Abstract
Objectives
We sought to evaluate the association of physical activity with chronic myocardial damage, assessed by elevated high sensitivity troponin T (hs-cTnT), in individuals with and without obesity.
Background
Physical activity is associated with reduced heart failure (HF) risk, particularly among individuals with obesity. The role of chronic myocardial damage in this association is uncertain.
Methods
We studied 9,427 participants in the Atherosclerosis Risk in Communities Study without cardiovascular disease, with body-mass index >18.5 kg/m2. Physical activity was categorized per AHA guidelines as: recommended, intermediate, or poor. We evaluated cross-sectional associations of physical activity and obesity with elevated hs-cTnT (≥14 ng/L). In prospective analyses, we quantified the association of elevated hs-cTnT with HF risk within cross-categories of baseline physical activity and obesity.
Results
Persons with poor physical activity were more likely to have elevated hs-cTnT than those with recommended (OR 1.39; 95% CI: 1.15–1.68). In cross-categories of physical activity and obesity, using the non-obese/recommended activity group as the reference, persons with obesity and poor activity were most likely to have elevated hs-cTnT (OR 2.46; 95% CI: 1.91–3.19), whereas the obese/recommended activity group had a weaker association (OR 1.68; 95% CI: 1.28–2.21; p<0.001 for interaction between physical activity and obesity). In prospective analyses, elevated hs-cTnT was strongly associated (p<0.001) with incident HF in all obesity/physical activity cross-categories (p>0.2 for interaction).
Conclusions
Physical activity is inversely associated with chronic subclinical myocardial damage. Physical activity may lessen the association between obesity and subclinical myocardial damage, which could represent a mechanism by which physical activity reduces HF risk.
Keywords: Heart Failure, Obesity, Physical Activity, Troponin T, Epidemiology
Introduction
Physical activity is widely appreciated as a key component of strategies to reduce heart failure (HF) risk (1). Physical activity has been linked to a lower likelihood of HF among individuals with obesity, a group at particularly elevated HF risk (2,3). Notably, the reduced HF risk associated with physical activity among individuals with obesity is not fully explained by traditional risk factors for cardiovascular disease (CVD), suggesting the protective association of physical activity against HF may be partially mediated through non-traditional mechanisms.
Obesity has potent associations with abnormalities of myocardial structure and function and subsequent HF (4) via unclear pathways (5). An increasingly appreciated risk factor for the development of HF is subclinical myocardial damage, as reflected by levels of cardiac troponin T measured using novel high sensitivity assays (hs-cTnT) (6,7). Indeed, among individuals without prior CVD, obesity has been independently linked to elevated hs-cTnT levels, and the combination of obesity and elevated hs-cTnT is associated with markedly increased HF risk (8). It is presently unknown whether higher physical activity is associated with lower levels of subclinical myocardial damage in the presence or absence of obesity. Decreased myocardial damage could represent a pathway by which physical activity is related to lower HF risk.
We therefore tested the hypothesis that physical activity has an inverse association with elevated hs-cTnT in a community based population of men and women without clinical CVD, and that higher physical activity lessens the association between obesity and subclinical myocardial damage. To evaluate the clinical importance of these associations, we also assessed the link between elevated hs-cTnT and subsequent HF risk within groups defined by physical activity and obesity status.
Methods
Study Population
The Atherosclerosis Risk in Community (ARIC) Study is a multicenter, prospective, observational cohort investigation of CVD and related conditions in middle-aged men and women. The study design has been previously described (9). Briefly, a total of 15,792 individuals aged 45–64 years, were enrolled between 1987 and 1989 from 4 US communities. Participants were examined at baseline, at three subsequent visits occurring approximately every three years, and at a fifth visit conducted from 2011–2013. Physical activity was assessed in all participants at Visit 3 (1993–1995), whereas measurements of hs-cTnT were obtained for all participants at Visit 4 (1996–1998). We therefore performed non-concurrent cross-sectional analyses evaluating the associations of physical activity and obesity status at Visit 3 with prevalent hs-cTnT levels at Visit 4. In prospective analyses, we additionally assessed the risk of incident HF associated with elevated hs-cTnT within cross-categories of physical activity and obesity.
