Abstract
Hepatocyte-like cells (HLCs) are generated from either various human pluripotent stem cells (hPSCs) including induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs), or direct cell conversion, mesenchymal stem cells as well as other stem cells like gestational tissues. They provide potential cell sources for biomedical applications. Liver transplantation is the gold standard treatment for the patients with end stage liver disease, but there are many obstacles limiting this process, like insufficient number of donated healthy livers. Meanwhile, the number of patients receiving a liver organ transplant for a better life is increasing. In this regard, HLCs may provide an adequate cell source to overcome these shortages. New molecular engineering approaches such as CRISPR/ Cas system applying in iPSCs technology provide the basic principles of gene correction for monogenic inherited metabolic liver diseases, as another application of HLCs. It has been shown that HLCs could replace primary human hepatocytes in drug discovery and hepatotoxicity tests. However, generation of fully functional HLCs is still a big challenge; several research groups have been trying to improve current differentiation protocols to achieve better HLCs according to morphology and function of cells. Large-scale generation of functional HLCs in bioreactors could make a new opportunity in producing enough hepatocytes for treating end-stage liver patients as well as other biomedical applications such as drug studies. In this review, regarding the biomedical value of HLCs, we focus on the current and efficient approaches for generating hepatocyte-like cells in vitro and discuss about their applications in regenerative medicine and drug discovery.
Keywords: Hepatocyte, Cell Therapy, Gene Therapy, Drug Discovery
Introduction
Nowadays human primary hepatocytes are regularly used, as the most important and efficient cells in the liver organ for biomedical applications, e.g. cell therapy and drug studies (1,3). Some evidences reported application of hepatocytes for cell therapy clinical trial of various liver disorders (4,5). Although limited access to sufficient human functional hepatocyte, due to the lack of availability of healthy donors as well as difficulties in hepatocyte long-term maintenance, are major problems in using these specialized cells (6).
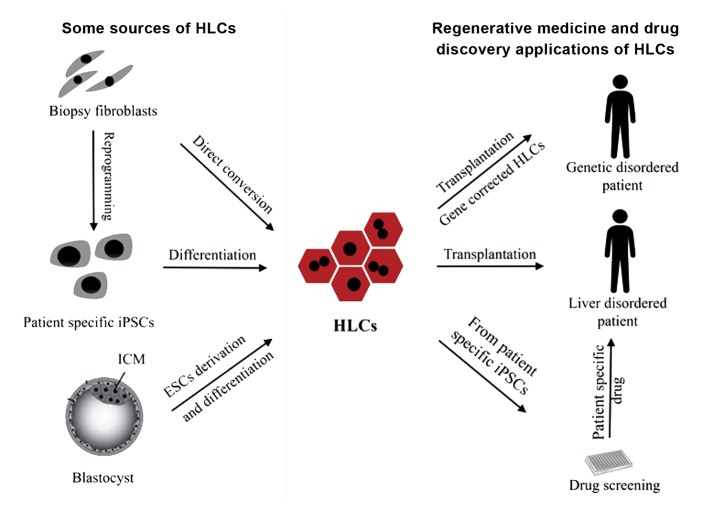
Primary human hepatocytes, usually derived from the livers, are immunologically rejected for transplantation and therefore the yield and quality of the isolated hepatocytes are a limiting factor in any biomedical application (3, 7). Researchers has currently introduced another source of hepatocyte as possible substitute, known as hepatocyte-like cells (HLCs), which has been produced in vitro. HLCs are usually derived from human pluripotent stem cell (hPSCs), including human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), gestational stem cells and mesenchymal stromal cells. Direct cell conversion is another method to generate HLCs (6, 8). Protocols to generate higher quality of HLCs are continuously improving and different research groups are working in this regard. Moreover, scaling up production of HLCs, using three-dimensional system (3D) in bioreactors, resulted in generating enough cells for any biomedical application (9, 10). In this review, we briefly described different methods to produce HLCs in vitro and explained some of their applications in research and regenerative medicine. Figure 1 presents regenerative medicine, drug study, some sources and applications of HLCs.
Fig.1.
Main sources of HLCs and their applications in regenerative medicine and drug discovery. Diagram of some sources of HLC (Left): biopsy derived fibroblasts from liver disease patient can directly be converted into HLCs, by overexpression of liver specific transcription factors (TFs). Patient specific iPSCs generated by overexpression of Yamanaka factors (Oct4, Sox2, Klf4 and c-Myc) can also be differentiated to HLCs for further applications. Embryonic stem cells from ICM of blastocyst are other sources of HLCs.
Diagram of some potential biomedical applications of HLC (Right): HLCs can be used for patients with end-stage liver disease. In addition, using iPSCs technology, monogenic disorders can be corrected in metabolic liver diseases at genome level and then healthy patient specific iPSC-derived HLCs could be a source for transplantation and decreasing signs of the disease. Drug screening after disease modeling, using patient specific iPSC-derived HLCs, to achieve new drugs for specific patients and individual drug administrations are another application of HLCs in the personalized medicine field.
HLCs; Hepatocyte-like cells and iPSCs; Induced pluripotent stem cells.
Different types of produced hepatocyte-like cells in vitro
Human embryonic stem cells-derived hepatocytese
ESCs, derived from the inner cell mass of blastocysts are immortalize cell type with ability to differentiate into all somatic cell lineages (11, 12). These primitive and highly undifferentiated cells were firstly isolated from mouse embryos (mESCs) (11) and the first hESCs line was successfully derived from in vitro fertilized human embryos (13). It has been shown that these cells with a high level of self-renewal ability and possibility to produce nearly all cell types, including "hepatocyte", can be used as an important tool for basic and clinical researches (14). There are two ways to produce HLCs through hESC: spontaneous differentiation and directed differentiation.
In the first approach, hESCs are aggregated to form human embryoid bodies (hEBs). These cell aggregates spontaneously start to differentiate into the three germ layers, including endodermal cells (15, 16). It has been shown that hESC can differentiate into hepatic-like cells through the EB formation, thus albumin-expressing cells have subsequently been detected in EBs (17, 18). Due to the low efficiency of spontaneous differentiation of hESCs, possibility of miscellaneous differentiation into any other cells and possibility of differentiation into non-homogeneous population of cells, scientists focused on the directed differentiation of hESCs into HLCs (14).
