Abstract
Many N,N-dialkylated tryptamines show psychoactive properties in humans and the number of derivatives involved in multidisciplinary areas of research has grown over the last few decades. Whereas some derivatives form the basis of a range of medicinal products, others are predominantly encountered as recreational drugs, and in some cases, the areas of therapeutic and recreational use can overlap. In recent years, 5-methoxy-N,N-diallyltryptamine (5-MeO-DALT) has appeared as a new psychoactive substance (NPS) and ‘research chemical’ whereas 4-acetoxy-DALT and the ring-unsubstituted DALT have only been detected very recently. Strategies pursued in the authors’ laboratories included the preparation and biological evaluation of previously unreported N,N-diallyltryptamines (DALTs). This report describes the analytical characterization of seventeen DALTs. Fifteen DALTs were prepared by a microwave-accelerated Speeter and Anthony procedure following established procedures developed previously in the authors’ laboratories. In addition to DALT, the substances included in this study were 2-phenyl-, 4-acetoxy-, 4-hydroxy-, 4,5-ethylenedioxy-, 5-methyl-, 5-methoxy-, 5-methoxy-2-methyl-, 5-ethoxy-, 5-fluoro-, 5-fluoro-2-methyl-, 5-chloro-, 5-bromo-, 5,6-methylenedioxy-, 6-fluoro-, 7-methyl, and 7-ethyl-DALT, respectively. The DALTs were characterized by nuclear magnetic resonance spectroscopy (NMR), gas chromatography (GC) quadrupole and ion trap (EI/CI) mass spectrometry (MS), low and high mass accuracy MS/MS, ultraviolet diode array detection and GC solid-state infrared analysis, respectively. A comprehensive collection of spectral data was obtained that are provided to research communities who face the challenge of encountering newly emerging substances where analytical data are not available. These data are also relevant to researchers who might wish to explore the clinical and non-clinical uses of these substances.
Keywords: New psychoactive substances, tryptamines, DALT, chemistry, forensic
Introduction
Many N,N-dialkylated tryptamine derivatives show psychoactive properties in humans.[1-5] Naturally occurring N,N-dimethyltryptamines, such as psilocybin, have been used for religious purposes since antiquity. Recently, N,N-dialkyltryptamines have also been the focus of attention due to increasing research efforts in clinically important areas [6-10] including potential treatment options for cluster headaches.[11-14] Non-medical and recreational use of both synthetic and naturally occurring derivatives and occurrences of untoward effects have been also been observed in recent years.[15,16]
One of the many potential and yet less explored substitution patterns is the synthetic N,N-diallyl substituted tryptamine template. The synthesis of the ring-unsubstituted tryptamine derivative N,N-diallyltryptamine (DALT) (1) (Figure 1) was first published in 1959[17] and indications about its psychoactive properties emerged in 1962, when it was briefly noted by Szára and Hearst.[18] In the following years, Szára mentioned a ‘psychotropic dose’ of 60 mg.[19] Subsequently, Alexander T. Shulgin synthesized both DALT (1) and 5-MeO-DALT (7) (A.T. Shulgin, personal communication). Although DALT (1) appeared to have few distinct effects at oral doses up to 42 mg[20] (80 mg has also been noted elsewhere[21,22]), 5-MeO-DALT (7) was found to produce short-lived, psychoactive effects at a dosage range of 12-20 mg. Remarkably, oral administration led to a comparatively fast onset of effects. Some of the information shared by Shulgin and Shulgin appeared on various Internet sites in 2004, which coincided with the marketing of 5-MeO-DALT (7) by chemical suppliers, presumably in response to data shared by Shulgin and Shulgin.[20,23-27] Reports linked to the detection of 5-MeO-DALT (7) began to surface in 2007[28-31] and continued to emerge until the present day. In recent years, 5-MeO-DALT (7) has been frequently discussed within the context of being a new psychoactive substance (NPS) where many of these substances are advertised as ‘research chemicals’ and available for purchase from Internet retailers or shops.[32,33] As far as the availability of analytical data are concerned, the majority of available reports describing the detection and characterization DALT derivatives focus on 5-MeO-DALT (7), reflecting its appearance in forensically related casework and/or from retail purchases[17,20,27,31,34-61] (Table 1).
Figure 1.
Structures of ring-substituted N,N-diallyltryptamines (1) – (17) characterized in this study.
Table 1.
Reports that describe the analysis of N,N-diallyltryptamines
| Compound a | Techniques b | Comment | Ref. |
|---|---|---|---|
| DALT (1) | Elemental analysis | Synthesis employing 3-(2-bromoethyl)indole and N,N-diallylamine | [17] |
| DALT (1) | GC-MS, IR | Synthesis employing N,N-dialkylation of tryptamine | [20] |
| 5-MeO-DALT (7) | GC-MS, IR | Synthesis employing N,N-dialkylation of 5-methoxytryptamine | [27] |
| 5-MeO-DALT (7) | GC-MS, LC-MS, LC-PDA | Analytical characterization | [31] |
| 5-MeO-DALT (7) c | GC-MS, IR, 1H NMR c | Synthesis employing N,N-dialkylation of 5-methoxytryptamine c | [34] |
|
d4-DALT (1) d4-5-MeO-DALT (7) |
1H and 13C NMR | Synthesis of deuterated standards via microwave-accelerated Speeter and Anthony procedure | [35] |
| 5-MeO-DALT (7) | GC-MS, LC-PDA | Five out of 29 tryptamine products purchased between April 2005 and March 2008 were found to contain 5-MeO-DALT (7). | [36] |
| 5-MeO-DALT (7) | LC-MECD | Analytical characterization | [37] |
| 5-MeO-DALT (7) c | LC-MS c | Analytical characterization c | [38] |
| 5-EtO-DALT (9) d4-5-EtO-DALT (9) |
GC-EI-IT-MS, GC-CI-IT-MS/MS, 1H and 13C NMR | Synthesis via microwave-accelerated Speeter and Anthony procedure | [39] |
| 5-MeO-DALT (7) c | LC-MS, LC-PDA c | Analytical characterization c | [40] |
| DALT (1) | LC-UV, 1H and 13C NMR, IR | Synthesis employing 3-(2-bromoethyl)indole and N,N-diallylamine | [41] |
| 5-MeO-2-Me-DALT (8) | GC-EI-IT-MS, GC-CI-IT-MS/MS, 1H and 13C NMR | Synthesis via microwave-accelerated Speeter and Anthony procedure | [42] |
| 5-MeO-DALT (7) | LC-DAD, LC-MS | Detection in postmortem femoral blood | [43] |
| 5-MeO-DALT (7) d | – d | Synthesis employing N,N-dialkylation of 5-methoxytryptamine d | [44] |
| 5-MeO-DALT (7) | 1H and 13C NMR, UV, IR | Analytical characterization and receptor binding assays | [45] |
| 5-MeO-DALT (7) | GC-MS, LC-MS, NMR, DART-TOF-MS | Liquid (n = 111) and powdered (n = 13) products purchased via the Internet between September 2009 and February 2012. Tryptamines including 5-MeO-DALT (7) detected in 31% of the samples. | [46] |
| 5-MeO-DALT (7) | GC-MS | Detection in herbal mixture also containing phenazepam and two synthetic cannabinoids | [47] |
| 5-MeO-DALT (7) | Presumptive color test | No reaction observed with sodium 1,2-naphthoquinone-4-sulphonate (NQS) | [48] |
| 5-MeO-DALT (7) | SRI-ToF-MS | Compounds derived from test purchases | [49] |
| 5-MeO-DALT (7) | GC-MS | Detection in herbal products (8 out of 75) containing synthetic cannabinoids collected between 2011 and June 2013. | [50] |
| 5-MeO-DALT (7) | LC-DAD, LC-MS/MS, LC-QTOF-MS/MS | One detection in casework in 2010 | [51] |
| 5-MeO-DALT (7) | Not reported | Case report featuring acute toxicity; no analytical confirmation | [52] |
| DALT (1) 2-Ph-DALT (2) 5-Me-DALT (6) 5-MeO-DALT (7) 5-MeO-2-Me-DALT (8) 5-EtO-DALT (9) d4-5-EtO-DALT (9) MD-DALT (14) 7-Me-DALT (16) 7-Et-DALT (17) 5-BnO-DALT e |
LC-LIT-MS | Method development and detection in human urine and plasma | [53] |
| 5-MeO-DALT (7) 5-MeO-DALT-TMS f |
GC-MS, LC-QqQ-MS, 1H NMR | Synthesis employing N,N-dialkylation of 5-methoxytryptamine and characterization. | [54] |
| 5-MeO-DALT (7) | LC-CLND | Analysis of seized samples collected between 2011 – 2013. | [55] |
| 5-MeO-DALT (7) | LC-DAD, LC-MS/MS, LC-QTOF-MS/MS | 5-MeO-DALT (7) remained stable for over 21 days in both blood and plasma stored at room temperature | [56] |
| 5-MeO-DALT (7) | GC-MS, LC-HR-MS | Detection in a seized sample | [57] |
| 5-MeO-DALT (7) | Portable NIR | Presumptive testing and application to forensic samples | [58] |
| DALT (1) d4-DALT (1) 5-MeO-DALT (7) |
GC-MS, LC-HR-MS, LC-LIT-MS | Identification of phase I and II metabolites in rat urine and in pooled human liver microsomes and initial CYP activty screening | [59] |
| 5-MeO-DALT (7) | DLLME, LC-MS/MS | Detection in spiked blood samples | [60] |
| DALT (1) ED-DALT (5) 5-Me-DALT (6) 5-MeO-DALT (7) 5-F-DALT (10) 5-F-2-Me-DALT (11) 5-Cl-DALT (12) 5-Br-DALT (13) MD-DALT (14) 6-F-DALT (15) 7-Me-DALT (16) |
LC-HR-MS, LC-QqQ-MS/MS | Cytochrome P450 inhibition assays and determination of in vivo CYP1A2 inhibition by 5-MeO-DALT (7) (caffeine as test substrate) | [61] |
Substances other than 5-MeO-DALT (7) have also been studied in a number of references cited in this table.
