Abstract
Background:
Echocardiographic right ventricular (RV) function assessment is difficult and still a gray area despite rapid advancement of imaging modalities. The aim of this study is to assess the role of echocardiographic RV outflow tract (RVOT) function in the form of RVOT fractional shortening (RVOT FS) and RVOT systolic excursion (RVOT SE) for the assessment of RV function.
Methods:
We studied ninety individuals divided equally into two groups. The control group included 45 normal healthy individuals and age-matched patient group included 45 patients with RV dysfunction which was defined by tricuspid annular plane systolic excursion (TAPSE) <16 mm and RV fractional area change (RV FAC) ≤35%. Echocardiography was performed to measure RVOT FS and RVOT SE and correlate them with other parameters of RV function including TAPSE, RV FAC, peak systolic velocity of the lateral tricuspid annulus (S’) using pulsed tissue Doppler, and pulmonary acceleration time (PAcT).
Results:
RVOT FS showed positive correlation with TAPSE (r = 0.75, P = 0.02), RV FAC (r = 0.45, P = 0.003), and PAcT (r = 0.39, P = 0.00) and negative correlation with left atrial dimensions (LADs) (r = −0.359, P = 0.017) and left ventricular end-diastolic dimensions (r = −0.304, P = 0.042). RVOT FS <32% was 93% sensitive and 98% specific to identify patients with impaired RV function. However, RVOT SE showed weak correlation with echocardiographic RV parameters. RVOT SE <5 mm was 80% sensitive and 76% specific to identify patients with impaired RV function.
Conclusion:
RVOT FS is a simple valuable parameter that can be used for the assessment of RV function. However, RVOT SE is less accurate than RVOT FS in RV function assessment.
Keywords: Echocardiography, right ventricle, right ventricular outflow tract
Introduction
Right ventricular (RV) function is an important predictor of mortality and quality of life in patients with left ventricular (LV) failure, myocardial infarction, congenital heart disease, and pulmonary hypertension.[1]
Echocardiographic assessment of RV functions has been gaining importance in recent years after being fairly neglected in the past. Several quantitative methods for the assessment of RV have been validated after mainly depending on qualitative assessment in the past and are now recommended as standard by both the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imagining (ECAVI).[2,3]
However, there are limitations to echocardiographic techniques currently used in clinical and academic practice to evaluate RV function.[4] Additional measurements of RV outflow tract (RVOT) fractional shortening (RVOT FS) may add great value.[5]
The aim of this study is to evaluate the usefulness of the RVOT FS and the RVOT systolic excursion (RVOT SE) obtained by M-mode echocardiography in the assessment of RV function.
Methods
Study design
The study was performed in Ain Shams University Hospitals, Cairo, Egypt, for 1 year extending from March 2014 to February 2015.
The study was conducted prospectively on ninety individuals divided equally into two groups according to their RV function based on tricuspid annular plane systolic excursion (TAPSE) and RV fractional area change (RV FAC) according to the recent guidelines of ASE.[2] These groups were control group (45 individuals) and patient group (45 patients). Control group represented normal healthy controls with completely normal echocardiographic findings included normal RV function (TAPSE was ≥16 mm and RV FAC was ≥35%), and patient group represented patients with impaired RV function whatever the etiology (TAPSE was <16 mm and RV FAC was <35%). Individuals were excluded from the study if they were younger than 18 years, older than 64 years, had atrial fibrillation, or had poor echocardiographic windows.
Clinical history and examination
Focused relevant history and physical examination were done. Standard files of individuals in the patient group were reviewed to document the etiology of RV dysfunction.