In this approach, several protocols have been developed to differentiate ES cells toward HLCs sequentially. In these protocols a series of growth factors and some other soluble factors which participate during liver development have been used in a stepwise manner, mimicking in vivo liver development (15, 19-22). Generally, these protocols have some specific steps. The first step is "Endoderm induction" whereby mainly activin is used. The second step is "Hepatic specification" using some factors like bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs). "Hepatoblast (hepatic stem cell) expansion" and "Hepatic maturation" are respectively the other steps, developed by using specific growth factors like hepatocyte growth factor (HGF), epidermal growth factor (EGF), oncostatin M (OSM) and Dexamethasone (DEX) (8). The progress in each step is usually evaluated by specific markers. Bile duct cells are excluded based on their specific markers in order to have a more homogenous population of hepatocytes (23). Siller et al. (24) recently presented a growthfactor- free protocol using only small molecules to induce HLC differentiation to pluripotent stem cells. CHIR99021, as potent pharmacological glycogen synthase kinase-3 (GSK-3)-specific inhibitor activating wingless-type MMTV integration site family (Wnt) signaling pathway during hepatocyte differentiation (25), was used to induce definitive endoderm formation, followed by treatment with dimethyl sulfoxide (DMSO), dexamethasone, hydrocortisone-21-hemisuccinate and Ile-(6) aminohexanoic amide (dihexa), as a small molecule that is an agonist of HGF (26), to drive hepatic maturation. The polygonal HLCs were generated, which expressed hepatic specific markers like albumin, AFP and alpha-1 antitrypsin (A1AT) with proper functionalities. In addition, some other groups have tried to improve current hepatic differentiation protocols with different strategies like using other appropriate cells for co-culture, monolayer culture or using 3D cell aggregates, through differentiation protocols (9, 27-31).
Induced pluripotent stem cells-derived hepatocytes
HLCs can be efficiently produced by iPSCs. iPSCs were introduced by the forced expression of a set of transcription factors (Oct4, Sox2, Klf4, and c-Myc genes) using a retroviral vector in somatic cells. These pluripotent reprogrammed cells were called iPSCs. Like ESC lines, iPSCs can differentiate into all three cell lineages including endoderm, while they have intensive proliferation in vitro (32, 33). In other studies, researches were focused on alternative ways to generate iPSC lines, different from integrative viral-mediated strategies, e.g. using excisable viral vectors (34), RNA-Sendai virus vectors (35), episomal plasmids transfections (36), miRNA (37) or mRNA transfections (38) as well as using only chemical compounds (39).
There are many studies which show that iPSCs can differentiate into HLCs (24, 40-43). These generated HLCs had some characteristics of human hepatocytes, particularly in morphology and phenotype (44), but regarding functional assays and metabolic activity, these cells were similar to immature hepatocytes (44, 45). Therefore, numbers of research group have been trying to increase the efficiency of differentiation of iPSCs to HLCs in 2D and 3D with different differentiation protocols (9, 24, 40, 46, 47). In both 2D and 3D differentiation protocols, the extracellular matrix, additional cell-cell interactions, the media and supplements, i.e. growth factors and cytokines, ensure the successful differentiation of iPSCs to HLCs (48). Recently, it has been shown that in 3D culture, the maturity of HLCs derived from iPSCs was increased (46). Moreover, spatially patterning of the cells, known as self- organization, to give rise to "organoid structures" has been introduced in 3D cultures (49). These structures can be expanded without limiting, cryopreserved as biobanks and easily manipulated using techniques established for 2D culture (50). Takebe et al. (29) in 2013 demonstrated when human iPSCs were co-cultured with endothelial and mesenchymal cells, they were self-organized in vitro into structures like small liver organoids, also called "liver buds", while they could be transplanted. Moreover, in other studies, researchers have tried to manipulate current protocols for more hepatocyte maturity, including drug metabolism activity (51).
Gestational stem cell-derived hepatocytes
Another source of HLCs is gestational stem cells derived from umbilical cord, umbilical cord blood, placenta, and amniotic fluid. This type of stem cells, which is easily accessible, can generate other cell lineages in vitro and in vivo (52, 53). Studies showed that human umbilical cord stem cells could differentiate into HLCs in vitro, with hepatocyte-like morphology and high-level expression of hepatic lineage markers. These cells could differentiate into HLCs in vivo either after injection into the NOD-SCID mice with induced liver damage (54, 55). In addition, human placenta-derived multipotent cells can also differentiate into other cell types including HLCs with primary hepatocyte characteristics (56). Gestational stem cells do not form teratomas or teratocarcinomas in humans, while they have a high proliferation rate and differentiation potential. Because of the plasticity and accessibility of these stem cells, many cord blood banks have been established for the collection and storage of these cells for future applications (57).
Mesenchymal stromal cells derived hepatocytes
Generation of HLCs from mesenchymal stromal cells (MSCs) using different sources such as bone marrow (BM-MSCs), umbilical cord blood (UC-MSCs), stem cell-derived (ESC-MSCs) and adipose tissue MSCs (Ad-MSCs) have been previously described (58). These types of stem cells are fibroblast-like, plastic-adherent and multipotent cells, rapidly expanding in vitro under standard conditions. MSCs have low immunogenicity and possess immunomodulatory properties, so they are commonly used in cirrhosis (59-63). They can differentiate into HLCs, expressing particular hepatic genes and presenting some metabolic activities (64). Culturing BM-MSCs in hepatocyte-conditioned medium without any growth factors can induce hepatic cell differentiation (65). Combination of HGF, nicotinamide and dexamethasone in MSCs culture medium could induce hepatic fate in MSCs (66). Moreover, other growth factors like insulin-like growth factor-I in combination with liver specifi c factors have been reported to differentiate MSCs into HLCs (67).
Direct cell conversion to hepatocyte-like cells
Direct conversion of adult cells like fibroblasts to other mature or progenitor somatic cells is an alternative way to bypass the pluripotent iPSC step, mainly by ectopic expression of particular cell-specific transcription factors (TFs). In this regard, functional cells are directly generated, which are useful in advanced clinical applications as well as basic science studies (68, 69). By this new method, known as "transdifferentiation", different research groups successfully converted mouse and human fibroblasts to other lineages, including neurons, cardiomyocytes and hepatocytes, the latter one was also called induced hepatocytes (iHeps). Sekiya and Suzuki (70) showed that a combination of Hnf4a with Foxa1, Foxa2 or Foxa3 could convert mouse adult fibroblasts into iHeps and this generated cells could repopulate and save the genetically modified mice model, with deficiency in the fumarylacetoacetate hydrolase (Fah) activity, leading to the accumulation of metabolites of tyrosine that are toxic to native hepatocytes.