Of 12,887 individuals who attended Visit 3, 9,427 were eligible for this study. We excluded individuals who were missing data on physical activity or BMI at Visit 3 (n=40) or hs-cTnT at Visit 4 (n=1,796), those with self-reported CVD, a CVD clinical event (HF or coronary heart disease, including coronary revascularization procedures), or silent myocardial infarction at or prior to Visit 4 (n=1,492), those not of black or white race (n=38) and those with a BMI <18.5 kg/m2 (n=94). All participants provided informed consent, and the study protocol was approved by the institutional review boards at each study site. Measurement of Study Variables
The primary exposure was physical activity, measured through a modified Baecke questionnaire (10) at Visit 3 (1993–1995). The questionnaire asked questions on participation in up to 4 sports or exercise physical activity within the previous year and the number of hours/week and months/year spent on each sport. As has been done in prior ARIC analyses (11,12), we converted the Baecke sports indices into minutes per week of moderate or vigorous exercise. Each activity was converted into metabolic equivalents of task (METS) based on the Compendium of Physical Activities. Moderate activities were defined as those involving a workload of 3–6 METS and vigorous activities were those involving a workload of >6 METS. We subsequently categorized physical activity according to the AHA guidelines as “recommended” (≥75 min/wk of vigorous intensity or ≥150 min/wk of any combination of moderate + vigorous intensity), “intermediate” (1–74 min/wk of vigorous intensity or 1–149 min/wk of any combination of moderate + vigorous intensity), or “poor” (0 min/wk of moderate or vigorous exercise) (13). We also modeled physical activity as a continuous variable in METS*min/wk and used this continuous variable to generate quartiles of physical activity.
Body Mass Index (BMI) was calculated from measured height and weight at Visit 3 and categorized as non-obese (BMI 18.5 to < 30 kg/m2) and obese (BMI ≥30 kg/m2). Information on additional covariates of interest was obtained through history, physical exam and laboratory data at Visit 3. Smoking status was categorized as current and non-current smoker, and alcohol consumption quantified in grams per week. Diabetes was defined as fasting glucose ge;126mg/dL, non-fasting glucose 200mg/dL, self-reported history of diabetes, or use of hypoglycemic agents. Systolic blood pressure (SBP) was measured three times and the mean of the second and third measurements was used for analyses.
Total cholesterol, high-density lipoprotein cholesterol, and triglycerides were measured using standardized enzymatic assays. Renal function was assessed using estimated glomerular filtration rate (eGFR) calculated based on serum creatinine at Visit 4 using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (14). N-terminal pro-brain natriuretic peptide (NT-proBNP) and high sensitivity C-reactive protein (hsCRP) were measured from plasma collected at Visit 4, and frozen at −80° C.
The primary outcome in cross-sectional analyses was elevated hs-cTnT, defined as a level ge;14ng/L, a cut point that has been used in several prior analyses (6,7). Hs-cTnT levels were measured in 2011 from Visit 4 plasma samples that had been stored at −80oC, using a high sensitivity assay (Elecsys troponin T, Roche Diagnostics, Indianapolis, IN). As previously reported, the reliability coefficient for hs-cTnT measurements among the ARIC study participants was 0.94 (15). The between-assay coefficient of variation for control materials was 6.9% for mean hs-cTnT concentrations of 29 ng/l.
Incident Heart Failure
In prospective analyses, the outcome of interest was incident HF, defined as the first hospitalization or death related to HF. HF deaths were identified by hospital discharge codes and death certificates, with ICD-9 code 428 being used to identify hospitalizations and deaths early in follow up, and ICD-10 code I-50 for HF deaths in later follow-up. Information on hospitalizations was obtained from participants via yearly telephone calls, and vital records were examined for all deaths. HF events occurring from 2005 onwards were additionally adjudicated by an expert panel (16).