Transthoracic echocardiography
Standard transthoracic echocardiography with machine integrated electrocardiogram recording was performed for all individuals in both groups using Vivid S5 GE healthcare equipped with 3 MHZ transducer. A standard study following standardized protocols[2] was performed for all individuals by an echocardiographer accredited by the ECAVI to obtain the following measurements: LV dimensions were measured by M-mode from the parasternal short-axis view at level of papillary muscles; LV ejection fraction (LV EF) was measured by Simpson's method of discs;[2] and anteroposterior diameter of the left atrium was measured by M-mode in the parasternal long axis view.[6]
Assessment of right ventricular areas
From RV focused apical four chambers view, manual tracing of the RV endocardial border was performed starting from the lateral tricuspid annulus along the free wall to the apex back to the medial tricuspid annulus along the interventricular septum at end diastole and at end systole to measure RV end-diastolic area (RVEDA) and RV end-systolic area (RVESA), respectively (trabeculations, papillary muscles, and moderator band were included in the cavity area). We assumed a normal reference range of 10–25 cm2 for RVEDA and 4–14 cm2 for RVESA.[1,2,6]
Estimation of right ventricular systolic function
RVFAC was defined as (RVEDA − RVESA)/RVEDA × 100. We assumed the normal value of RVFAC to be ≥35%.[2,6]
TAPSE was measured by M-mode from the apical four-chamber view as a distance of SE of the lateral RV annular segment of the tricuspid valve along its longitudinal plane. Total displacement was measured by the leading edge to leading edge convention and expressed in millimeters. We assumed the normal value of TAPSE to be ≥16 mm.[2,6]
Pulsed wave tissue Doppler imaging
Pulsed wave tissue Doppler imaging images were acquired by placing the region of interest in the RV free wall at the level of tricuspid valve annulus. Gains were optimized and low wall filter settings were selected in addition to a Doppler velocity range of −20–+20 cm/s with a swipe speed of 50 mm/s.
Having proper alignment with ultrasound beam was considered mandatory. Peak velocities of the following waves were measured: S’ is the major positive (systolic) wave, e’ is the first negative (diastolic) wave, and a’ is the second negative wave.
We assumed the normal values of S’ velocity to be ≥9.5 cm/s, of e’ to be ≥7.8 cm/s, and of e’/a’ to be ≥0.52.[2,6]
Estimation of right ventricular outflow tract systolic function
RVOT FS was measured by M-mode from parasternal short-axis view at aortic valve level with magnified images of RVOT, and the cursor was aligned perpendicular to the anterior RVOT wall.[5,7] Then, RVOT end-diastolic (RVOT ED) and RVOT end-systolic (RVOT ES) diameters were measured.[5]
RVOT FS was defined as RVOT FS = 100 × (RVOT ED − RVOT ES)/(RVOT ED).
RVOT SE was measured from the same image as mentioned above. It was defined as the SE of the endocardial surface of the anterior RVOT wall relative to the transducer.[5]
In all individuals of both study groups, all RVOT measurements were taken for five consecutive beats then averaged.
Statistical analysis
All data were analyzed using SPSS software, version 19. Copyright, SPSS Inc. SPSS Inc products are registered trademarks of SPSS Inc. as part of IBM company, Chicago, USA. Continuous variables were presented as mean ± standard deviation (SD) and categorical variables as absolute numbers and percentages. Comparison of demographic, clinical, and echocardiographic data between both groups was performed using independent t-test for continuous variables and Chi-square for categorical variables. Pearson's correlation coefficients were calculated to illustrate certain relationships. Receiver operating characteristic curve was used to obtain cutoff values of continuous or nominal variables. P < 0.05 was considered significant.
Results
Baseline characteristics
The age within control group was 37.5 ± 10.6 years while it was 45.7 ± 14.34 years in the patient group, with insignificant difference between them (P > 0.05). Control group included 31 (68.8%) males and 14 (31.1%) females and patient group included 22 (48.8%) males and 23 (51.1%) females, with no significant difference between both groups regarding gender distribution (P > 0.05). Both pulse rate and systolic blood pressure were significantly higher among patient group than in control group while there was no significant difference in respect to diastolic blood pressure [Table 1].
Table 1.
Demographic and clinical data between both study groups
| Demographic and clinical data | Controls | Patients | P |
|---|---|---|---|
| Age (years) | 35.3±9.202 | 44.7±12.3 | 0.15 |
| Male (%) | 31 (69) | 22 (49) | 0.54 |
| Female (%) | 14 (31) | 23 (51) | |
| Pulse (b/m) | 78.11±12.023 | 91.27±11.833 | 0.0005 |
| SBP (mmHg) | 118.56±9.331 | 126.89±15.01 | 0.025 |
| DBP (mmHg) | 75.89±5.963 | 73.67±11.302 | 0.247 |
SBP: Systolic blood pressure, DBP: Diastolic blood pressure
Certain characteristics among patient group
The etiology of RV dysfunction among patient group was heterogenous, but ischemic heart disease was the most common etiology affecting 16 (35.5%) patients, followed by rheumatic valve heart disease affecting 15 (33.3%) patients. Other less common causes included dilated cardiomyopathy in 5 (11.1%) patients, congenital heart disease in 4 (8.8%) patients, chronic lung disease or pulmonary vascular disease in 4 (8.8%) patients, and constrictive pericarditis in one (2.2%) patient.