GC-MS: gas chromatography mass spectrometry: IR: infrared spectroscopy; NMR: nuclear magnetic resonance spectroscopy; LC: various forms of high performance liquid chromatography; PDA: photo diode array detection; MECD: multi-channel electrochemical detection; GC-EI-IT-MS: GC electron ionization ion t rap mass spectrometry; GC-CI-IT-MS/MS: GC chemical ionization ion trap tandem mass spectrometry; UV: ultraviolet spectroscopy; DART: direct analysis in real time; TOF: time-of-flight; SRI: selective reagent ionization; QTOF: quadrupole time-of-flight; HR-MS: high-resolution MS; LIT: linear ion trap; QqQ: triple quadrupole; CLND: chemiluminescence nitrogen detection; NIR: near infrared spectroscopy; DLLME: dispersive liquid/liquid microextraction.
Reference in abstract form [written in Japanese] via SciFinder®.
Patent source written in Chinese.
5-BnO-DALT: 5-benzyloxy-DALT; not included in the present study.
TMS: Trimethylsilyl derivative.
Strategies pursued in the authors’ laboratories include the preparation of previously unreported DALT analogs in order to make available the analytical data to researchers who encounter these types of new psychoactive substances (NPS) and ‘research chemicals’. Furthermore, the psychoactive properties associated with a range of N,N-dialkylated tryptamines make the DALT compounds a desirable target for pharmacological and pharmacokinetic investigations as reported recently for some of the compounds characterized in the present study.[59,61-63] In addition to 5-MeO-DALT (7),[30] the two additional DALT analogs 4-AcO-DALT (3)[64] and DALT (1),[65] also described in the present study, have been detected by the European Early-Warning System and reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCCDA), which suggested that the development of new DALT analogs might be a likely prospect within the ‘research chemical’ context.
Research communities confronted with the NPS phenomenon face a number of challenges, which include the lack of analytical data when dealing with newly emerging substances. The present study addresses the need for providing a comprehensive collection of analytical data for DALT analogs (1) – (17) (Figure 1). The majority of spectral data described in this report are described for the first time. Synthesized compounds were characterized by nuclear magnetic resonance spectroscopy (NMR), gas chromatography (GC) quadrupole and ion trap (EI/CI) mass spectrometry (MS), and low and high mass accuracy MS/MS, ultraviolet diode array detection and GC solid-state infrared analysis, respectively.
Experimental
Materials
4-AcO-DALT (3) and 4-OH-DALT (4), sold as the fumarate salt, were from Scientific Supplies (London, UK). 5-MeO-2-Me-DALT HCl (8) and 5-EtO-DALT HCl (9) were available from previous studies.[39,42]
The synthesis of N,N-diallyltryptamines (DALTs) reported in this study adopted the well-established procedure of Speeter and Anthony.[66] As summarized in the Supporting Information, the substituted indole starting material (a) was acylated to give the acid chloride intermediate (b) followed by amination with N,N-diallylamine to the yield glyoxalylamide (c). The reduction with lithium aluminum hydride provided the DALT analogs. The reduction of the corresponding glyoxalylamide (c) (0.3 mmol) was carried out under microwave-accelerated conditions as described in detail by the authors previously.[35,39,42] High accuracy electrospray ionization mass spectra of the protonated molecules and their key product ions are summarized in Table 3. All 1H and 13C NMR data for intermediates (c) and DALTs (1) – (17) are presented as Supporting Information.
Table 3.
Positive electrospray high-resolution MS and MS/MS data
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [M+H]+ |
|
|
||||||||||
| No. | R | R’ | Formula | Theor. | Found | Δ (ppm) | Formula | Theor. | Found | Δ (ppm) | Found | Δ (ppm) |
| 1 | H | H | C16H21N2+ | 241.16993 | 241.16978 | −0.58 | C10H10N+ | 144.08078 | 144.08072 | −0.39 | 110.09638 | −0.40 |
| 2 | H | Ph | C22H25N2+ | 317.20123 | – | – | C16H14N+ | 220.11208 | 220.11179 | −1.32 | 110.09634 | −0.81 |
| 3 | 4-AcO | H | C18H23N2O2+ | 299.17540 | 229.17523 | −0.58 | C12H12NO2+ | 202.08626 | 202.08627 | 0.09 | 110.09646 | 0.29 |
| 4 | 4-OH | H | C16H21N2O+ | 257.16484 | 257.16479 | −0.17 | C10H10NO+ | 160.07569 | 160.07588 | 1.20 | 110.09648 | 0.50 |
| 5 | 4,5-ED a | H | C18H23N2O2+ | 299.17540 | 299.17459 | −2.72 | C12H12NO2+ | 202.08626 | 202.08606 | −0.97 | 110.09640 | −0.26 |
| 6 | 5-CH3 | H | C17H23N2+ | 255.18558 | 255.18533 | −0.95 | C11H12N2+ | 158.09643 | 158.09634 | −0.52 | 110.09637 | −0.54 |
| 7 | 5-OCH3 | H | C17H23N2O+ | 271.18049 | 271.17999 | −1.83 | C11H12NO+ | 174.09134 | 174.09113 | −1.24 | 110.09633 | −0.88 |
| 8 | 5-OCH3 | CH3 | C18H25N2O+ | 285.19614 | 285.19565 | −1.72 | C12H14NO+ | 188.10699 | 188.10666 | −1.76 | 110.09628 | −1.30 |
| 9 | 5-OC2H5 | H | C18H25N2O+ | 285.19614 | 285.19574 | −1.40 | C12H14NO+ | 188.10699 | 188.10678 | −1.11 | 110.09631 | −1.09 |
| 10 | 5-F | H | C16H20FN2+ | 259.16050 | 259.16010 | −1.57 | C10H9FN+ | 162.07135 | 162.07117 | −1.15 | 110.09633 | −0.88 |
| 11 | 5-F | CH3 | C17H22FN2+ | 273.17615 | 273.17578 | −1.36 | C11H11FN+ | 176.08700 | 176.08679 | −1.20 | 110.09633 | −0.88 |
| 12 | 5-Cl | H | C16H2035ClN2+ C16H2037ClN2+ |
275.13095 277.12800 |
275.13092/277.12759 | −0.12 −1.48 |
C10H935ClN+ | 178.04180 -- |
178.04187 -- |
0.44 -- |
110.09645 | 0.23 |
| 13 | 5-Br | H | C16H2079BrN2+ C16H2081BrN2+ |
319.08044 321.07839 |
319.07999/321.07779 | −1.41 −1.87 |
C10H979BrN+ -- |
221.99129 -- |
221.99121 -- |
−0.35 -- |
110.09642 | −0.05 |
| 14 | 5,6-MD b | H | C17H21N2O2+ | 285.15975 | 285.15918 | −2.02 | C11H10NO2+ | 188.07061 | 188.07062 | −0.07 | 110.09638 | −0.40 |
| 15 | 6-F | H | C16H20FN2+ | 259.16050 | 259.16013 | −1.46 | C10H9FN+ | 162.07135 | 162.07117 | −1.26 | 110.09628 | −1.30 |
| 16 | 7- CH3 | H | C17H23N2+ | 255.18558 | 255.18524 | −1.31 | C11H12N+ | 158.09643 | 158.09621 | −1.38 | 110.09631 | −1.09 |
| 17 | 7- C2H5 | H | C18H25N2+ | 269.20123 | 269.20068 | −2.01 | C12H14N+ | 172.11208 | 172.11194 | −0.80 | 110.09637 | −0.47 |
4,5-(OCH2CH2O): 4,5-ethylenedioxy
5,6-(OCH2O): 5,6-methylenedioxy.