In another study, fibroblasts were converted to iHeps by the transduction of Hnf1a plus Gata4 and Foxa3, and inactivation of p19Arf. This iHeps could repopulate the livers of Fah- /- mice, increasing the survival rate in the recipients (71). Similar technique was used to generate human induced hepatocyte (hiHeps) by the forced expression of HNF1α, HNF4α, and HNF6 with ATF5, PROX1, C/EBPβ as the maturation factors and P53-shRNA (72). In another study by increasing the expression of FOXA3, HNF1α, HNF4α and FOXA2, iHeps were generated (73). In both studies, in vitro gene expression profiles of hiHeps were similar to mature human hepatocytes and they showed in vivo functionality in FRG (Fah-/-/Rag2- /-/Il2rg) mouse model. In another strategy, Yamanaka factors were used for generation of an epigenetic instability, along with the small molecules and/or a cocktail of Hnf4a, Cebpa and Nr1i2 under hepatic inducing conditions (74). In addition, it has been shown that Kdm2b as an epigenetic modulator with HNF4α and Foxa3 could accelerate generation of iHeps in hepatic media (75).
Applications of in vitro produced hepatocyte-like cells
Applications of hepatocyte-like cells in cell therapy of acquired liver diseases
There are many patients suffering from liver disease worldwide. Many of these patients with acquired liver diseases, such as acute liver failures (ALF), fulminant hepatic failure and chronic liver diseases can benefit from cell therapy, specially hepatocyte transplantation (2, 76). In acute liver failure and fulminant hepatic failure, liver metabolic functions are seriously deteriorated following the loss of hepatocytes mass caused by toxins, drugs and hepatotrophic viruses. In chronic liver diseases, severe alteration of hepatic microarchitecture is followed by generation of fibrotic areas (77). Recently fibrosis regression was reported by in vivo hepatocyte transplantation. In these studies myofibroblasts, recruited in fibrotic areas, were reprogrammed into hepatocytes (78, 79).
Up to now many clinical trials with hepatocyte transplantation have been successfully performed with cost effeciecy and simply doing cell administration by intravascular injection rather than surgery (1, 4, 80-84). Moreover, it is possible to use cryopreserved hepatocytes and may even be transplanted them repeatedly (85). Due to the limitations for cell therapy with hepatocyte, such as insufficient numbers and low viability of cells, in vitro generated HLCs offer a new arena for basic studies and a potential source for possible use in therapy in the future (6, 14).
Human iPSCs derived hepatocyte-like cells (iPSCs-HLCs) presented a new platform for the liver cell therapy, but there is no registered clinical trial using these cells for cell therapy (86). However, in a mouse model it has been shown that iPSCs-HLCs could efficiently be engraftd into the liver with normal function (40). These cells were applied in lethal fulminant hepatic failure in non-obese diabetic severe combined immunodeficient mice and rescued them after cell therapy (87). Because of the ethical advantages of iPSCs and using autologous starting cells, as an important step toward "personalized medicine", it seems that iPSCs-HLCs have potential of clinical applications in future of liver diseases (6, 88). Recently, iPSCs have been generated without viral vectors and transgene-free sequences by non-integrating episomal vectors (36). Transdifferentiation of MSCs into HLCs has already been reported and these generated HLCs has been characterized in vitro and in vivo (58). Animal model studies showed that in vitro pre-differentiated MSCs could boost liver repopulation and functionality of hepatic cells (58, 89). Moreover, co-transplantation of iPSCs-HLCs and MSCs could be a suitable option for the treatment of end-stage liver disease, due to the paracrine effects of drived MSC trophic factors (76). In Table 1, current cell sources for liver cell therapy, as well as some HLCs from various sources, as potential cell types appropriate for cell therapy, are described.
Table 1.
Cell therapy of various liver disease with potential pluripotent stem cells-derived HLCs and other appropriate cells
| Cell sources | Role in disease types | Clinical trial | Disadvantage | Reference |
|---|---|---|---|---|
| Hepatocytes | Metabolic liver disorderLiver disorders in infantsAutoimmune liver disorders | Yes | Possibility of infection with hepatitis virusesDecreased engraftmentability in injured liverLimited access | (90) |
| hESCs-HLCs | Liver disordersMetabolic liver disorder | No | Unknown maintenance in long-term | (91) |
| hiPSCs-HLCs | Liver disordersMetabolic liver disorder | No | No fully function Unknown maintenance in long-term | (92) |
| MSCs | Liver disordersCirrhosis | There are numbers of clinical reports | Some negative results in clinical studies | (93) |
HLC; Hepatocyte-like cells, hESCs; Human embryonic stem cells, hiPSCs; Human induced pluripotent stem cells, and MSCs; Mesenchymal stem cells.
Challenges in cell therapy with hepatocyte-like cells
The ESCs/ iPSCs derived HLCs with current protocols have a fetal-like phenotype, rather than a mature hepatocyte phenotype, however, it is possible to induce more maturation in vivo (45). Heterogeneous populations of HLCs, including differentiated and undifferentiated cells may increase the risk of tumorogenicity due to the high proliferation capacity of undifferentiated cells (94, 95). Then enrichment strategies create a new platform for generating HLCs for future clinical and pharmaceutical application (9). Furthermore, for any potential clinical use of HLCs derived from pluripotent cells, the iPSCs and ESCs should be generated in good manufacturing practice (GMP) condition as an appropriate system for protection of products and control them according to accridated quality standards. Therefore, many research groups are working to improve the protocol for generating HLCs from these pluripotent cells. Up to now, some studies showed that transplantation of ESC-derived HLCs in animal models could improve hepatic function (19, 96-101), but ethical concerns and regulatory issues, immunologic rejection and tumorogenicity are still the main limiting factors. To avoid time consuming procedure in the generation of HLCs from pluripotent stem cells under monolayer culture, we need to produce HLCs in 3D suspension culture for the clinical applications (102). Vosough et al. (9) described a scalable stirred-suspension bioreactor culture of functional HLCs from the hPSCs. After intrasplenic transplantation of these HLCs in acute liver injury, an increased survival rate and efficient engraftment of functional cells were observed.