Twenty-two (48.8%) patients were in New York Heart Association (NYHA) Class II, 18 (40%) patients were in NYHA Class III, and 5 (11.1%) patients were in ambulatory Class IV.
Echocardiographic parameters
All measured LV echocardiographic parameters were significantly different between both groups as shown in Table 2.
Table 2.
Left ventricular echocardiographic parameters between both study groups
| LV echocardiographic parameters | Controls | Patients | P |
|---|---|---|---|
| LAD (mm) | 32.61±2.847 | 50.52±10.674 | 0.0005 |
| LVSWT (mm) | 7.49±0.920 | 8.47±1.502 | 0.004 |
| LVEDD (mm) | 47.68±3.248 | 58.29±11.841 | 0.000 |
| LVPWT (mm) | 7.18±0.777 | 8.58±1.617 | 0.000 |
| LVESD (mm) | 29.57±2.453 | 45.44±14.842 | 0.000 |
| LV EF (%) | 67.47±3.659 | 41.44±16.148 | 0.000 |
LAD: Left atrial dimension, LVSWT: Left ventricular septal wall thickness, LVEDD: Left ventricular end-diastolic dimension, LVESD: Left ventricular end-systolic dimension, LV EF: Left ventricle ejection fraction, LVPWT: Left ventricular posterior wall thickness
As expected, all echocardiographic parameters of RV function were significantly different between both groups (P < 0.01). They were lower in patient group in comparison to the control group as shown in Table 3.
Table 3.
Difference of right ventricular echocardiographic parameters between study groups
| RV echocardiographic parameters | Controls | Patients | P |
|---|---|---|---|
| TAPSE (mm) | 22.87±2.33 | 11.8±1.7 | 0.0005 |
| RV EDA (cm2) | 15.84±1.68 | 24.10±6.33 | 0.000 |
| RV ESA (cm2) | 8.10±1.53 | 18.62±5.28 | 0.000 |
| RV FAC (%) | 48.99±6.93 | 22.77±5.67 | 0.000 |
| S’ of lateral tricuspid annulus (cm/s) | 14.96±1.85 | 8.69±1.86 | 0.000 |
| PAcT (ms) | 138.78±11.23 | 90.60±19.18 | 0.000 |
TAPSE: Tricuspid annular plane systolic excursion, RV: Right ventricular, EDA: End-diastolic area, ESA: End-systolic area, FAC: Fractional area change, S’: Peak systolic velocity of lateral tricuspid annulus, PAcT: Pulmonary acceleration time
Right ventricular outflow tract echocardiographic parameters
We found that RVOT FS was significantly lower among patient group than control group (21.17% ± 6.11% vs. 48.90% ± 10.12% with P < 0.01). In addition, RVOT SE was also significantly lower among patient group (3.47 ± 1.45 mm vs. 5.82 ± 2.43 mm with P < 0.01) [Table 4 and Figure 1].
Table 4.
Difference of right ventricular outflow tract fractional shortening and right ventricular outflow tract systolic excursion between both study groups
| RVOT echocardiographic parameters | Controls | Patients | P |
|---|---|---|---|
| RVOT EDD (mm) | 31.00±4.13 | 38.60±8.59 | 0.0005 |
| RVOT ESD (mm) | 16.04±4.65 | 30.44±7.62 | 0.000 |
| RVOT FS (%) | 48.90±10.12 | 21.17±6.11 | 0.000 |
| RVOT SE (mm) | 5.82±2.43 | 3.47±1.45 | 0.000 |
RVOT: Right ventricular outflow tract, EDD: End-diastolic dimension, ESD: End-systolic dimensions, FS: Fractional shortening, SE: Systolic excursion
Figure 1.