Statistical Analysis
We used multivariable logistic regression models to evaluate the adjusted associations of physical activity categories with myocardial damage, as assessed by elevated hs-cTnT. We used the highest (”recommended”) physical activity category (individuals performing ≥75 min/wk of vigorous intensity or ≥150 min/wk of any combination of moderate + vigorous intensity exercise physical activity) as the reference group. We constructed two models, one with confounders and a second one also including potential mediators of the effects of physical activity on subclinical myocardial damage. Model 1 included age, sex, race, smoking status, and alcohol intake. Model 2 included all variables from Model 1 as well as SBP, anti-hypertensive medication use, diabetes mellitus, total cholesterol, HDL-cholesterol, and triglycerides. We constructed a third adjustment model that included the variables in Model 2 as well as additional predictors of future HF such as heart rate, eGFR, NT-proBNP, and hsCRP. Covariates were selected a-priori, based on previously published data and known risk factors for HF. We conducted sensitivity analyses with physical activity modeled as quartiles. We additionally evaluated the continuous association of physical activity (in (MET*minute)/week) with elevated hs-cTnT by scaling per 1-SD and constructing restricted cubic spline models. We tested for multiplicative interactions with age (≥ or < 65 years), race and gender on the association between physical activity and hs-cTnT.
To evaluate the association between physical activity and hs-cTnT in the presence and absence of obesity, we conducted analyses stratified by obesity status (non-obese and obese), using regression models including the same covariates outlined above. We also created cross-categories of physical activity (recommended, intermediate, poor) and obesity status (non-obese, obese) to assess the combined association of these two exposure variables with elevated hs-cTnT, using a common reference group of non-obese individuals with recommended physical activity. We performed additional analyses using abdominal obesity, defined by World Health Organization criteria as a waist circumference of ≥ 88 cm for women and ≥ 102 cm for men, as an alternative measure of adiposity.
In prospective analyses, the baseline was ARIC visit 4 (the time point of hs-cTnT measurement) with follow up for incident HF through December 31st, 2012. We constructed Poisson and Cox proportional hazards regression models, adjusting for covariates at Visit 3, to estimate the adjusted incidence rates and hazard ratios with associated 95% confidence intervals for incident HF within each cross-category of physical activity and obesity. To evaluate the clinical implications of the associations of physical activity and obesity with elevated hs-cTnT, we then constructed regression models to estimate the incidence rates and hazard ratios for HF associated with elevated hs-cTnT within each of the cross-categories of physical activity and obesity status. We created an interaction term and performed likelihood ratio tests to test for heterogeneity in the associations between elevated hs-cTnT and incident HF across the cross-categories.
Statistical analyses were performed with Stata version 13.1. All p-values presented are 2-sided.
Results
Characteristics of the study population according to physical activity categories are presented in Table 1. Physical activity categories were fairly evenly distributed across the study population, with 43% of individuals reporting recommended, 23% intermediate, and 34% poor levels of physical activity. Among those with and without obesity, 33% and 47%, respectively, performed recommended levels of physical activity. Compared to those with recommended physical activity, participants with lower levels of physical activity were more likely to be women, African-Americans, current smokers, had higher SBP, prevalence of diabetes and BMI. Lower physical activity was also associated with lower HDL-cholesterol levels, and higher heart rate, eGFR and hsCRP. There were no significant differences in NT-proBNP levels across categories of physical activity.
Table 1.
Characteristics of Study Population According to Physical Activity Categories at Baseline, the Atherosclerosis Risk in Communities Study (1993–1995)
| Physical Activity Category | ||||
|---|---|---|---|---|
| Recommended n=4043 (43%) | Intermediate n=2178 (23%) | Poor n=3206 (34%) | p value | |
| Age, years (SD) | 60.6 (5.8) | 60.0 (5.6) | 59.6 (5.5) | <0.0001 |
| Female sex % | 51.8 | 65.0 | 60.5 | <0.0001 |
| African American % | 15.2 | 20.8 | 28.6 | <0.0001 |
| Current smoker % | 12.5 | 14.6 | 21.0 | <0.0001 |
| Alcohol intake, g/wk (SD) | 42.0 (90.7) | 37.4 (94.8) | 37.9 (116.0) | 0.13 |
| Anti-hypertensive meds % | 29.1 | 31.4 | 34.9 | <0.0001 |
| SBP, mmHg (SD) | 122.3 (17.5) | 123.2 (18.4) | 125.1 (19.0) | <0.0001 |
| Diabetes, % | 10.2 | 13.0 | 15.1 | <0.0001 |
| HDL-cholesterol, mg/dl (SD) | 53.7 (18.4) | 54.2 (18.7) | 51.7 (17.0) | <0.0001 |
| Total cholesterol, mg/dl (SD) | 207.2 (37.0) | 209.4 (37.1) | 207.5 (37.4) | 0.06 |
| Triglycerides, mg/dl (SD) | 137.4 (93.9) | 139.5 (78.9) | 141.3 (83.5) | 0.16 |
| BMI, kg/m2 (SD) | 27.5 (4.6) | 28.5 (5.1) | 29.4 (6.0) | <0.0001 |
| Heart rate, beats per min (SD) | 64.2 (9.5) | 66.0 (9.6) | 66.4 (9.8) | <0.0001 |
| eGFR, ml/min/1.73m2 (SD) | 85.7 (14.5) | 86.6 (15.8) | 87.9 (16.5) | <0.0001 |
| NT-proBNP, pg/ml (SD) | 155.5 (3019.2) | 128.3 (532.0) | 113.9 (368.0) | 0.67 |
| hsCRP, mg/L (SD) | 3.7 (6.0) | 4.2 (5.7) | 5.2 (7.5) | <0.0001 |
Physical activity categories defined as: poor (0 min/wk moderate or vigorous), intermediate (1–74 min/wk of vigorous or 1–149 min/wk of moderate + vigorous), and recommended (≥75 min/wk of vigorous or ≥150 min/wk of moderate + vigorous).