Instrumentation
Gas chromatography-mass spectrometry (GC-MS)
Electron ionization (EI) mass spectra (70 eV) were recorded using a Finnigan TSQ 7000 triple stage quadrupole mass spectrometer coupled to a gas chromatograph (Trace GC Ultra, Thermo Electron) using a CTC CombiPAL (CTC Analytics, Switzerland) autosampler. The emission current was 200 μA and the scan time was 1 s spanning a scan range between m/z 29 – m/z 600. The ion source temperature was maintained at 175 °C. Samples were introduced via GC with splitless injection using a fused silica capillary DB-1 column (30 m × 0.25 mm, film thickness 0.25 μm). The temperature program consisted of an initial temperature of 80 °C, held for 1 min, followed by a ramp to 280 °C at 15 °C/min. The final temperature was held for 21 min. The injector temperature was 220 °C. The transfer line temperature was maintained at 280 °C and the carrier gas was helium in constant flow mode at a flow rate of 1.0 mL/min. Approximately 2 mg were dissolved in 1.5 mL methanol. For analysis, 1 μL sample solutions were injected into the GC-MS system.
Gas chromatography solid-state infrared analysis (GC-sIR)
The methanolic solution was measured on a GC-solid phase-IR-system consisting of an Agilent GC 7890B (Waldbronn, Germany) with probe sampler Agilent G4567A and a DiscovIR-GC™ (Spectra Analysis, Marlborough, Massachusetts, USA). The column eluent was cryogenically accumulated on a rotating ZnSe disk that was cooled by liquid nitrogen. The IR spectra were directly recorded through the IR-transparent ZnSe disk using a nitrogen cooled MCT detector. GC parameters: the injection was carried out in splitless mode with an injection port temperature of 240 °C and a DB-1 fused silica capillary column (30 m × 0.32 mm i.d., 0.25 μm film thickness). The carrier gas was helium with a flow rate of 2.5 mL/min; oven temperature program: 80 °C for 2 min, ramped to 290 °C at 20 °C/min, and held at the final temperature for 25 min. The transfer line heater was set at 280 °C. IR conditions: oven temperature, restrictor temperature, disc temperature, and Dewar cap temperatures were 280 °C, 280°C, −40 °C, and 35 °C, respectively. The vacuum was 0.2 mTorr, disc speed 3 mm/s, spiral separation was 1 mm, wavelength resolution 4 cm−1 and IR range 650–4000 cm−1. Acquisition time was 6s/file with 64 scans/spectrum. Data were processed using GRAMS/AI Ver. 9.1 (Grams Spectroscopy Software Suite, Thermo Fischer Scientific) followed by implementation of the OMNIC Software, Ver. 7.4.127 (Thermo Electron Corporation).
High-resolution electrospray ionization mass spectrometry (HR-ESI-MS)
High-resolution mass spectral and MS/MS analyses were performed on an LTQ/Orbitrap™ Discovery mass spectrometer (Thermo Scientific, Bremen, Germany). This hybrid system consists of a linear ion trap (LTQ™) coupled to an Orbitrap™ Fourier transform mass spectrometer for accurate mass measurements. Samples were dissolved in acetonitrile/water (1:1, containing 0.1% formic acid) and infused at a rate of 5 μL/min. Measured accurate masses were within ± 5 ppm of the theoretical masses. The following conditions were used: drying gas (N2) 10 L/min, capillary temperature 310 °C, spray voltage 4 V, capillary voltage, 22 V and tube lens 77 V. A normalized collision energy™ (NCE) of 45% (of a maximum of 5 eV) was used for CID. Full scan high-resolution (30,000 FWHM) spectra (m/z 75 – 400) were acquired in positive electrospray ionization (ESI) mode. Mass calibrations were performed using solutions of caffeine, L-methionyl-arginyl-phenylalanylalanine acetate × H20 (MRFA), Ultramark 1621®, sodium docecyl sulfate and sodium taurocholate.
Diode array detection (DAD)
HPLC-DAD analyses were performed on an Agilent 1200 HPLC system equipped with the following modules: G1312B BinPump SL, G13798 degasser, G1367D HiP ALS SL plus autosampler, a G1316B column compartment (set at 35 °C), and a G1315C diode array detector (Agilent, Waldbronn, Germany). Data acquisition rate was 80 Hz with the scan rate set between 210 – 400 nm (spectrum step 1 nm). The injection volume was 10 μL (0.1 mg/mL analyte solution). A Synergi Max-RP (250 × 4.6 mm, 4 μm) column from Phenomenex (Macclesfield, United Kingdom) was used and analytes were eluted under gradient conditions. Mobile phase A consisted of 25 mM triethylammonium phosphate (TEAP) buffer solution and mobile phase B comprised of 70% acetonitrile and 30% water containing 25 mM TEAP. The gradient elution commenced at 70% A and decreased to 5% within 5 min. This was then held until 12 min and returned to initial conditions by a 5 min post time.
Liquid chromatography-mass spectrometry (LC-MS)
LC-MS analyses were performed on an Agilent 1100 HPLC system equipped with a G13795 degasser, G1312A BinPump, a G1313A ALS and G1316A column oven (COLCOM) (Agilent, Little Island, Cork, Ireland). Separation was obtained on a Kinetex phenyl-hexyl column (2.6 μm, 100 × 2.10 mm) Phenomenex (Macclesfield, Cheshire, United Kingdom). The analytes were eluted under isocratic conditions using amobile phase of 97%water and 3%acetonitrile (both containing 0.1% formic acid). The Agilent single quadrupole MSD settings were as follows: positive electrospray mode, capillary voltage 3500 V, drying gas (N2) 12 L/min at 350 °C, and nebulizer gas (N2) pressure 50 psi. In-source collision-induced dissociation experiments were carried out with an increased fragmentor voltage of 110 V. Samples were dissolved in acetonitrile/water (1:1, containing 0.1% formic acid) at a concentration of 10 μg/mL. The injection volume was 0.5 μL, flow rate was 0.4 mL/min and the column temperature was set at 30 °C. Total run time was 25 min.