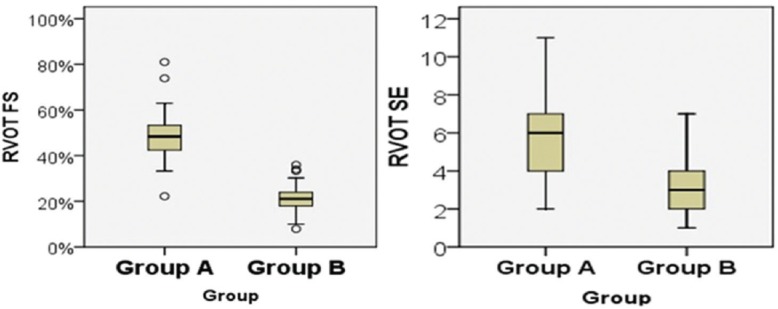
The left panel showing the difference of right ventricular outflow tract fractional shortening between study groups while the right panel showing the difference of right ventricular outflow tract systolic excursion between study groups.
Correlations
As regard demographic data within study population
Both RVOT FS and RVOT SE showed no significant correlation with age or gender within study groups. No significant correlation between RVOT echocardiographic parameters and arterial blood pressure either systolic or diastolic blood pressure (r = 0.15 and P = 0.16).
As regard echocardiographic measurements among patient group
RVOT FS showed moderate positive correlation of high significance with RV FAC (r = 0.45, P = 0.003) and pulmonary acceleration time (PAcT) (r = 0.39, P = 0.00) and high positive correlation with TAPSE (r = 0.75, P = 0.02), while RVOT FS showed moderate negative correlation of high significance with left atrial dimension (LAD) (r = −0.359, P = 0.017) and weak negative correlation with LV ED dimension (LVEDD) (r = −0.304, P = 0.042) [Table 5 and Figure 2]. However, it did not show significant correlation with S’ of lateral tricuspid annulus or LV EF.
Table 5.
Correlation of right ventricular outflow tract fractional shortening with other echocardiographic variables
| Variable | Pearson coefficient (r) | P |
|---|---|---|
| RV FAC | 0.45 | 0.003 |
| PAcT | 0.386 | 0.009 |
| TAPSE | 0.75 | 0.02 |
| S’ of lateral tricuspid annulus | 0.26 | 0.084 |
| LAD | −0.359 | 0.017 |
| LV EF | 0.192 | 0.207 |
| LVEDD | −0.304 | 0.042 |
RV FAC: Right ventricular fractional area change, TAPSE: Tricuspid annular plane systolic excursion, S’: Peak systolic velocity of lateral tricuspid annulus, PAcT: Pulmonary acceleration time, LAD: Left atrial diastolic dimension, LV EF: Left ventricular ejection fraction, LVEDD: Left ventricular end-diastolic dimension
Figure 2.
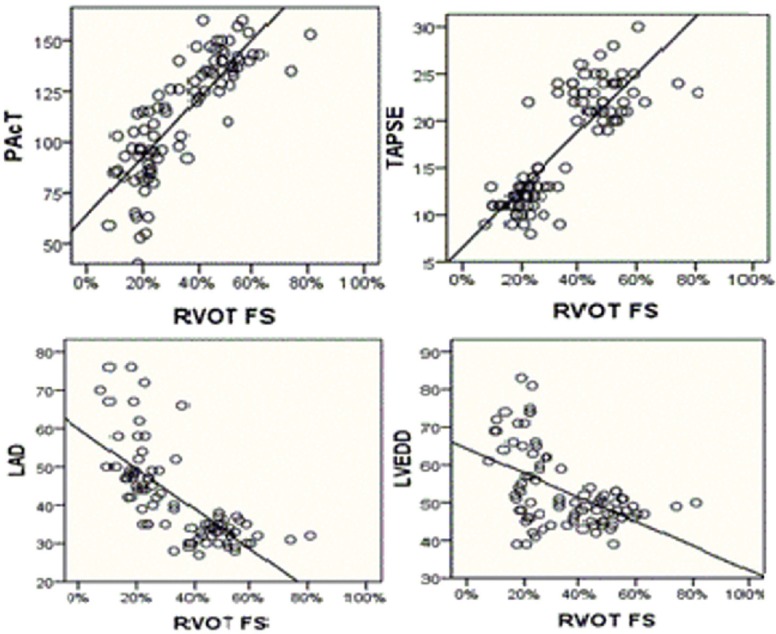
Correlation of right ventricular outflow tract fractional shortening with other echocardiographic parameters.
