes or obesity
Acknowledgments
Funding sources:
The Boston Birth Cohort is supported in part by March of Dimes PERI grants (20-FY02-56); the National Institutes of Health (NIH) grants (R21ES011666, 2R01HD041702, R01HD086013); and the Maternal and Child Health Bureau (R40MC27443). Nymisha Chilukuri is supported in part by the Johns Hopkins School of Medicine Dean’s Year of Research Program.
Role of funding sources: The funding agencies had no involvement in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vahanian SA, Lavery JA, Ananth CV, Vintzileos A. Placental implantation abnormalities and risk of preterm delivery: A systematic review and meta-analysis. Am J Obstet Gynecol. 2015;213(4):S78–S90. doi: 10.1016/j.ajog.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 2.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet. 2005;366(9499):1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 3.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Longtine MS, Nelson DM. Placental dysfunction and fetal programming: The importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med. 2011;29(3):187–96. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ptacek I, Sebire N, Man J, Brownbill P, Heazell A. Systematic review of placental pathology reported in association with stillbirth. Placenta. 2014;35(8):552–62. doi: 10.1016/j.placenta.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Ananth CV, Friedman AM. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol. 2014;38(3):151–8. doi: 10.1053/j.semperi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–93. [PubMed] [Google Scholar]
- 8.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95(2):115–28. [PubMed] [Google Scholar]
- 9.Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol. 2015;213(4 Suppl):S14–20. doi: 10.1016/j.ajog.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padmanabhan N, Jia D, Geary-Joo C, et al. Mutation in folate metabolism causes epigenetic instability and transgenerational effects on development. Cell. 2013;155(1):81–93. doi: 10.1016/j.cell.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(5):303–4. doi: 10.1016/j.placenta.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 13.DeSisto CL. Prevalence estimates of gestational diabetes mellitus in the United States, pregnancy risk assessment monitoring system (PRAMS), 2007–2010. Preventing Chronic Disease. 2014;11:130415. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 15.Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4):S21–8. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Zuckerman B, Pearson C, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 18.Nachman RM, Mao G, Zhang X, et al. Intrauterine inflammation and maternal exposure to ambient PM2. 5 during preconception and specific periods of pregnancy: The Boston Birth Cohort. Environ Health Perspect. 2016;124(10):1608. doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langston C, Kaplan C, Macpherson T, Manci E. Practice guideline for examination of the placenta. Arch Pathol Lab Med. 1997;121(5):449. [PubMed] [Google Scholar]
- 20.Benirschke K, Kaufman P, Baergen R. Pathology of the human placenta. 3rd. New York: Springer-Verlag; 1995. [Google Scholar]
- 21.Benirschke K, Kaufman P. Pathology of the human placenta. 4th. New York: Springer; 2000. [Google Scholar]
- 22.Benirschke K, Kaufman P, Baergen R. Pathology of the human placenta. 5th. New York: Springer; 2006. [Google Scholar]
- 23.Redline RW. The clinical implications of placental diagnoses. Semin Perinatol. 2015;39(1):2–8. doi: 10.1053/j.semperi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338(3):147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 25.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 26.McDonald SD, Han Z, Mulla S, Beyene J, Knowledge Synthesis Group Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636–50. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 28.Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94(11):4284–91. doi: 10.1210/jc.2009-1231. [DOI] [PubMed] [Google Scholar]
- 29.Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes-a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutcheon JA, Lisonkova S, Joseph K. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183(1):S1–S22. [PubMed] [Google Scholar]
- 33.World Health Organization. Obesity: Preventing and managing the global epidemic. World Health Organization; 2000. [PubMed] [Google Scholar]
- 34.Beebe LA, Cowan LD, Altshuler G. The epidemiology of placental features: associations with gestational age and neonatal outcome. Obstet Gynecol. 1996;87:771–8. doi: 10.1016/0029-7844(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 35.Maloney KF, Heller D, Baergen RN. Types of maternal hypertensive disease and their association with pathologic lesions and clinical factors. Fetal Pediatr Pathol. 2012;31(5):319–23. doi: 10.3109/15513815.2012.659391. [DOI] [PubMed] [Google Scholar]
- 36.Ogge G, Chaiworapongsa T, Romero R, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39(6):641–52. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189(4):1173–7. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 38.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parks WT. Placental hypoxia: the lesions of maternal malperfusion. Semin Perinatol. 2015;39(1):9–19. doi: 10.1053/j.semperi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61(12):1254–60. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- 41.Morgan TK. Impact of obesity on uteroplacental immunology and placental pathology. NeoReviews. 2016;17(2):e70–9. [Google Scholar]
- 42.Huang L, Liu J, Feng L, Chen Y, Zhang J, Wang W. Maternal prepregnancy obesity is associated with higher risk of placental pathological lesions. Placenta. 2014;35(8):563–9. doi: 10.1016/j.placenta.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Bar J, Schreiber L, Saruhanov E, Ben-Haroush A, Golan A, Kovo M. Placental histopathological findings in obese and nonobese women with complicated and uncomplicated pregnancies. Arch Gynecol Obstet. 2012;286(6):1343–7. doi: 10.1007/s00404-012-2450-z. [DOI] [PubMed] [Google Scholar]
- 44.Huynh J, Dawson D, Roberts D, Bentley-Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta. 2015;36(2):101–14. doi: 10.1016/j.placenta.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