Results and discussion
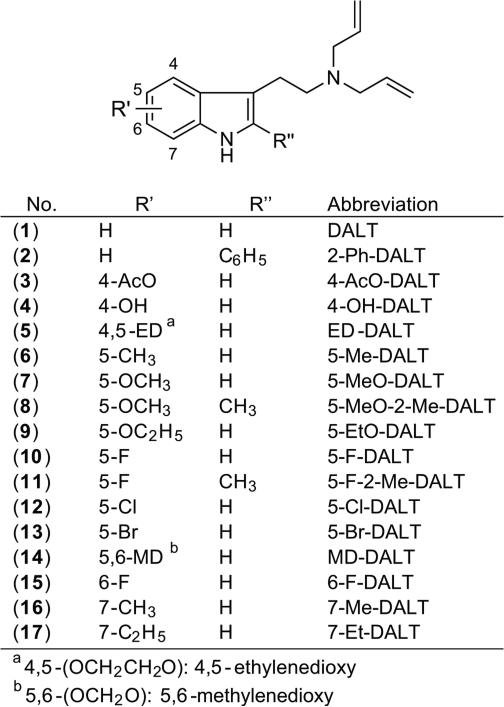
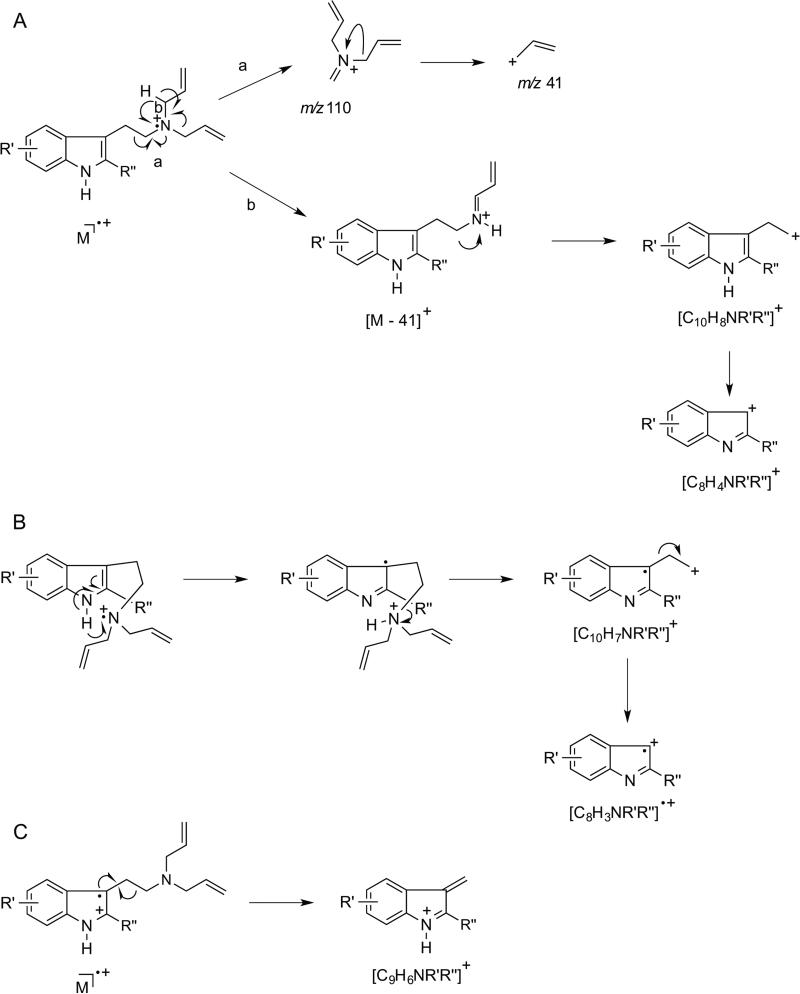
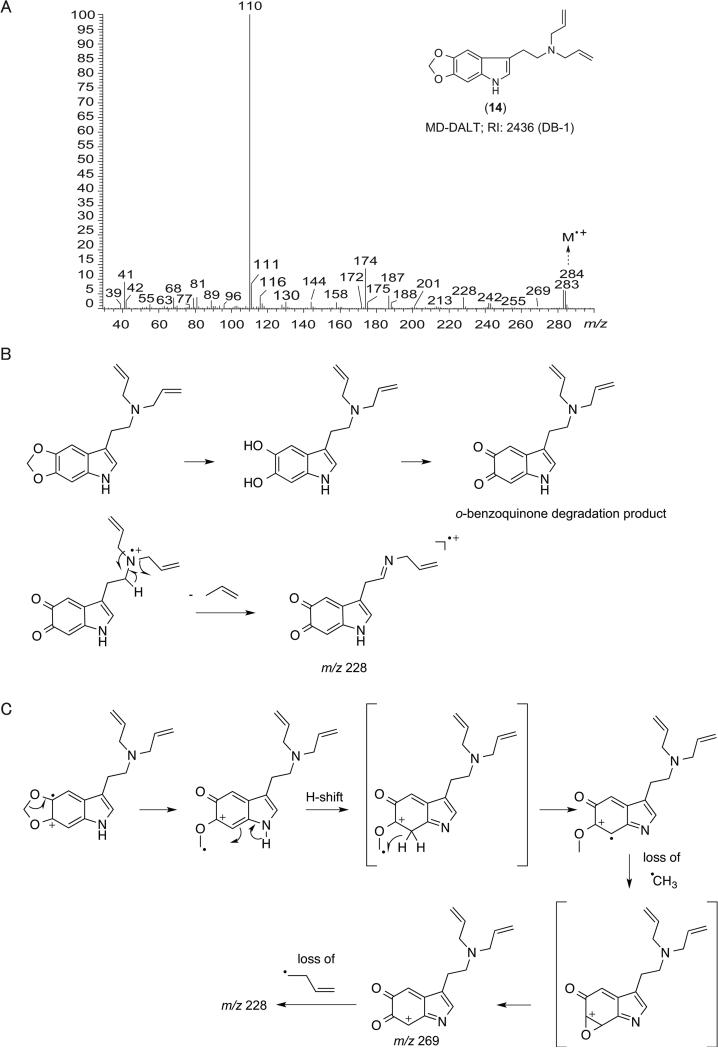
Electron ionization (EI) quadrupole mass spectra recorded for DALTs (1) – (17) featured a fragmentation behavior similar to other N,N-dialkyltryptamines[67,68] and a generalized scheme is proposed in Figure 2 to encompass the key features. The associated main fragments are summarized in Table 2 and all EI quadrupole mass spectra are provided as Supporting Information. Consistent with previously published reports about 5-MeO-DALT (7) and some of the other DALT analogs (Table 1), base peak formation of the iminium ion was observed at m/z 110 via alpha-cleavage (Figure 2A, pathway ‘a’). The allyl ion at m/z 41 was also frequently observed, possibly formed by secondary fragmentation of m/z 110 or directly from the ionized allyl double bond in the intact molecular ion. An [M – 41]+ species, albeit occasionally low in relative abundance, is proposed to fragment further into [C10H8NR’R”]+ and [C8H4NR’R”]+, respectively (Figure 2A, pathway ‘b’). Formation of [C10H7NR’R”]+ and [C8H3NR’R”]+ ions might have followed the proposed mechanism shown in Figure 2B whereas detection of [C9H6NR’R”]+ might have resulted from alpha-cleavage instigated from ionization of C2 – C3 double bond of the indole ring (Figure 2C). Implementation of GC ion trap mass spectrometry showed some variations, for example, in relative abundance of fragment ions when compared those formed under quadrupole mass spectrometry conditions, which might have reflected the tendency to display self-ionization phenomena within the ion trap. For completeness, GC ion trap MS (GC-IT-MS) data using both EI and chemical ionization methods have been included as Supporting Information. Interestingly, an ion at m/z 228 was observed in the EI mass spectrum of MD-DALT (14) (Table 2, Figure 3A) and it was speculated that the associated loss of 56 amu from the molecular ion might have arisen from mechanisms proposed in Figure 3B and 3C. In the first scenario, MD-DALT (14) would have been required to degrade into an ortho-benzoquinone derivative before being subjected to a neutral loss off propene (Figure 3B). An alternative pathway might have involved an H-shift and rearrangement, possibly including an epoxide and benzoquinone intermediate. A subsequent loss of a methyl radical followed by a loss of an allyl radical could have accounted for m/z 228 (Figure 3C).
Figure 2.
Proposed, generalized mass spectral fragmentation pathways for (1) – (17) recorded under electron ionization conditions (see also Table 2).
Table 2.