RVOT SE showed weak positive correlation with RV FAC, TAPSE, and PAcT and also weak negative correlation with LAD, LVEDD, and LV EF, without statistical significance [Table 6].
Table 6.
Correlation of right ventricular outflow tract systolic excursion with other echocardiographic variables
| Variable | Pearson coefficient (r) | P |
|---|---|---|
| RV FAC | 0.206 | 0.176 |
| PAcT | 0.252 | 0.095 |
| TAPSE | 0.03 | 0.84 |
| S’ of lateral tricuspid annulus | −0.38 | 0.803 |
| LAD | −0.15 | 0.316 |
| LV EF | −0.113 | 0.462 |
| LVEDD | −0.22 | 0.144 |
RV FAC: Right ventricular fractional area change, TAPSE: Tricuspid annular plane systolic excursion, S’: Peak systolic velocity of lateral tricuspid annulus, PAcT: Pulmonary acceleration time, LAD: Left atrial diastolic dimension, LV EF: Left ventricular ejection fraction, LVEDD: Left ventricular end-diastolic dimension
RVOT FS ≤32% was the best cutoff value that differentiates patients with RV systolic dysfunction from healthy individuals with normal RV systolic function. It was 93% sensitive and 98% specific [Figure 1]. We also found that RVOT SE <5 mm was the best cutoff value that differentiate patients from controls but with sensitivity of 80% and specificity of 76%.
Discussion
Echocardiographic assessment of the right side of heart is gaining importance in the current clinical practice and research with guidelines recently published specifically to address this purpose.[2]
This is because of growing evidence of its effects on clinical outcome, morbidity, and mortality of several cardiac conditions.[8,9]
RVOT represents up to 20% of RV volume and contributes to up to 15% of total RV stroke volume.[6,10,11] RVOT has also important role in some patients with congenital heart diseases or arrhythmias.[7,10] Surgeons frequently estimate RV function during surgery by looking at the RVOT contraction.[12,13]
This study aimed to assess the usefulness of RVOT function for assessment of the whole RV function using RVOT SE and RVOT FS parameters.
The main findings of this study were that patients with impaired RV systolic function had significant impairment of RVOT systolic function (manifested by reduction in RVOT FS and RVOT SE) in comparison to healthy controls with normal RV systolic function. RVOT FS <32% was 93% sensitive and 98% specific to identify patients with impaired RV function. While RVOT SE <5 mm was 80% sensitive and 76% specific to identify patients with impaired RV function. To the best of our knowledge, no study previously assessed both RVOT FS and RVOT SE and simultaneously compared between them among patients with impaired RV systolic function and correlating both with other RV echocardiographic parameters.
In the current study, it was found that pulse rate and systolic blood pressure were significantly higher in patient group with impaired RV function in comparison to control group. These differences were because of 37.8% of patients were hypertensive and near 50% were noncompliant on medical treatment due to economic basis.
As regard the etiology of RV failure in patient group, ischemic heart disease was the most common in 35.6% of patients and rheumatic valvular heart disease was the second cause in 33.3%, followed by less common other causes. Prevalence of coronary artery disease in Egypt is about 8.3% according to Almahmeed et al.[14] However, rheumatic heart disease is still a major health problem in Egypt due to poor socioeconomic standards.[15]
In the current study, echocardiographic parameters of LV function were significant difference between both groups and this was attributed to that the same pathology affecting RV simultaneously affecting LV and also due to interventricular dependence.
In the current study, we found that RVOT diastolic dimensions in healthy controls was 31 ± 4.4 mm while RVOT systolic dimensions was 16.04 ± 4.65 mm. Similar findings were reported in a study to assess RVOT FS in 81 patients with reduced LV EF. In the study, RVOT diameter was 3.1 cm at end diastole (range, 1.9–5.3 cm) and 2.1 cm at end systole (range, 0.4–4.5 cm) in normal healthy controls.[16]
As regards RVOT FS, we found that RVOT FS was significantly lower in patient group in comparison to healthy controls (21.17% ± 6.11% vs. 48.9 ± 10.12% with P < 0.001).