SD indicates standard deviation; SBP, systolic blood pressure; HDL, high-density lipoprotein; BMI, body mass index; hs-cTnT, high sensitivity cardiac Troponin T; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-brain natriuretic peptide; hsCRP, high-sensitivity C-reactive protein.
Within the study population, 7.2% of individuals had elevated hs-cTnT levels. The prevalence of elevated hs-cTnT increased with lower physical activity levels, being found among 6.8%, 7.0%, and 7.7% of individuals with recommended, intermediate, and poor activity, respectively.
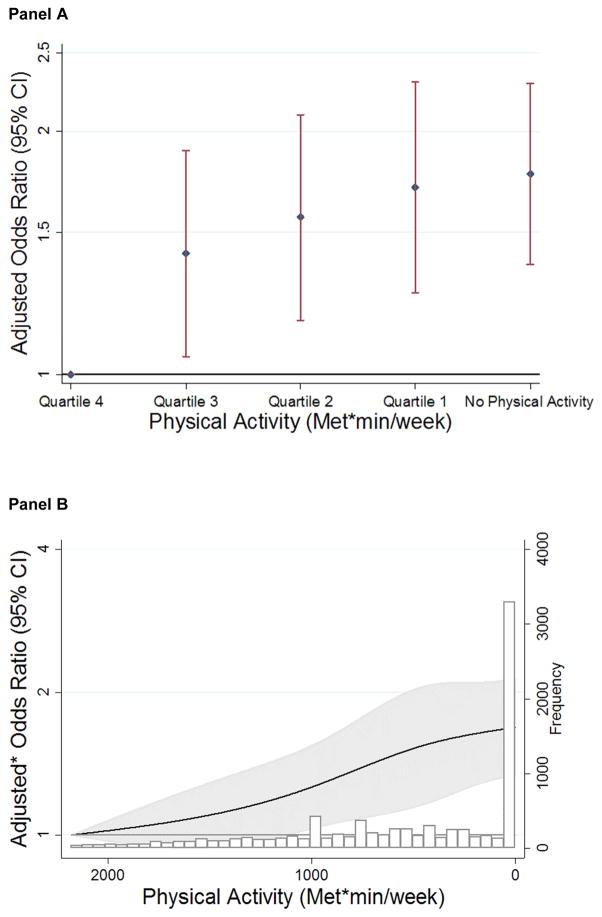
In logistic regression analyses, lower categories of physical activity were associated with significantly higher odds of subclinical myocardial damage. Relative to those with recommended physical activity, persons with poor physical activity were more likely to have elevated hs-cTnT (OR 1.39, 95% CI 1.15–1.68) after adjusting for confounders (Table 2, Model 1). Notably, a significant inverse association between physical activity and elevated hs-cTnT remained after additional adjustment for several potential CVD mediators (OR 1.31; 95% CI: 1.08–1.59) (Table 2, Model 2). We noted similar trends, however of only borderline statistical significance after additional adjustment for heart rate, eGFR, NT-proBNP and hsCRP (OR 1.21; 95% CI: 0.99–1.49) (Table 2, Model 3). When physical activity was modeled continuously, its association with elevated hs-cTnT remained significant (p=0.02). In analyses modeling physical activity as quartiles, we observed a significant association between lower physical activity quartiles and a higher likelihood of myocardial damage (Figure 1, Panel A). A graded association between lower levels of physical activity and higher odds of subclinical myocardial damage was also demonstrated in restricted cubic spline models (Figure 1, Panel B). Results were similar across age, race and gender subgroups, with no significant interaction between physical activity and these demographic variables on the outcome of elevated hs-cTnT.