Applications of hepatocyte-like cells in cell therapy of acquired liver diseases
iPSC technology provides the possibility of "gene correction" on patient somatic cells. The gene correction on iPSCs can be applied for patients with monogenic inherited metabolic liver diseases, like Alpha-1 antitrypsin deficiency and Wilson’s disease (mutation in ATP7B gene). After ex vivo gene correction, iPSCs can differentiate into hepatocyte and then be transplanted to the patient (76). In gene correction of Alpha-1 antitrypsin deficiency in iPSCs, scientists showed that a combination of two targeted gene technologies, zinc finger nucleases (ZFNs) and PiggyBac (PB) technology, which were significantly efficient gene-targeting technology, could correct a point mutation (Glu342Lys) and then corrected iPSCs-HLCs could restore the structure and function in vitro and in vivo (103). In another study, researchers tried to generate iPSCs from a Chinese patient with Wilson’s disease (WD) that bears the R778L Chinese hotspot mutation in the ATPase Cu2+ transporting beta polypeptide (ATP7B) gene.
After gene correction using a self-inactivating lentiviral vector that expresses codon-optimized ATP7B, these iPSCs were differentiated into HLCs with copper metabolism capacity, which reversed the functional defect in vitro. These studies could introduce a new way for generating disease modeling valuable for screening alleviate compounds or gene therapy approaches (104).
Recently, some new technologies such as clustered regularly interspaced short palindromic repeats (CRISPR)/Cas based RNA-guided DNA endonucleases , as a new and powerful genome editing tool, allow precise gene editing in liverbased monogenic disorders in animal models by either permanently deleting/inserting specific genetic sequences or adding/removing epigenetic information temporarily with minimal off-target modifications (105, 106). In a new study, researchers used dual adeno-associated virus (AAV) vectors to deliver the CRISPR/Cas9 components, one expressing Cas9 and the other expressing a guide RNA as well as the donor DNA, to newborn mice with a partial deficiency in the urea cycle disorder enzyme, ornithine transcarbamylase (OTC), which resulted in improvment of their survival rate. They limited any off-target activity by using a liver-specific promoter for Cas9, ensuring its expression only within liver cells (107). In another study, viral and non-viral delivery systems were used. So, gene editing was accomplished with a combination of lipid nanoparticle–mediated delivery of Cas9 mRNA with adeno-associated viruses encoding a sgRNA and a repair template, to induce repair of a human hereditary tyrosinemia disease gene in adult mouse models. In this report, disease symptoms, such as weight loss and liver damage, were rescued. In addition, the efficiency of correction was reported less than 6% of hepatocytes after a single application (108). These presented results and efficacy of corrections were suggested potential utility of CRISPR-mediated gene repair for genetic diseases.
Applications of hepatocyte-like cells in cell therapy of acquired liver diseases
HLCs, obtained from various sources, have cytochrome P450 (CYPs) activity, which is crucial for metabolism of xenobiotics and drugs (Table 2) (45, 73, 109). Although one of the main goal of improving differentiation protocols in different studies is increasing the functionality of generating cells in drug metabolism and appropriate CYPs activity. Recent studies tried to show an increasing activity of HLCs in drug metabolism (51, 72, 109). To measure the capability of HLCs for drug metabolism, particular substances were introduced, including phenacetin (CYP1A), bupropion (CYP2B6), diclofenac (CYP2C9) and midazolam (CYP3A) (109). Before evaluating the HLCs, they should be treated with an appropriate inducer, like phenobarbital and rifampicin. In addition, mRNA and protein expression of important CYPs in the presence and absence of inducers can be checked in HLCs (70, 109-112).
If researchers can generate HLCs with the ability of drug metabolism, these cells might be replaced with primary hepatocyte as a "gold standard" for drug metabolism and drug toxicity tests (86). Activity and expression of drug transporters are another characteristic that sometimes were assessed in generating HLCs in various studies to evaluate the quality of them compared to primary hepatocytes. Therefore the activity of the uptake transporters such as organic anion transporting polypeptides (OATPs), OATP1B1 and Na(+)-taurocholate cotransporting polypeptide (NTCP) as well as the efflux transporter bile salt export pump (BSEP) were evaluated (109).
Another important application of HLCs, especially with iPSC technology, is in discovery of new and safe drugs, small molecules and components to alleviate the respective property after high-throughput screening. In this way, HLCs produced by patient specific iPSCs were checked by the array of drugs or components to find new drugs or investigating toxicity of drugs. Moreover, testing drugs for iPSC that belong to an individual, admirably help to choose the best drug among various candidates and also determine the best dose of the chosen drug for every patient (14, 86, 113). Undoubtedly, this is the best way for "personalized drug administration" which will guide toward the future medicine. In addition, recently bioengineering tools such as microfluidicbased cell culture device opened new window to drug studies with HLCs (114). This controlled system allows exact spatial and frequent delivery of media, drugs and signaling factors to live cells (115). Giobbe et al. (116) reported a microfluidic system on the chip to differentiate PSCs into HLCs to predict drug toxicity. Further more, by providing a controlled microenvironment for generating HLCs, microfluidic systems make it feasible to study the candidate or new drug metabolism and drug-response screening in high-throughput testing (117, 118).
Recent developments in humanized liver models present a promising horizon in the future preclinical applications of generated hepatocytes in vivo, especially for drug discovery (3). It has been reported that human hepatocyte can be transplanted into the animal models and they can be repopulated in the host liver (119). Highly immunodeficient FRG [Fah(/) Rag2(/) Il2rg (/)] mice are currently the best model for repopulation of human hepatocyte in animals. This model is T, B and NK cells deficient, in addition to deficiency in the fumarylacetoacetate hydrolase (Fah) activity, as an enzyme catalyzing the last step of tyrosine metabolism (120). Researchers reported that FRG mice liver can be repopulated efficiently with human hepatocyte and display a serum lipoprotein profile similar to human apolipoproteins.
Table 2.