RVOT FS was also able to identify patients with impaired RV systolic function with adequate accuracy. RVOT FS cutoff value of <32% was 93% sensitive and 98% specific to identify patients with impaired RV systolic function. Similar results were demonstrated in a study reported by Asmer et al.,[12] where RVOT FS was significantly lower in patients with impaired RV function than in individuals with preserved RV function (17% ± 7% vs. 47% ± 7% with P < 0.0001) and RVOT FS cutoff value <30% was 95% sensitive and 100% specific to diagnose impaired RV systolic function.
In the current study, RVOT FS in healthy controls was 48.90% ± 10.12% which was slightly lower than that reported by a study done by Lindqvist et al.[5] In their study, they assessed the role of RVOT FS for the assessment of RV function aiming mainly to assess its relation with pulmonary artery systolic pressure. They reported that RVOT FS was 61% ± 13% in healthy controls.
In the current study, RVOT SE was significantly lower in patients than in healthy controls (3.47 ± 1.4 mm vs. 5.82 ± 2.43 mm with P < 0.001). The best cutoff value of RVOT SE was <5 mm with 80% sensitivity and 76% specificity. However, there was a considerable overlap of RVOT SE values between both group individuals. This was somewhat different from that reported by Asmer et al.[12] They reported that RVOT SE was significantly lower in patients with impaired RV systolic function than in individuals with normal RV function (1.7 ± 1.1 mm vs. 9.6 ± 1.5 mm with P < 0.001). RVOT SE in their study was different within each group from that found in our study as reported above. They also reported that the best cutoff value of RVOT SE was <6 mm with 100% sensitivity and 100% specificity to diagnose impaired RV systolic function, which completely separated patients with impaired RV function from patients with preserved RV function and this fact was not proven here in the current study.
Regarding correlation of RVOT FS with other echocardiographic parameters within patient group, we found that RVOT FS was strongly positively correlated with TAPSE (r = 0.75 with P = 0.02), which was as that reported by Lindqvist et al.[5] In their study, RVOT FS was positively correlated with TAPSE (r = 0.66 with P < 0.0001).
Furthermore, RVOT FS correlated positively with RVOT FAC (r = 0.45 with P = 0.003) which was comparable to that reported by Yamaguchi et al.[16] (r = 0.37 with P = 0.0008).
In 1924, Keith[17] described the cavity of RV infundibulum and summarized that RVOT role was to serve as a protection for the pulmonary circulation during RV pressure rise in systole. In addition, it was reported that RVOT FS closely correlates with events happening near the RVOT region as PAcT and pulmonary artery pressure.
In the current study, we found that RVOT FS showed correlation with indirect parameters of pulmonary vascular resistance (PAcT and LAD). It correlated positively with PAcT (r = 0.39 with P = 0.00) which was weaker than that reported by Lindqvist et al.[5] (r = 0.8 with P < 0.0001). This can be explained by inclusion of healthy controls in their study in the correlation in addition to the difference of inclusion criteria of their study groups. RVOT FS also correlated negatively with LAD (r = −0.359 with P = 0.017) which was comparable to that reported by Yamaguchi et al.[16] (r = −0.45 with P < 0.001).
In the current study, RVOT FS did not correlate with LV EF (r = 0.192 with P = 0.207), which was different from that reported by Yamaguchi et al.[16] (r = 0.33 with P = 0.0028) and from that reported by Asmer et al.[12] (r = 0.55 with P < 0.0003). This might be attributed to the difference in etiology of cardiac disease among patient group in these studies. In their studies, cardiac disease was mostly of nonvalvular etiology being mainly due to ischemic heart disease or DCM (64%) in the former and of ischemic origin in the latter (75%).
In addition, it was found in our study that RVOT SE did not correlate significantly with RVOT FS, RV echocardiographic parameters, LAD, LV EF.