Electron ionization mass spectral data
| No. | R′ | R″ | M.+ | [M-41]+ | [C9H6NR′R″]+ | [C10H8NR′R″]+, [C10H7NR′R″].+ | [C8H4NR′R″]+, [C8H3NR′R″].+ | Other fragments |
|---|---|---|---|---|---|---|---|---|
| 1 | H | H | 240 | 199 | 130 | 144, 143 | 116, 115 | -- |
| 2 | H | Ph | 316 | 275 | 206 | 220, 219 | 192, 191 | -- |
| 3 | 4-AcO | H | 298 | 257 | 188 | 202, 201 | 174, 173 | 256 (M - C2H2O), 239 (M - .OAc), 160 (188 - CO), 146 (188 - C2H2O) |
| 4 | 4-OH | H | 256 | 215 | 146 | 160, 159 | 132, 131 | -- |
| 5 | 4,5-ED a | H | 298 | 257 | 188 | 202, 201 | 176, 175 | 160 (188 - C2H4), 132 (160 - CO) |
| 6 | 5-CH3 | H | 254 | 213 | 144 | 158, 157 | 130, 129 | -- |
| 7 | 5-OCH3 | H | 270 | 239 | 160 | 174, 173 | 146, 145 | 239 (M - .OCH3), 145 (160 - .CH3), 240 (M - CH2O), 130 (160 - CH2O), 117 (145 - CO) |
| 8 | 5-OCH3 | CH3 | 284 | 243 | 174 | 188, 187 | 160, 159 | 253 (M - .OCH3), 159 (174 - .CH3), 144 (174 - CH2O), 131 (159 - CO) |
| 9 | 5-OC2H5 | H | 284 | 241 | 174 | 188, 187 | 160, 159 | 269 (M - .CH3), 239 (M - C2H5O.), 146 (174 - C2H4) |
| 10 | 5-F | H | 258 | 217 | 148 | 162, 161 | 134, 133 | -- |
| 11 | 5-F | CH3 | 272 | 231 | 162 | 176, 175 | 148, 147 | -- |
| 12 | 5-Cl | H | 274/276 | 233/235 | 164/166 | 178/180, 177/179 | 150/152, 149/147 | 239 (M - Cl.) |
| 13 | 5-Br | H | 318/320 | 277/279 | 208/210 | 222/220, 221/223 | 194/192, 193/191 | 239 (M - Br.) |
| 14 | 5,6-MD b | H | 284 | 243 | 174 | 188, 187 | 160, 159 | 228, 144 (174 - CH2O) |
| 15 | 6-F | H | 258 | 217 | 148 | 162, 161 | 134, 133 | -- |
| 16 | 7-CH3 | H | 254 | 213 | 144 | 158, 157 | 130, 129 | -- |
| 17 | 7-C2H5 | H | 268 | 227 | 158 | 172, 171 | 144, 143 | 239 (M - C2H5.) |
4,5-(OCH2CH2O): 4,5-ethylenedioxy
5,6-(OCH2O): 5,6-methylenedioxy.
Figure 3.
A: Electron ionization mass spectrum recorded for MD-DALT (14). B and C: Two proposed alternative mass spectral fragmentation pathways that may account for the formation of m/z 228.
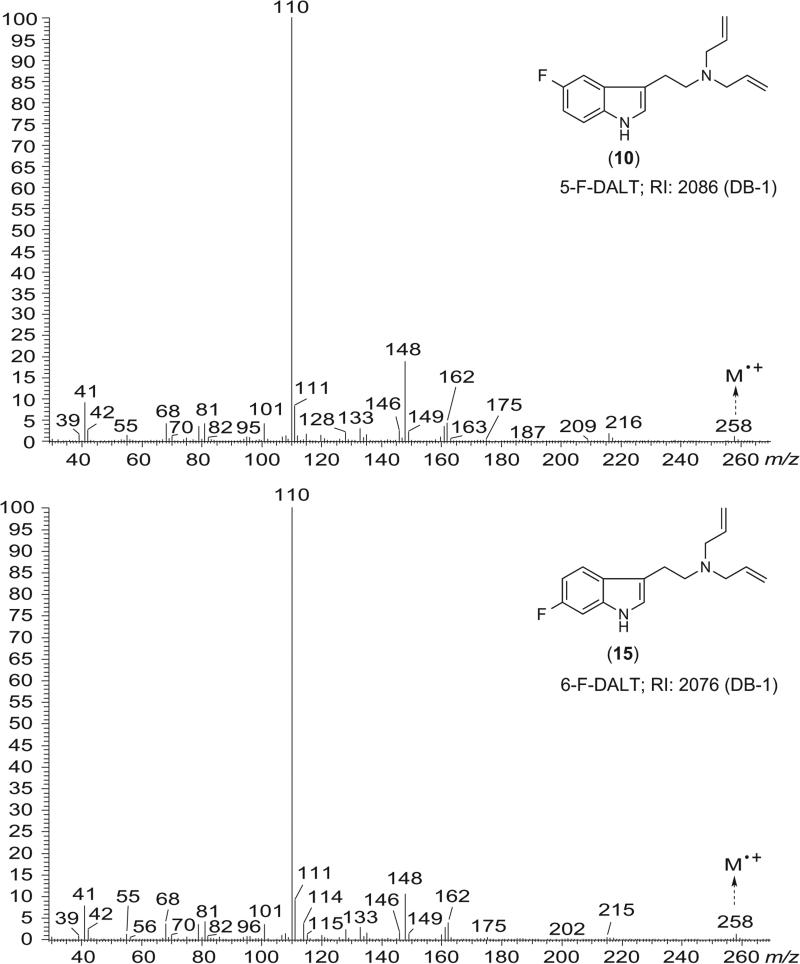
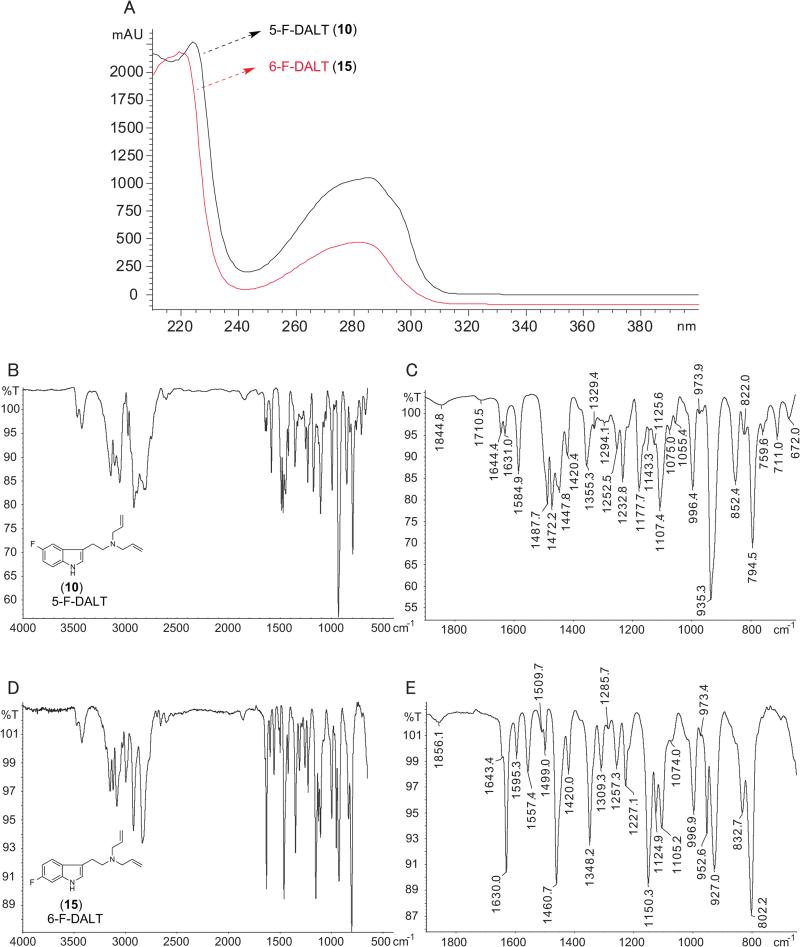
One of the challenges that might be encountered when identifying a new psychoactive substance on the market includes the consideration of potential isomers,[69,70] especially if not all of these substances are commercially available for analytical comparisons. From this perspective, it has become increasingly helpful to consider supporting implementation of analytical procedures by synthesis of the isomers of interest to support the verification process.[71-74] Figure 4 shows the similarity of EI mass spectra recorded for 5-F-DALT (10) and 6-F-DALT (15). As expected, these similarities precluded unambiguous identification and although the GC retention index values were slightly dissimilar under the conditions used but not sufficient for unambiguous identification without having both standards (see also supplemental GC-IT-MS data). An inspection of the photodiode array spectra obtained for both substances revealed only minor differences of potential significance, such as distinct shoulders at lower wavelengths between 200 nm and 230 nm (Figure 5A). The photo diode array spectra (PDA) of all DALTs are given as Supporting Information. The recorded PDA spectrum of 5-MeO-DALT (7) was consistent with one reported in the literature.[31]
Figure 4.