Examples of recently reported CYP enzymes activity and drug metabolisms in generated HLCs from different sources
| Cell sources | CYP enzymes/Drug metabolism | Method of analysis | Inducer | Reference |
|---|---|---|---|---|
| hESC | CYP1A1, CYP1A2, CYP3A4, CYP7A1, CYP1B1, CYP2B6, CYP2C9,CYP2C19, CYP2D6, CYP2E1 | PCR | No | (121) |
| hESC, hiPSC | CYP1A2, CYP3A4, CYP3A7, CYP2D6, CYP2C9, CYP2C19 | Immunohistochemistry/ luminescence based kit | Phenobarbital, rifampicin and acetaminophen | (122) |
| iPSCs | CYP3A4 | luminescence based kit | No | (46) |
| iPSCs | CYP1A1 | EROD | Ethoxyresorufin, dicumarol | (123) |
| Fibroblast (direct conversion) | CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP2C9, CYP2C19, CYP1A2, CYP3A4Testosterone, midazolam, phenacetin, bupropion, diclofenac, S-mephenytoin | qPCR, IF,HPLC-MS | Rifampicin, b-naphthoflavone, phenobarbital | (72) |
| Fibroblast (direct conversion) | CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP3A4/phenacetin, coumarin, dextromethorphan | qPCR,LC-MS/MS | 3-methylcholanthrene,phenobarbital, or rifampicin | (73) |
| AT-MSC | CYP1A1, CYP1A2, CYP2A1, CYP2C7, CYP2C12, CYP2E1, CYP3A1Phenacetin, coumarin, chlorzoxazone | qPCR,LC-MS/MS | No inducer,3-methylcholanthrene, phenobarbital,and acetone | (124) |
| Human umbilical cord-derived MSC | CYP3A4 | Liquid chromatography | Midazolam | (125) |
HLCs; Hepatocyte-like cells, hESC; Human emberyonic stem cell, iPSCs; Induced pluripotent stem cells, AT-MSC; Adipose-derived mesenchymal stem cells, qPCR; Quantitative polymerase chain reaction, IF; Immunofluorescence, HPLC-MS; Liquid chromatography-mass spectrometry, and LC-MS/MS; Liquid chromatography tandem-mass spectrometry.
They displayed that these humanized FRG mice have capacity to be a suitable model for atherosclerosis and cholesterol metabolism. Moreover and interestingly, in these mice drug metabolizing enzyme system were humanized either (126). Hickey et al. (127) represented a new Fah(/) pigs, as a large animal model. Fah deficiency is an utero lethal difficulty in pigs that is correctable with administration of 2-(2-nitro- 4-trifluoromethylbenzoyl)-1,3 cyclohexanedione (NTBC). After withdrawing NTBC, FAH−/− pigs died due to acute liver failure. This animal model may be suitable platform to generate HLCs with further functionality. Another research group tried to improve repopulation efficacy of humanized liver in chimeric mice with transduction of HLCs by adenovirus vector (Ad-FNK) to express FNK. Overexpression of FNK resulted in apoptosis inhibition in HLCs. In this study, Ad-FNKtransduced human iPSC-HLCs transplanted into urokinase-type plasminogen activator-transgenic severe combined immunodeficiency (uPA/SCID) mice and assessed the effectiveness of repopulation. Human albumin levels, human hepatocyte-related genes and proteins in the transplanted mice were significantly increased in this model (128). Briefly, human pluripotent stem cell-derived HLCs are appropriate and promising sources for the generation of humanized liver models.
Achieving adequate and appropriate HLCs, is an important route for cell therapy of patients with end-stage liver failure (48). There are some potential sources that researchers have focused on it in recent years for obtaining functional HLCs, including mainly stem cells-based sources like MSCs, pluripotent stem cells, hESC and induced pluripotent stem cell (iPSC) lines, as well as direct lineage conversion of adult somatic cells to HLCs as a new strategy (129). HLCs exhibit many phenotypes and some functional traits of mature hepatocytes (6). Up to now there is no registered clinical trial using HLCs, but there are some considerable potential advantages of HLCs against primary hepatocytes, especially for iPSC-HLCs, including potential of large scale production, patient-specificity of iPSC-HLCs preventing transplanted cell immunorejection, possibility of gene editing of autologous iPSCHLCs with non-integrated tools to treat inherited genetic liver diseases prior to differentiation and transplantation, especially with helping new tools such as the most widely used engineered CRISPR/ Cas system (77, 107, 108). The CRISPR/Cas9 method, as a powerful genome editing system, successfully corrected point mutations in A1AT deficiency disease-specific iPSCs (105). Moreover, PCSK9 gene was mutated by CRISPR/Cas9 system in mouse liver for changing the lipid profile of animals, i.e. decreasing plasma cholesterol level and increasing LDL receptors (130). In another study, an adenovirus based CRISPR/Cas9 system for in vivo gene editing precisely knocked-out the CEBPα gene, as an important transcription factor for metabolic genes in the liver organ (131).
Various studies have confirmed the feasibility of generating and cultivating human pluripotent stem cells in stirred suspension bioreactors (9, 132). Using this technology helped us to move forward to practical applications, requiring a large number of cells for treatment and high- throughput drug screening (48).
HLCs have been shown to be a powerful in vitro system not only to study patient-specific disease model and some human liver disease, i.e. viral hepatitis and plasmodium infection (133- 136), but also to drug study, especially with the help of modern techniques such as microfluidicbased cell culture platforms. This system either allows to improve HLCs generation by providing monitoring of culture parameters or helping to co-culture of HLCs with the other cells, even from other organs, make it possible to investigate unintended systemic side-effects of therapeutic agents and their metabolites (137). Moreover, human pluripotent stem cells-derived HLCs provide infinite and genetically defined sources for humanized liver models. These animal models will provide many in vitro applications, including highthroughput drug screening, toxicology and further applications in liver assist devices (138).
Finding potential sources and strategies for generating HLCs open a new window in liver regenerative medicine, but many considerable experimental challenges remain to be solved including finding new methods or biomolecules improving the efficiency as well as extending differentiation of these cells and metabolic activity of generated HLCs. Besides, finding new improved methods to enrich, purify and large-scale production of HLCs are necessary. On the other hand, development of suitable animal models and efficient HLCs delivery strategies should be considered for improvement of future clinical use (139).
Conclusion
In vitro generation of HLCs using pluripotent or non-pluripotent cells via differentiation and direct conversion to hepatocytes provides potential applications in regenerative medicine, via cell and gene therapies for liver diseases and drug discovery. At this time, considerable experiments continue to increase the functionality of generated HLCs to introduce them as a suitable replacement for primary hepatocytes.
Acknowledgments
We express our gratitude to all members of the Hepatocyte Program at Royan Institute for their kind cooperation. This review has not received any financial support. The authors have no conflict of interest.