Table 2.
Odds Ratios (95% CIs) for Elevated hs-cTnT Associated with Lower Physical Activity Categories
| Physical Activity Category | P for trend † | |||
|---|---|---|---|---|
| Recommended n=4043 | Intermediate n=2178 | Poor n=3206 | ||
| Model 1 | Reference (1) | 1.34* (1.08–1.66) | 1.39* (1.15–1.68) | <0.001 |
| Model 2 | Reference (1) | 1.25 (1.00–1.57) | 1.31* (1.08–1.59) | 0.001 |
| Model 3 | Reference (1) | 1.15 (0.91–1.45) | 1.21 (0.99–1.49) | 0.02 |
Statistically significant
Calculated using continuous physical activity, in (MET*min)/week
Model 1: adjusted for age, race, sex, smoking status, and alcohol use.
Model 2: adjusted for Model 1 + SBP, anti-hypertensive medication use, diabetes, total cholesterol, HDL-cholesterol, and triglycerides.
Model 3: adjusted for Model 2+ heart rate, eGFR, NT-proBNP, and hsCRP.
Figure 1.
Panel A
Association of Quartiles* of Physical Activity with Odds Ratios (and 95% CIs) for Elevated Hs-cTnT
Reference: Quartile 4: ≥1365.9 (mets*min)/week
Adjusted for age, race, sex, smoking status, and alcohol use
*Cutpoints for quartiles of physical activity: Quartile 1: 0–453.6 (mets*min)/wk; Quartile 2: 453.6–846.3 (mets*min)/wk; Quartile 3: 846.3–1364.9 (mets*min)/wk; Quartile 4: ≥1365.9 (mets*min)/wk
Panel B
Continuous Association Between Physical Activity and Elevated Hs-cTnT Among Participants Without Clinical CVD in Restricted Cubic Spline Models
Reference: 95th percentile, 2182 (MET*min)/week
* Adjusted for age, race, sex, smoking status, and alcohol use
We found similar results in analyses stratified by obesity status. A significant, inverse, relationship was observed between physical activity modeled continuously and elevated hs-cTnT among both obese (p=0.01) and non-obese (p=0.02) participants. Each 1-SD lower physical activity level (781 (MET*min)/week) was significantly associated with a higher likelihood of elevated hs-cTnT, in persons who were obese (OR 1.22, 95% CI 1.05–1.42) and non-obese (OR 1.14, 95% CI 1.02–1.27).
In analyses evaluating the association of cross-categories of physical activity and obesity status with elevated hs-cTnT after adjusting for confounders (Table 3, Model 1), using the non-obese and recommended activity group as the reference, we found that persons in the obese and poor physical activity group were most likely to have elevated hs-cTnT (OR 2.46; 95% CI: 1.91–3.19). However, the combination of obesity and recommended physical activity levels had a weaker association with elevated hs-cTnT (OR 1.68; 95% CI: 1.28–2.21). After further adjustment for CVD mediators (Table 3, Model 2), the combination of obesity and recommended physical activity levels was no longer significantly associated with elevated hs-cTnT (OR 1.20; 95% CI: 0.90–1.61). Similar trends were noted after additional adjustment for heart rate, eGFR, NT-proBNP, and hsCRP (Table 3, Model 3). Notably, a statistically significant interaction between physical activity and obesity on the outcomes of hs-cTnT was seen in all models. Analogous patterns were seen when waist circumference, categorized using World Health Organization criteria for abdominal obesity, was used as an alternative metric for adiposity (Supplemental Table 1).
Table 3.