Study limitations
Limitations of the current study are that it comes from a single medical center with a relatively small number of patients. RVOT anterior wall visualization with M-mode can be suboptimal in some patients and that can hamper RVOT FC or RVOT SE measurements. Furthermore, an oblique cut of RVOT may underestimate RVOT FC. Furthermore, applying emerging echocardiographic techniques to the RV such as strain and three-dimensional of RV should be considered in future studies to add more insight into changes affecting RV function.
Conclusion
RVOT FS is noninvasive and easy applicable echocardiographic parameter that can provide a comprehensive evaluation of RV systolic function with other recommended echocardiographic parameters. RVOT SE is not accurate as sole parameter, but its high values can be used as indicator for normal RV systolic function.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Marcu CB, Beek AM, Van Rossum AC. Cardiovascular magnetic resonance imaging for the assessment of right heart involvement in cardiac and pulmonary disease. Heart Lung Circ. 2006;15:362–70. doi: 10.1016/j.hlc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–5. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 4.Triantafyllou K, Kranidis A, Karabinos E, Grassos H, Babalis D. Clinical implications of the echocardiographic evaluation of right ventricular function on the long axis using newer techniques. Hellenic J Cardiol. 2010;51:42–8. [PubMed] [Google Scholar]
- 5.Lindqvist P, Henein M, Kazzam E. Right ventricular outflow-tract fractional shortening: An applicable measure of right ventricular systolic function. Eur J Echocardiogr. 2003;4:29–35. doi: 10.1053/euje.2002.0177. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 7.Geva T, Powell AJ, Crawford EC, Chung T, Colan SD. Evaluation of regional differences in right ventricular systolic function by acoustic quantification echocardiography and cine magnetic resonance imaging. Circulation. 1998;98:339–45. doi: 10.1161/01.cir.98.4.339. [DOI] [PubMed] [Google Scholar]
- 8.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–54. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 9.Bleeker GB, Steendijk P, Holman ER, Yu CM, Breithardt OA, Kaandorp TA, et al. Assessing right ventricular function: The role of echocardiography and complementary technologies. Heart. 2006;92(Suppl 1):i19–26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugishita Y, Watanabe M, Fisher SA. The development of the embryonic outflow tract provides novel insights into cardiac differentiation and remodeling. Trends Cardiovasc Med. 2004;14:235–41. doi: 10.1016/j.tcm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Dell’Italia LJ. The right ventricle: Anatomy, physiology, and clinical importance. Curr Probl Cardiol. 1991;16:653–720. doi: 10.1016/0146-2806(91)90009-y. [DOI] [PubMed] [Google Scholar]
- 12.Asmer I, Adawi S, Ganaeem M, Shehadeh J, Shiran A. Right ventricular outflow tract systolic excursion: A novel echocardiographic parameter of right ventricular function. Eur Heart J Cardiovasc Imaging. 2012;13:871–7. doi: 10.1093/ehjci/jes055. [DOI] [PubMed] [Google Scholar]
- 13.Arya A, Piorkowski C, Sommer P, Gerds-Li JH, Kottkamp H, Hindricks G. Idiopathic outflow tract tachycardias. Herz Kardiovaskuläre Erkr. 2007;32:218–25. doi: 10.1007/s00059-007-2980-5. [DOI] [PubMed] [Google Scholar]
- 14.Almahmeed W, Arnaout MS, Chettaoui R, Ibrahim M, Kurdi MI, Taher MA, et al. Coronary artery disease in Africa and the Middle East. Ther Clin Risk Manag. 2012;8:65–72. doi: 10.2147/TCRM.S26414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorour KA. Rheumatic heart disease in Egypt: Gloomy past and promising future. Egypt Heart J. 2014;66:139–42. [Google Scholar]
- 16.Yamaguchi M, Tsuruda T, Watanabe Y, Onitsuka H, Furukawa K, Ideguchi T, et al. Reduced fractional shortening of right ventricular outflow tract is associated with adverse outcomes in patients with left ventricular dysfunction. Cardiovasc Ultrasound. 2013;11:19. doi: 10.1186/1476-7120-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith A. Schorstein lecture on the fate of the bulbus cordis in the human heart. Lancet. 1924;204:1267–73. [Google Scholar]