References
- 1.Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40(6):878–886. doi: 10.1016/j.jhep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, Strom SC. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res. 2015;54(3-4):162–177. doi: 10.1159/000369552. [DOI] [PubMed] [Google Scholar]
- 3.Gramignoli R, Tahan V, Dorko K, Skvorak KJ, Hansel MC, Zhao W, et al. New potential cell source for hepatocyte transplantation: discarded livers from metabolic disease liver transplants. Stem Cell Res. 2013;11(1):563–573. doi: 10.1016/j.scr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7(5):288–298. doi: 10.1038/nrgastro.2010.44. [DOI] [PubMed] [Google Scholar]
- 5.Mazariegos G, Shneider B, Burton B, Fox IJ, Hadzic N, Kishnani P, et al. Liver transplantation for pediatric metabolic disease. Mol Genet Metab. 2014;111(4):418–427. doi: 10.1016/j.ymgme.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: from liver transplantation to cell factory. J Hepatol. 2015;62(1 Suppl):S157–169. doi: 10.1016/j.jhep.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 7.Strom SC, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K, et al. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006;15(Suppl 1):S105–110. doi: 10.3727/000000006783982395. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MD, Ware BR, Khetani SR. Stem cell-derived liver cells for drug testing and disease modeling. Discov Med. 2015;19(106):349–358. [PMC free article] [PubMed] [Google Scholar]
- 9.Vosough M, Omidinia E, Kadivar M, Shokrgozar MA, Pournasr B, Aghdami N, et al. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cells Dev. 2013;22(20):2693–2705. doi: 10.1089/scd.2013.0088. [DOI] [PubMed] [Google Scholar]
- 10.Sivertsson L, Synnergren J, Jensen J, Bjorquist P, Ingelman-Sundberg M. Hepatic differentiation and maturation of human embryonic stem cells cultured in a perfused three-dimensional bioreactor. Stem Cells Dev. 2013;22(4):581–594. doi: 10.1089/scd.2012.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivertsson L, Synnergren J, Jensen J, Bjorquist P, Ingelman-Sundberg M. Hepatic differentiation and maturation of human embryonic stem cells cultured in a perfused three-dimensional bioreactor. Stem Cells Dev. 2013;22(4):581–594. doi: 10.1089/scd.2012.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 13.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 14.Behbahan IS, Duan Y, Lam A, Khoobyari S, Ma X, Ahuja TP, et al. New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Rev. 2011;7(3):748–759. doi: 10.1007/s12015-010-9216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 16.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000;97(21):11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72(5):230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 18.Lavon N, Benvenisty N. Study of hepatocyte differentiation using embryonic stem cells. J Cell Biochem. 2005;96(6):1193–1202. doi: 10.1002/jcb.20590. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26(5):1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 20.Gerbal-Chaloin S, Funakoshi N, Caillaud A, Gondeau C, Champon B, Si-Tayeb K. Human induced pluripotent stem cells in hepatology: beyond the proof of concept. Am J Pathol. 2014;184(2):332–347. doi: 10.1016/j.ajpath.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Baharvand H, Hashemi SM, Shahsavani M. Differentiation of human embryonic stem cells into functional hepatocyte-like cells in a serum-free adherent culture condition. Differentiation. 2008;76(5):465–477. doi: 10.1111/j.1432-0436.2007.00252.x. [DOI] [PubMed] [Google Scholar]
- 22.Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol. 2006;50(7):645–652. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Liu J, Liu Y, Li Z, Gao WQ, He Z. Generation, characterization and potential therapeutic applications of mature and functional hepatocytes from stem cells. J Cell Physiol. 2013;228(2):298–305. doi: 10.1002/jcp.24150. [DOI] [PubMed] [Google Scholar]
- 24.Siller R, Greenhough S, Naumovska E, Sullivan GJ. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Reports. 2015;4(5):939–952. doi: 10.1016/j.stemcr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sineva GS, Pospelov VA. Inhibition of GSK3beta enhances both adhesive and signalling activities of beta-catenin in mouse embryonic stem cells. Biol Cell. 2010;102(10):549–560. doi: 10.1042/BC20100016. [DOI] [PubMed] [Google Scholar]
- 26.McCoy AT, Benoist CC, Wright JW, Kawas LH, Bule-Ghogare JM, Zhu M, et al. Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents. J Pharmacol Exp Ther. 2013;344(1):141–154. doi: 10.1124/jpet.112.199497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavon N. Generation of hepatocytes from human embryonic stem cells. Methods Mol Biol. 2010;640:237–246. doi: 10.1007/978-1-60761-688-7_11. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa S, Surapisitchat J, Virtanen C, Ogawa M, Niapour M, Sugamori KS, et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development. 2013;140(15):3285–3296. doi: 10.1242/dev.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 30.Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9(2):396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 31.Farzaneh Z, Pakzad M, Vosough M, Pournasr B, Baharvand H. Differentiation of human embryonic stem cells to hepatocyte-like cells on a new developed xeno-free extracellular matrix. Histochem Cell Biol. 2014;142(2):217–226. doi: 10.1007/s00418-014-1183-4. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 34.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 40.Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, et al. Highly efficient generation of human hepatocytelike cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19(11):1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 42.Ghodsizadeh A, Taei A, Totonchi M, Seifinejad A, Gourabi H, Pournasr B, et al. Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem Cell Rev. 2010;6(4):622–632. doi: 10.1007/s12015-010-9189-3. [DOI] [PubMed] [Google Scholar]