Adjusted Odds Ratios* (95% CIs) for Elevated hs-cTnT According to Cross- Categories of Physical Activity and Obesity Status at Baseline
| Model 1 | |||||
|---|---|---|---|---|---|
|
| |||||
| Physical Activity Category | p for trend† | p for interaction‡ | |||
| Recommended | Intermediate | Poor | |||
|
| |||||
| Non-obese | 1 (Reference) n=3,043 |
1.22 (0.92–1.62) n=1,459 |
1.20 (0.93–1.54) n=1,935 |
0.02 | <0.001 |
| Obese | 1.68* (1.28–2.21) n=1,935n=1,000 |
2.37* (1.74–3.23) n=1,935n=719 |
2.46* (1.91–3.19) n=1,935n=1,271 |
0.01 | |
|
| |||||
| Model 2 | |||||
|
| |||||
| Physical Activity Category | p for trend† | p for interaction‡ | |||
| Recommended | Intermediate | Poor | |||
|
| |||||
| Non-obese | Reference (1) n=3,043 |
1.17 (0.88–1.55) n=1,459 |
1.18 (0.91–1.52) n=1,935 |
0.03 | 0.011 |
| Obese | 1.20 (0.90–1.61) n=1,000 |
1.66* (1.20–2.29) n=719 |
1.73* (1.32–2.27) n=1,271 |
0.01 | |
|
| |||||
| Model 3 | |||||
|
| |||||
| Physical Activity Category | p for trend† | p for interaction‡ | |||
| Recommended | Intermediate | Poor | |||
|
| |||||
| Non-obese | Reference (1) n=1,271n=3,043 |
0.99 (0.73–1.34) n=1,271n=1,459 |
1.03 (0.79–1.35) n=1,271n=1,935 |
0.32 | 0.003 |
| Obese | 1.11 (0.81–1.49) n=1,000 |
1.56* (1.11–2.18) n=719 |
1.63* (1.23–2.15) n=1,271 |
0.01 | |
Statistically significant
Calculated using continuous physical activity, in (MET*min)/week
interaction term across cross-categories calculated using likelihood ratio tests
Using a common reference group of non-obese with poor physical activity Non-obese: BMI 18.5 to <30 kg/m2
Obese: ≥ 30 kg/m2
Model 1: adjusted for age, race, sex, smoking status, and alcohol use.
Model 2: adjusted fort Model 1 variables + SBP, anti-hypertensive medication use, diabetes, total cholesterol, HDL-cholesterol, and triglycerides.
Model 3: adjusted for Model 2+ heart rate, eGFR, NT-proBNP, and hsCRP.
In prospective analyses, we evaluated the association between elevated hs-cTnT and subsequent HF risk within each cross-category of physical activity and obesity status. Over a median 15 years of follow-up, there were 1,178 HF events in the study population. As shown in Supplemental Table 2, the adjusted incidence rates for HF (per thousand person years) were lowest in the subgroup that were non-obese with recommended activity (5.75), while the highest rates were seen in the obese/poor activity subgroup (14.09). A similar pattern was seen in Cox regression analyses (Supplemental Figure 1), with the highest HF risk among the cross-categories seen for those with obesity and poor activity (HR 2.55, 95% CI: 2.14–3.04).
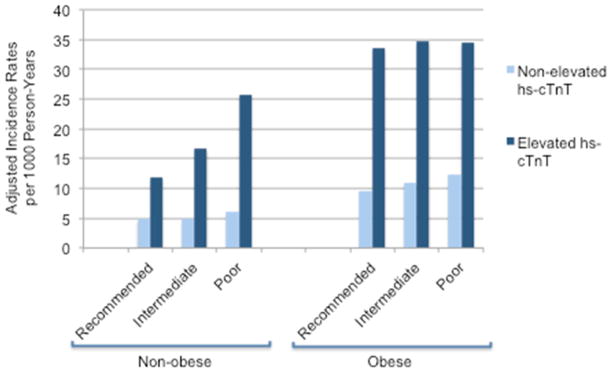
Within each cross-category of physical activity and obesity status, the presence of elevated hs-cTnT was strongly associated with higher incidence rates for HF relative to non-elevated hs-cTnT (Figure 2). Analogously, in multivariable Cox regression analyses, elevated hs-cTnT was strongly associated with significantly increased HF risk compared to non-elevated hs-cTnT within each obesity-physical activity cross-category (Supplemental Table 3). A test for an interaction in the association between elevated hs-cTnT and incident HF across the cross-categories was not significant (p for interaction =0.21). Among those with obesity and poor physical activity, the subgroup with the highest likelihood of myocardial damage, elevated hs-cTnT was associated with a 3-fold higher risk of HF compared to non-elevated hs-cTnT (HR 3.19; 95% CI: 2.28–4.47).