Electron ionization mass spectra recorded for the two isomers 5-F-DALT (10) and 6-F-DALT (15).
Figure 5.
A. Implementation of photodiode array detection for both isomers 5-F-DALT (10) and 6-F-DALT (15). B and C: Gas chromatography solid-state infrared data recorded for (10). D and E: Gas chromatography solid-state infrared data recorded for (15). Isomers (10) and (15) could be differentiated due to differences observed in the partial spectra C and E.
The most promising data was obtained from GC solid-state infrared analysis (GC-sIR) as shown in Figure 5B – 5E. The expanded spectral regions shown in Figure 5C (5-F-DALT (10)) and Figure 4E (6-F-DALT (15)) confirmed that distinct differences could be noted between the two positional isomers. In case of 6-F-DALT (15), the more prominent differences included the sharp signals at wavenumbers 1630.0, 1460.7 1348.2, 1150.3 and 802.2 cm−1, respectively. The advantage of employing a GC-sIR procedure was the formation of solid and amorphous free base material following elution of the analyte from the GC column. Consequently, IR spectra obtained from this procedure are comparable to standard spectra of the free bases recorded from attenuated total reflectance IR devices and are especially useful in identification of the correct isomers in analysis of mixtures. The GC-sIR spectra of all DALTs (1) – (17) are provided as Supplementary Information.
Positive electrospray (ES+) high-resolution MS and MS/MS data for all DALTs are summarized in Table 3 and, consistent with literature reported previously (Table 1), two key product ions were detected. The detected product ions were comparable to those observed when exposing the DALTs to analysis by LC positive mode single quadrupole mass spectrometry and in-source collision-induced dissociation. All mass spectra have been provided as Supporting Information.
The present study provided a comprehensive set of spectral data obtained from seventeen N,N-diallyltryptamines (DALTs) in order to facilitate the identification and exploration of these newly emerging substances. Another challenge frequently encountered with new psychoactive substances is the lack of information regarding their biological properties, which triggered a first set of studies carried out in the authors’ laboratories in the areas of metabolism[59] and cytochrome P450 inhibition.[61] Receptor binding data for 5-MeO-DALT (7)[45] and additional DALTs substituted at the 5-position have also appeared recently.[63] The fact that a range of closely related N,N-dialkylated tryptamines (e.g. sumatriptan) show important therapeutic applications demonstrates that there is a need to disentangle the different pharmacological features associated with the tryptamine template. [5,75-81] Anecdotal reports suggest that 5-MeO-DALT (7) might provide relief from cluster headaches.[82] However, the extent to which this might apply to this particular compound or other DALTs warrants further investigation.
Conclusion
The new psychoactive substances phenomenon is an area of investigation that attracts attention from multi-disciplinary stakeholders who face the challenge of keeping up-to-date with newly emerging substances where few data are available that aid their identification. The characterization of seventeen N,N-diallyltryptamines yielded a comprehensive set of analytical data that were collected to serve these research communities that are involved with the study of psychoactive substances including both clinical and non-clinical applications.
Supplementary Material
Acknowledgements
Dr. Halberstadt is supported by awards from NIMH (K01 MH100644) and NIDA (R01 DA002925).
References
- 1.Hoffer A, Osmond H. The Hallucinogens. Academic Press, Inc.; Orlando, Florida: 1967. [Google Scholar]
- 2.Brimblecombe RW, Pinder RM. Hallucinogenic Agents. Wright-Scientechnica; Bristol, England: 1975. [Google Scholar]
- 3.Jacobs BL, editor. Hallucinogens: Neurochemical, Behavioral and Clinical Perspectives. Raven Press; New York: 1984. [Google Scholar]
- 4.Shulgin A, Shulgin A. TIHKAL: The Continuation. Transform Press; Berkeley: 1997. [Google Scholar]
- 5.Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 2015;277:99. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiat. 2011;68:71. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- 7.Bogenschutz MP, Pommy JM. Therapeutic mechanisms of classic hallucinogens in the treatment of addictions: from indirect evidence to testable hypotheses. Drug Test. Anal. 2012;4:543. doi: 10.1002/dta.1376. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J. Psychopharmacol. 2014;28:983. doi: 10.1177/0269881114548296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J. Psychopharmacol. 2015;29:289. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- 10.Kraehenmann R, Preller KH, Scheidegger M, Pokorny T, Bosch OG, Seifritz E, Vollenweider FX. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol. Psychiatry. 2015;78:572. doi: 10.1016/j.biopsych.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Sewell RA, Halpern JH, Harrison GP. Response of cluster headache to psilocybin and LSD. Neurology. 2006;66:1920. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- 12.Sewell RA, Halpern JH. Response of cluster headache to psilocybin and LSD. In: Winkelman MJ, Roberts TB, editors. Psychedelic Medicine: New Evidence for Hallucinogenic Substances as Treatments. Vol. 1. Praeger; Westport, CT, USA: 2007. p. 97. [Google Scholar]
- 13.Di Lorenzo C, Coppola G, Di Lorenzo G, Bracaglia M, Rossi P, Pierelli F. The use of illicit drugs as self-medication in the treatment of cluster headache: results from an Italian online survey. Cephalalgia. 2015 doi: 10.1177/0333102415583145. DOI: 10.1177/0333102415583145. [DOI] [PubMed] [Google Scholar]
- 14.Schindler EA, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA. Indoleamine hallucinogens in cluster headache: results of the Clusterbusters medication use survey. J. Psychoactive Drugs. 2015;47:372. doi: 10.1080/02791072.2015.1107664. [DOI] [PubMed] [Google Scholar]
- 15.Araújo AM, Carvalho F, Bastos MD, de Pinho PG, Carvalho M. The hallucinogenic world of tryptamines: an updated review. Arch. Toxicol. 2015;89:1151. doi: 10.1007/s00204-015-1513-x. [DOI] [PubMed] [Google Scholar]