- 43.Asgari S, Moslem M, Bagheri-Lankarani K, Pournasr B, Miryounesi M, Baharvand H. Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rev. 2013;9(4):493–504. doi: 10.1007/s12015-011-9330-y. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014;32(2):504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baxter M, Withey S, Harrison S, Segeritz CP, Zhang F, Atkinson-Dell R, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62(3):581–589. doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gieseck RL 3rd, Hannan NR, Bort R, Hanley NA, Drake RA, Cameron GW, et al. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One. 2014;9(1):e86372–e86372. doi: 10.1371/journal.pone.0086372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19(11):1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 48.Hannoun Z, Steichen C, Dianat N, Weber A, Dubart-Kupperschmitt A. The potential of induced pluripotent stem cell derived hepatocytes. J Hepatol. 2016;65(1):182–199. doi: 10.1016/j.jhep.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Wan AC. Recapitulating cell-cell interactions for organoid construction - are biomaterials dispensable? Trends Biotechnol. 2016;34(9):711–721. doi: 10.1016/j.tibtech.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18(3):246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 51.Kondo Y, Iwao T, Nakamura K, Sasaki T, Takahashi S, Kamada N, et al. An efficient method for differentiation of human induced pluripotent stem cells into hepatocytelike cells retaining drug metabolizing activity. Drug Metab Pharmacokinet. 2014;29(3):237–243. doi: 10.2133/dmpk.dmpk-13-rg-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82(3):252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 53.Gilchrist ES, Plevris JN. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transpl. 2010;16(2):118–129. doi: 10.1002/lt.21965. [DOI] [PubMed] [Google Scholar]
- 54.Sancho-Bru P, Najimi M, Caruso M, Pauwelyn K, Cantz T, Forbes S, et al. Stem and progenitor cells for liver repopulation: can we standardise the process from bench to bedside? Gut. 2009;58(4):594–603. doi: 10.1136/gut.2008.171116. [DOI] [PubMed] [Google Scholar]
- 55.Piscaglia AC, Di Campli C, Zocco MA, Di Gioacchino G, Novi M, Rutella S, et al. Human cordonal stem cell intraperitoneal injection can represent a rescue therapy after an acute hepatic damage in immunocompetent rats. Transplant Proc. 2005;37(6):2711–2714. doi: 10.1016/j.transproceed.2005.06.076. [DOI] [PubMed] [Google Scholar]
- 56.Chien CC, Yen BL, Lee FK, Lai TH, Chen YC, Chan SH, et al. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells. 2006;24(7):1759–1768. doi: 10.1634/stemcells.2005-0521. [DOI] [PubMed] [Google Scholar]
- 57.Piscaglia AC, Campanale M, Gasbarrini A, Gasbarrini G. Stem cell-based therapies for liver diseases: state of the art and new perspectives. Stem Cells Int. 2010;2010:259461–259461. doi: 10.4061/2010/259461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsolaki E, Yannaki E. Stem cell-based regenerative opportunities for the liver: state of the art and beyond. World J Gastroenterol. 2015;21(43):12334–12350. doi: 10.3748/wjg.v21.i43.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10(4):459–466. [PubMed] [Google Scholar]
- 60.Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21(10):1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 61.Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: shortterm and long-term outcomes. Hepatology. 2011;54(3):820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 62.Zhao W, Li JJ, Cao DY, Li X, Zhang LY, He Y, et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18(10):1048–1058. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vosough M, Moossavi S, Mardpour S, Akhlaghpoor S, Azimian V, Jarughi N, et al. Repeated intraportal injection of mesenchymal stem cells in combination with pioglitazone in patients with compensated cirrhosis: a clinical report of two cases. Arch Iran Med. 2016;19(2):131–136. [PubMed] [Google Scholar]
- 64.Liu WH, Song FQ, Ren LN, Guo WQ, Wang T, Feng YX, et al. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 2015;19(3):511–520. doi: 10.1111/jcmm.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007;102(1):52–63. doi: 10.1002/jcb.21275. [DOI] [PubMed] [Google Scholar]
- 66.Chivu M, Dima SO, Stancu CI, Dobrea C, Uscatescu V, Necula LG, et al. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl Res. 2009;154(3):122–132. doi: 10.1016/j.trsl.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 67.Ayatollahi M, Soleimani M, Tabei SZ, Kabir Salmani M. Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I. World J Stem Cells. 2011;3(12):113–121. doi: 10.4252/wjsc.v3.i12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pournasr B, Khaloughi K, Salekdeh GH, Totonchi M, Shahbazi E, Baharvand H. Concise review: alchemy of biology: generating desired cell types from abundant and accessible cells. Stem Cells. 2011;29(12):1933–1941. doi: 10.1002/stem.760. [DOI] [PubMed] [Google Scholar]
- 69.Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012;14(9):892–899. doi: 10.1038/ncb2567. [DOI] [PubMed] [Google Scholar]
- 70.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475(7356):390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 71.Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475(7356):386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 72.Du Y, Wang J, Jia J, Song N, Xiang C, Xu J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell. 2014;14(3):394–403. doi: 10.1016/j.stem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14(3):370–384. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Pournasr B, Asghari-Vostikolaee MH, Baharvand H. Transcription factor-mediated reprograming of fibroblasts to hepatocyte-like cells. Eur J Cell Biol. 2015;94(12):603–610. doi: 10.1016/j.ejcb.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Zakikhan K, Pournasr B, Nassiri-Asl M, Baharvand H. Enhanced direct conversion of fibroblasts into hepatocyte-like cells by Kdm2b. Biochem Biophys Res Commun. 2016;474(1):97–103. doi: 10.1016/j.bbrc.2016.04.076. [DOI] [PubMed] [Google Scholar]
- 76.Yu Y, Wang X, Nyberg SL. Application of induced pluripotent stem cells in liver diseases. Cell Med. 2014;7(1):1–13. doi: 10.3727/215517914X680056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldman O, Gouon-Evans V. Human pluripotent stem cells: myths and future realities for liver cell therapy. Cell Stem Cell. 2016;18(6):703–706. doi: 10.1016/j.stem.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 78.Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, et al. Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell. 2016;18(6):797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 79.Rezvani M, Espanol-Suner R, Malato Y, Dumont L, Grimm AA, Kienle E, et al. In vivo hepatic reprogramming of myofibroblasts with AAV vectors as a therapeutic strategy for liver fibrosis. Cell Stem Cell. 2016;18(6):809–816. doi: 10.1016/j.stem.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weber A, Groyer-Picard MT, Franco D, Dagher I. Hepatocyte transplantation in animal models. Liver Transpl. 2009;15(1):7–14. doi: 10.1002/lt.21670. [DOI] [PubMed] [Google Scholar]
- 81.Ito M, Nagata H, Miyakawa S, Fox IJ. Review of hepatocyte transplantation. J Hepatobiliary Pancreat Surg. 2009;16(2):97–100. doi: 10.1007/s00534-008-0023-0. [DOI] [PubMed] [Google Scholar]
- 82.Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant. 2004;4(Suppl 6):7–13. doi: 10.1111/j.1600-6135.2004.0340.x. [DOI] [PubMed] [Google Scholar]