Figure 2.
Adjusted Incidence Rates for Incident HF Associated with Elevated and Non-Elevated hs-cTnT Within Each Cross-Category of Physical Activity and Obesity Status * p values for the incidence rate difference between elevated and non-elevated hs-cTnT within each obesity/physical activity cross-category are < 0.001 for all groups
Discussion
In this analysis of a bi-racial community-based sample of men and women without a history of CVD at baseline, we found that physical activity was inversely associated with chronic subclinical myocardial damage, as assessed by elevated hs-cTnT levels. This association was seen among individuals with and without obesity. Findings were consistent across demographic subgroups. When examining cross-categories of physical activity and obesity status, those with recommended physical activity and without obesity had the lowest likelihood of subclinical myocardial damage, while those with poor activity and obesity were most likely to have myocardial damage. Importantly, our results also suggest that recommended physical activity levels may lessen the association between obesity and myocardial damage. Furthermore, the presence of subclinical myocardial damage was significantly and similarly associated with incident HF within each cross-category of physical activity and obesity. The protective association of physical activity against subclinical myocardial damage may have implications for HF risk reduction, particularly among the high-risk group of individuals with excess weight.
Subclinical myocardial damage, as reflected by hs-cTnT is increasingly appreciated as a potent risk factor for the development of HF (6,7). In prior work, obesity has been linked to elevated hs-cTnT, and those individuals with both obesity and elevated hs-cTnT had a 9-fold higher risk of future HF than individuals with normal weight and undetectable hs-cTnT (8). While a prior study in an elderly population demonstrated an association between greater physical activity and lower hs-cTnT levels (17), there are limited data regarding how combinations of physical activity and obesity influence the likelihood of subclinical myocardial damage. Our study extends prior work by showing a graded inverse association between physical activity and hs-cTnT in a population that includes both middle-aged and elderly adults in the general community. Furthermore, we found a statistically significant interaction between physical activity and obesity on myocardial damage, indicating that this protective association may be stronger among individuals with obesity, a group at particularly high-risk for future HF.
In the current study, the association between higher physical activity and a lower likelihood of elevated hs-cTnT was seen even after accounting traditional CVD mediators but attenuated after adjustment additional predictors of HF including hsCRP. This suggests the associations of physical inactivity with myocardial damage may be related to pathways beyond the effects of physical activity on risk factor levels, which might include decreased inflammation (18). The mechanisms by which physical activity may lead to lower myocardial damage are incompletely understood. Laboratory studies have shown modulatory effects of physical activity on several myocellular defense mechanisms, with improved cardiac antioxidant capacity and mitochondrial respiratory function, ultimately leading to improved myocardial tolerance to noxious stimuli (19,20). Physical activity also has favorable effects on cardiac structure and function (21). Further investigation will be needed to understand whether these and other pathways mediate the association between physical inactivity and myocardial damage.
Clinical Implications
Our study has several important clinical implications. Given the high morbidity and mortality associated with HF, as well as its growing prevalence, there is an emerging focus on the early detection of high risk individuals such as those with obesity, who would most benefit from targeted prevention strategies (13). Given prior data demonstrating a potent association between obesity and incident HF that is unlikely to be addressed by solely controlling adiposity-associated cardiovascular risk factors such as hypertension, diabetes and dyslipidemia (22), promoting increased physical activity may be a particularly important strategy for HF risk reduction among individuals with obesity.
Additionally, a dose-response relationship between physical activity and myocardial damage was observed, with those individuals with AHA recommended levels of physical activity having lower likelihood of elevated hs-cTnT than those with poor physical activity. However, only 43% of the study population performed guideline-recommended levels of physical activity, and this number was even lower among individuals with obesity (33%). Given the strong association of subclinical myocardial damage with incident HF, further studies should explore whether hs-cTnT might be used as a marker of cardiovascular health in association with changes in physical activity. This could be particularly relevant among individuals with obesity, a group known to have higher levels of subclinical myocardial damage and increased HF risk.