- 16.Tittarelli R, Mannocchi G, Pantano F, Romolo FS. Recreational use, analysis and toxicity of tryptamines. Curr. Neuropharmacol. 2015;13:26. doi: 10.2174/1570159X13666141210222409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitali T, Mossini F. Sulla preparazione di alcune triptamine N'-disostituite. Boll. Sci. Fac. Chim. Ind. Bologna. 1959;17:84. [Google Scholar]
- 18.Szara S, Hearst E. The 6-hydroxylation of tryptamine derivatives: a way of producing psychoactive metabolites. Ann. N. Y. Acad. Sci. 1962;96:134. [Google Scholar]
- 19.Szara S. DMT (N,N-dimethyltryptamine) and homologues: clinical and pharmacological considerations. In: Efron DH, editor. Psychotomimetic Drugs. Raven Press; New York: 1970. p. 275. [Google Scholar]
- 20. TIHKAL Info. # 57. DALT. Available at: https://isomerdesign.com/PiHKAL/read.php?domain=tk&id=57 [28 December 2015]
- 21.Jacob III P, Shulgin AT. Struktur-Wirkungs-Beziehungen der klassischen Halluzinogene und ihrer Analoga. In: Dittrich A, Hofmann A, Leuner H, editors. Welten des Bewußtseins. Band 1. Ein interdisziplinärer Dialog. Verlag für Wissenschaft und Bildung; Berlin: 1993. p. 83. [Google Scholar]
- 22.Shulgin AT. Basic pharmacology and effects. In: Laing RR, editor. Hallucinogens. A Forensic Drug Handbook. Elsevier Science Ltd.; London: 2003. p. 67. [Google Scholar]
- 23. The Big & Dandy 5-MeO-DALT Thread (26 June 2004). Available at: http://www.bluelight.org/vb/threads/146249-The-Big-amp-Dandy-5-MeO-DALT-Thread [28 December 2015]
- 24. 5-MeO-DALT. Tryptamines reloaded (16 July 2004). De Hollandse Psychonaut > Drugs > Psychedelica. Available at: https://www.dhpforum.nl/forums/index.php?/topic/826-5-meo-dalt/ [28 December 2015]
- 25. 5-MeO-DALT. General discourse. The Hive archive. (05 July 2004). Available at: https://the-hive.archive.erowid.org/forum/showflat.pl?static=1&Cat=&Number=517583 [28 December 2015]
- 26.Shulgin A, Shulgin A, 5-MeO-DALT Entry from a forthcoming book. 2004 Available at: https://www.erowid.org/chemicals/5meo_dalt/5meo_dalt_info1.shtml [20 December 2015]
- 27. TIHKAL Info. # 56. 5-MeO-DALT. Available at: https://isomerdesign.com/PiHKAL/read.php?domain=tk&id=56 [28 December 2015]
- 28.Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007;559:132. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 29.Nonaka R, Nagai F, Ogata A, Satoh K. In vitro screening of psychoactive drugs by [35S]GTPγS binding in rat brain membranes. Biol. Pharm. Bull. 2007;30:2328. doi: 10.1248/bpb.30.2328. [DOI] [PubMed] [Google Scholar]
- 30.EMCDDA–Europol 2007 Annual Report on the implementation of Council Decision 2005/387/JHA In accordance with Article 10 of Council Decision 2005/387/JHA on information exchange, risk assessment and control of new psychoactive substances. EMCDDA; Lisbon: 2008. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_132894_EN_2007_Implementationreport.pdf [20 December 2015] [Google Scholar]
- 31.Kikura-Hanajiri R, Kawamura M, Uchiyama N, Ogata J, Kamakura H, Saisho K, Goda Y. Analytical data of designated substances (Shitei-Yakubutsu) controlled by the pharmaceutical affairs law in Japan, part I: GC MS and LC-MS. Yakugaku Zasshi. 2008;128:971. doi: 10.1248/yakushi.128.971. [DOI] [PubMed] [Google Scholar]
- 32.King LA, Kicman AT. A brief history of ‘new psychoactive substances’. Drug Test. Anal. 2011;3:401. doi: 10.1002/dta.319. [DOI] [PubMed] [Google Scholar]
- 33.Brandt SD, King LA, Evans-Brown M. The new drug phenomenon. Drug Test. Anal. 2014;6:587. doi: 10.1002/dta.1686. [DOI] [PubMed] [Google Scholar]
- 34.Zhao HW, Hong YY, Liu CC. Synthesis of N,N-diallyl-5-methoxytryptamine. Huaxue Shiji. 2006;28:307. [Google Scholar]
- 35.Brandt SD, Tirunarayanapuram SS, Freeman S, Dempster N, Barker SA, Daley PF, Cozzi NV, Martins CPB. Microwave-accelerated synthesis of psychoactive deuterated N,N-dialkylated-[α,α,β,β-D4]-tryptamines. J. Label. Compd. Radiopharm. 2008;51:423. [Google Scholar]
- 36.Takahashi M, Nagashima M, Suzuki J, Seto T, Yasuda I, Yoshida T. Creation and application of psychoactive designer drugs data library using liquid chromatography with photodiode array spectrophotometry detector and gas chromatography-mass spectrometry. Talanta. 2009;77:1245. doi: 10.1016/j.talanta.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 37.Min JZ, Yamashita K, Toyo'oka T, Inagaki S, Higashi T, Kikura-Hanajiri R, Goda Y. Simultaneous and group determination methods for designated substances by HPLC with multi-channel electrochemical detection and their application to real samples. Biomed. Chromatogr. 2010;24:1287. doi: 10.1002/bmc.1439. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M, Sakurai K, Watabe K, Kikura-Hanajiri R, Goda Y. Establishment of LC/MS library for screening analysis on “non-approved or unauthorized pharmaceuticals” and “designated substances”. Iyakuhin Iryo Kiki Regyuratori Saiensu. 2010;41:742. [Google Scholar]
- 39.Tearavarich R, Hahnvajanawong V, Dempster N, Daley PF, Cozzi NV, Brandt SD. Microwave-accelerated preparation and analytical characterization of 5-ethoxy-N,N-dialkyl-[α,α,β,β-H4]- and [α,α,β,β-D4]-tryptamines. Drug Test. Anal. 2011;3:597. doi: 10.1002/dta.223. [DOI] [PubMed] [Google Scholar]
- 40.Yokota Y, Takahashi S, Terasaki S, Tamura T. On the development of rapid detection method of medicines and designated drugs. Toyama-ken Yakuji Kenkyusho Nenpo. 2011;38:35. [Google Scholar]
- 41.Ascic E, Hansen CL, Le Quement ST, Nielsen TE. Synthesis of tetrahydro-β-carbolines via isomerization of N-allyltryptamines: a metal-catalyzed variation on the Pictet-Spengler theme. Chem. Commun. 2012;48:3345. doi: 10.1039/c2cc17704h. [DOI] [PubMed] [Google Scholar]
- 42.Brandt SD, Tearavarich R, Dempster N, Cozzi NV, Daley PF. Synthesis and characterization of 5-methoxy-2-methyl-N,N-dialkylated tryptamines. Drug Test. Anal. 2012;4:24. doi: 10.1002/dta.398. [DOI] [PubMed] [Google Scholar]
- 43.Corkery JM, Durkin E, Elliott S, Schifano F, Ghodse AH. The recreational tryptamine 5-MeO-DALT (N,N-diallyl-5-methoxytryptamine): a brief review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39:259. doi: 10.1016/j.pnpbp.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Song Z, Gu H, Xuliang X, Zhan J. Process for preparation of N,N-diallyl-5-methoxytryptamine hydrochloride. 2012 Patent No. CN102391170A.
- 45.Arunotayanun W, Dalley JW, Huang X-P, Setola V, Treble R, Iversen L, Roth BL, Gibbons S. An analysis of the synthetic tryptamines AMT and 5-MeO-DALT: emerging ‘novel psychoactive drugs’. Bioorg. Med. Chem. Lett. 2013;23:3411. doi: 10.1016/j.bmcl.2013.03.066. [DOI] [PubMed] [Google Scholar]
- 46.Kikura-Hanajiri R, Uchiyama N, Kawamura M, Goda Y. Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol. 2013;31:44. [Google Scholar]
- 47.Park Y, Lee C, Lee H, Pyo J, Jo J, Lee J, Choi H, Kim S, Hong RS, Park Y, Hwang BY, Choe S, Jung JH. Identification of a new synthetic cannabinoid in a herbal mixture: 1-butyl-3-(2-methoxybenzoyl)indole. Forensic Toxicol. 2013;31:187. [Google Scholar]
- 48.Philp M, Shimmon R, Stojanovska N, Tahtouh M, Fu SL. Development and validation of a presumptive colour spot test method for the detection of piperazine analogues in seized illicit materials. Anal. Methods. 2013;5:5402. [Google Scholar]
- 49.Acton WJ, Lanza M, Agarwal B, Juerschik S, Sulzer P, Breiev K, Jordan A, Hartungen E, Hanel G, Maerk L, Mayhew CA, Maerk TD. Headspace analysis of new psychoactive substances using a selective reagent ionisation-time of flight-mass spectrometer. Int. J. Mass Spectrom. 2014;360:28. doi: 10.1016/j.ijms.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung H, Choi H, Heo S, Kim E, Lee J. Synthetic cannabinoids abused in South Korea: drug identifications by the National Forensic Service from 2009 to June 2013. Forensic Toxicol. 2014;32:82. [Google Scholar]
- 51.Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci. Int. 2014;243:55. doi: 10.1016/j.forsciint.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Jovel A, Felthous A, Bhattacharyya A. Delirium due to intoxication from the novel synthetic tryptamine 5-MeO-DALT. J. Forensic Sci. 2014;59:844. doi: 10.1111/1556-4029.12367. [DOI] [PubMed] [Google Scholar]
- 53.Meyer MR, Caspar A, Brandt SD, Maurer HH. A qualitative/quantitative approach for the detection of 37 tryptamine-derived designer drugs, 5 β-carbolines, ibogaine, and yohimbine in human urine and plasma using standard urine screening and multi-analyte approaches. Anal. Bioanal. Chem. 2014;406:225. doi: 10.1007/s00216-013-7425-9. [DOI] [PubMed] [Google Scholar]
- 54.Nakazono Y, Tsujikawa K, Kuwayama K, Kanamori T, Iwata YT, Miyamoto K, Kasuya F, Inoue H. Simultaneous determination of tryptamine analogues in designer drugs using gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry. Forensic Toxicol. 2014;32:154. [Google Scholar]
- 55.Rasanen I, Kyber M, Szilvay I, Rintatalo J, Ojanperä I. Straightforward single-calibrant quantification of seized designer drugs by liquid chromatography-chemiluminescence nitrogen detection. Forensic Sci. Int. 2014;237:119. doi: 10.1016/j.forsciint.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Soh YNA, Elliott S. An investigation of the stability of emerging new psychoactive substances. Drug Test. Anal. 2014;6:696. doi: 10.1002/dta.1576. [DOI] [PubMed] [Google Scholar]
- 57.Strano Rossi S, Odoardi S, Gregori A, Peluso G, Ripani L, Ortar G, Serpelloni G, Romolo FS. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun. Mass Spectrom. 2014;28:1904. doi: 10.1002/rcm.6969. [DOI] [PubMed] [Google Scholar]
- 58.Tsujikawa K, Yamamuro T, Kuwayama K, Kanamori T, Iwata YT, Miyamoto K, Kasuya F, Inoue H. Application of a portable near infrared spectrometer for presumptive identification of psychoactive drugs. Forensic Sci. Int. 2014;242:162. doi: 10.1016/j.forsciint.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Michely JA, Helfer AG, Brandt SD, Meyer MR, Maurer HH. Metabolism of the new psychoactive substances N,N-diallyltryptamine (DALT) and 5-methoxy-DALT and their detectability in urine by GC-MS, LC-MSn, and LC HR-MS-MS. Anal. Bioanal. Chem. 2015;407:7831. doi: 10.1007/s00216-015-8955-0. [DOI] [PubMed] [Google Scholar]
- 60.Odoardi S, Fisichella M, Romolo FS, Strano-Rossi S. High-throughput screening for new psychoactive substances (NPS) in whole blood by DLLME extraction and UHPLC-MS/MS analysis. J. Chromatogr. B. 2015;1000:57. doi: 10.1016/j.jchromb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Dinger J, Woods C, Brandt SD, Meyer MR, Maurer HH. Cytochrome P450 inhibition potential of new psychoactive substances of the tryptamine class. Toxicol. Lett. 2016;241:82. doi: 10.1016/j.toxlet.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Meyer MR, Orschiedt T, Maurer HH. Michaelis-Menten kinetic analysis of drugs of abuse to estimate their affinity to human P-glycoprotein. Toxicol. Lett. 2013;217:137. doi: 10.1016/j.toxlet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 63.Cozzi NV, Daley PF. Receptor binding profiles and quantitative structure-affinity relationships of some 5-substituted-N,N-diallyltryptamines. Bioorg. Med. Chem. Lett. 2015 doi: 10.1016/j.bmcl.2015.12.053. DOI: 10.1016/j.bmcl.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 64.EMCDDA–Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA. EMCDDA; Lisbon: 2013. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_212366_EN_EMCDDA-Europol 2012 Annual Report_final.pdf [20 December 2015] [Google Scholar]
- 65.EMCDDA–Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA. EMCDDA; Lisbon: 2015. Available at: http://www.emcdda.europa.eu/attachements.cfm/att_240380_EN_TDAN15001ENN.pdf [20 December 2015] [Google Scholar]
- 66.Speeter ME, Anthony WC. The action of oxalyl chloride on indoles: A new approach to tryptamines. J. Am. Chem. Soc. 1954;76:6208. [Google Scholar]
- 67.Brandt SD, Martins CPB. Analytical methods for psychoactive N,N-dialkylated tryptamines. Trends Anal. Chem. 2010;29:858. [Google Scholar]
- 68.Martins CPB, Freeman S, Alder JF, Passie T, Brandt SD. The profiling of psychoactive tryptamine drug synthesis focusing on mass spectrometry. Trends Anal. Chem. 2010;29:285. [Google Scholar]
- 69.Elliott SP, Brandt SD, Freeman S, Archer RP. AMT (3-(2-aminopropyl)indole) and 5-IT (5-(2-aminopropyl)indole): an analytical challenge and implications for forensic analysis. Drug Test. Anal. 2013;5:196. doi: 10.1002/dta.1420. [DOI] [PubMed] [Google Scholar]
- 70.Elliott SP, Brandt SD, Wallach J, Morris H, Kavanagh PV. First reported fatalities associated with the ‘research chemical’ 2-methoxydiphenidine. J. Anal. Toxicol. 2015;39:287. doi: 10.1093/jat/bkv006. [DOI] [PubMed] [Google Scholar]
- 71.Brandt SD, Elliott SP, Kavanagh PV, Dempster NM, Meyer MR, Maurer HH, Nichols DE. Analytical characterization of bioactive N-benzyl-substituted phenethylamines and 5-methoxytryptamines. Rapid Commun. Mass Spectrom. 2015;29:573. doi: 10.1002/rcm.7134. [DOI] [PubMed] [Google Scholar]
- 72.McLaughlin G, Morris N, Kavanagh PV, Dowling G, Power JD, Twamley B, O'Brien J, Talbot B, Sitte HH, Brandt SD. Test purchase, synthesis and characterization of 3-fluorophenmetrazine (3-FPM) and differentiation from its ortho- and para-substituted isomers. Drug Test. Anal. 2015 doi: 10.1002/dta.1945. DOI: 10.1002/dta.1945. [DOI] [PubMed] [Google Scholar]
- 73.McLaughlin G, Morris N, Kavanagh PV, Power JD, O'Brien J, Talbot B, Elliott SP, Wallach J, Hoang K, Morris H, Brandt SD. Test purchase, synthesis, and characterization of 2-methoxydiphenidine (MXP) and differentiation from its meta- and para-substituted isomers. Drug Test. Anal. 2015 doi: 10.1002/dta.1800. DOI: 10.1002/dta.1800. [DOI] [PubMed] [Google Scholar]
- 74.Wallach J, Colestock T, Cicali B, Elliott SP, Kavanagh PV, Adejare A, Dempster NM, Brandt SD. Syntheses and analytical characterizations of N-alkyl-arylcyclohexylamines. Drug Test. Anal. 2015 doi: 10.1002/dta.1861. DOI: 10.1002/dta.1861. [DOI] [PubMed] [Google Scholar]
- 75.González-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Halberstadt AL, Geyer MA. Serotonergic hallucinogens as translational models relevant to schizophrenia. Int. J. Neuropsychopharmacol. 2013;16:2165. doi: 10.1017/S1461145713000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 2011;25:1548. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halberstadt AL, Nichols DE. Serotonin and serotonin receptors in hallucinogen action. In: Müller C, Jacobs BL, editors. Handbook of Behavioral Neurobiology of Serotonin, Chapter 4.7. Elsevier/Academic Press; Amsterdam: 2010. p. 621. [Google Scholar]
- 80.Nichols DE. Structure-activity relationships of serotonin 5-HT2A agonists. WIREs Membr. Transp. Signal. 2012;1:559. [Google Scholar]
- 81.Nichols DE, Nichols CD. Serotonin receptors. Chem. Rev. 2008;108:1614. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 82.Citizen Pain. Available at: http://www.citizen-pain.com/files.html [29 December 2015]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.