Our study does have certain limitations. Usual physical activity levels were assessed using a questionnaire at a single point in time. Self-reported activity levels are likely associated with some misclassification and may not fully capture the cumulative effect of regular physical activity on myocardial damage. Furthermore, we did not have information on fitness in the ARIC study. Hs-cTnT was measured from stored blood samples and degradation over time may have modestly affected the absolute values of hs-cTnT; however, given the high correlations observed in prior validity studies (15), relative measures of association should remain unbiased. The non-concurrence of physical activity and hs-cTnT measurements is also a limitation and we cannot establish temporality of the observed associations of physical activity with myocardial damage in this study. As with all observational studies, we cannot exclude the presence of residual confounding. Additionally, we cannot exclude possible false positive findings resulting from multiple testing in our multivariable analyses. Nonetheless, our findings within a large, well-characterized, community based bi-racial cohort offer new insights regarding the inter-relationship of physical activity and obesity with myocardial damage that are likely broadly generalizable.
In summary, among middle-aged and older adults without a history of clinical CVD, physical activity was inversely associated with subclinical myocardial damage. Additionally, higher physical activity may lessen the association between obesity and subclinical myocardial damage. These results may have important implications for HF prevention, particularly in persons with obesity.
Clinical Perspectives.
Obesity and subclinical myocardial damage are important risk factors for heart failure. Physical activity is associated with lower risk of heart failure among non-obese and obese individuals, through unknown mechanisms. In this analysis of ARIC, we found that physical activity was inversely associated with subclinical myocardial damage. Additionally, higher physical activity may lessen the association between obesity and subclinical myocardial damage. Given the strong association of hs-cTnT and heart failure, the current study supports promotion of physical activity as a key strategy for heart failure prevention, particularly among high-risk subgroups such as persons with obesity.
Translational Outlook.
Physical activity was inversely associated with subclinical myocardial damage among non- obese and obese individuals, which could represent a mechanism by which physical activity reduces heart failure risk. Further research is needed to elucidate the mechanisms underlying the association of physical activity and heart failure.
Acknowledgments
Source of funding: This work was supported by a Robert Wood Johnson Amos Medical Faculty Development Award and an NIH/NHLBI grant (K23HL12247) awarded to Dr. Ndumele and by an NIH/NIDDK grant (R01DK089174) awarded to Dr. Selvin. Dr. Selvin was also supported by NIH/NIDDK grant K24DK106414.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
Abbreviations list
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- HsCRP
high sensitivity C-reactive protein
- Hs-cTnT
high sensitivity cardiac Troponin T
- MET
metabolic equivalents of task
- NT-proBNP
N-terminal pro-Brain Natriuretic Peptide
- SBP
systolic blood pressure
Footnotes
Disclosures: Drs. Selvin and Ballantyne have served on an advisory board for Roche Diagnostics. Drs. Ballantyne and Nambi along with Roche and Baylor College of Medicine have filed a provisional patent (patent #61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pandey A, Garg S, Khunger M, et al. Dose-Response Relationship Between Physical Activity and Risk of Heart Failure: A Meta-Analysis. Circulation. 2015;132:1786–94. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 2.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–44. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndumele CE, Coresh J, Lazo M, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart Fail. 2014;2:600–7. doi: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 10.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 11.Autenrieth CS, Evenson KR, Yatsuya H, Shahar E, Baggett C, Rosamond WD. Association between physical activity and risk of stroke subtypes: the atherosclerosis risk in communities study. Neuroepidemiology. 2013;40:109–16. doi: 10.1159/000342151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in Atherosclerosis Risk in Communities. Med Sci Sports Exerc. 2013;45:901–7. doi: 10.1249/MSS.0b013e31827d87ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal SK, Avery CL, Ballantyne CM, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin Chem. 2011;57:891–7. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.deFilippi CR, de Lemos JA, Tkaczuk AT, et al. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J Am Coll Cardiol. 2012;60:2539–47. doi: 10.1016/j.jacc.2012.08.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ertek S, Cicero A. Impact of physical activity on inflammation: effects on cardiovascular disease risk and other inflammatory conditions. Arch Med Sci. 2012;8:794–804. doi: 10.5114/aoms.2012.31614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ascensao A, Ferreira R, Magalhaes J. Exercise-induced cardioprotection-- biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol. 2007;117:16–30. doi: 10.1016/j.ijcard.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 20.Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–82. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 21.!!! INVALID CITATION !!! (28–30).
- 22.Ndumele CE, Matsushita K, Lazo M, et al. Obesity and Subtypes of Incident. doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]