- 83.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19(1):39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 84.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338(20):1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 85.Stephenne X, Najimi M, Sokal EM. Hepatocyte cryopreservation: is it time to change the strategy? World J Gastroenterol. 2010;16(1):1–14. doi: 10.3748/wjg.v16.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yi F, Liu GH, Izpisua Belmonte JC. Human induced pluripotent stem cells derived hepatocytes: rising promise for disease modeling, drug development and cell therapy. Protein Cell. 2012;3(4):246–250. doi: 10.1007/s13238-012-2918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55(4):1193–1203. doi: 10.1002/hep.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y, Chan L, Nguyen HV, Tsang SH. Personalized medicine: cell and gene therapy based on patient-specific iPSC-derived retinal pigment epithelium cells. Adv Exp Med Biol. 2016;854:549–555. doi: 10.1007/978-3-319-17121-0_73. [DOI] [PubMed] [Google Scholar]
- 89.Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58(4):570–581. doi: 10.1136/gut.2008.154880. [DOI] [PubMed] [Google Scholar]
- 90.Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33(10):1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 91.Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26(4):894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51(1):329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012;8(3):972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]
- 94.Takayama K, Inamura M, Kawabata K, Katayama K, Higuchi M, Tashiro K, et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4alpha transduction. Mol Ther. 2012;20(1):127–137. doi: 10.1038/mt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sa-Ngiamsuntorn K, Wongkajornsilp A, Phanthong P, Borwornpinyo S, Kitiyanant N, Chantratita W, et al. A robust model of natural hepatitis C infection using hepatocyte-like cells derived from human induced pluripotent stem cells as a long-term host. Virol J. 2016;13:59–59. doi: 10.1186/s12985-016-0519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45(5):1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 97.Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires Activin A and Wnt3a signaling. Proc Natl Acad Sci USA. 2008;105(34):12301–12306. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136(3):990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brolen G, Sivertsson L, Bjorquist P, Eriksson G, Ek M, Semb H, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145(3):284–294. doi: 10.1016/j.jbiotec.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 100.Duan Y, Ma X, Zou W, Wang C, Bahbahan IS, Ahuja TP, et al. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28(4):674–686. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- 101.Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51(5):1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- 102.Yu Y, Wang X, Nyberg SL. Potential and challenges of induced pluripotent stem cells in liver diseases treatment. J Clin Med. 2014;3(3):997–1017. doi: 10.3390/jcm3030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yusa K, Rashid ST, Strick-Marchand H, Varela I, Liu PQ, Paschon DE, et al. Targeted gene correction of alpha1- antitrypsin deficiency in induced pluripotent stem cells. Nature. 2011;478(7369):391–394. doi: 10.1038/nature10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang S, Chen S, Li W, Guo X, Zhao P, Xu J, et al. Rescue of ATP7B function in hepatocyte-like cells from Wilson’s disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin. Hum Mol Genet. 2011;20(16):3176–3187. doi: 10.1093/hmg/ddr223. [DOI] [PubMed] [Google Scholar]
- 105.Smith C, Abalde-Atristain L, He C, Brodsky BR, Braunstein EM, Chaudhari P, et al. Efficient and allele-specific genome editing of disease loci in human iPSCs. Mol Ther. 2015;23(3):570–577. doi: 10.1038/mt.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu P, Li KE, Xu S. The future of iPS cells in advancing regenerative medicine. Genet Res (Camb) 2016;98:e4–e4. doi: 10.1017/S001667231600001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang Y, Wang L, Bell P, McMenamin D, He Z, White J, et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol. 2016;34(3):334–338. doi: 10.1038/nbt.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, et al. Therapeutic genome editing by combined viral and nonviral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34(3):328–333. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ulvestad M, Nordell P, Asplund A, Rehnstrom M, Jacobsson S, Holmgren G, et al. Drug metabolizing enzyme and transporter protein profiles of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochem Pharmacol. 2013;86(5):691–702. doi: 10.1016/j.bcp.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 110.Tasnim F, Phan D, Toh YC, Yu H. Cost-effective differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules. Biomaterials. 2015;70:115–125. doi: 10.1016/j.biomaterials.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Donato MT, Gomez-Lechon MJ. Fluorescence-based screening of cytochrome P450 activities in intact cells. Methods Mol Biol. 2013;987:135–148. doi: 10.1007/978-1-62703-321-3_12. [DOI] [PubMed] [Google Scholar]
- 112.Donato MT, Jimenez N, Castell JV, Gomez-Lechon MJ. Fluorescence-based assays for screening nine cytochrome P450 (P450) activities in intact cells expressing individual human P450 enzymes. Drug Metab Dispos. 2004;32(7):699–706. doi: 10.1124/dmd.32.7.699. [DOI] [PubMed] [Google Scholar]
- 113.Chun YS, Chaudhari P, Jang YY. Applications of patientspecific induced pluripotent stem cells; focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int J Biol Sci. 2010;6(7):796–805. doi: 10.7150/ijbs.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin C, Ballinger KR, Khetani SR. The application of engineered liver tissues for novel drug discovery. Expert Opin Drug Discov. 2015;10(5):519–540. doi: 10.1517/17460441.2015.1032241. [DOI] [PubMed] [Google Scholar]
- 115.Paguirigan A, Beebe DJ. Gelatin based microfluidic devices for cell culture. Lab Chip. 2006;6(3):407–413. doi: 10.1039/b517524k. [DOI] [PubMed] [Google Scholar]
- 116.Giobbe GG, Michielin F, Luni C, Giulitti S, Martewicz S, Dupont S, et al. Functional differentiation of human pluripotent stem cells on a chip. Nat Methods. 2015;12(7):637–640. doi: 10.1038/nmeth.3411. [DOI] [PubMed] [Google Scholar]
- 117.Du G, Fang Q, den Toonder JM. Microfluidics for cellbased high throughput screening platforms-a review. Anal Chim Acta. 2016;903:36–50. doi: 10.1016/j.aca.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 118.Wu MH, Huang SB, Lee GB. Microfluidic cell culture systems for drug research. Lab Chip. 2010;10(8):939–956. doi: 10.1039/b921695b. [DOI] [PubMed] [Google Scholar]
- 119.Strom SC, Davila J, Grompe M. Chimeric mice with humanized liver: tools for the study of drug metabolism, excretion, and toxicity. Methods Mol Biol. 2010;640:491–509. doi: 10.1007/978-1-60761-688-7_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25(8):903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park HJ, Choi YJ, Kim JW, Chun HS, Im I, Yoon S, et al. Differences in the epigenetic regulation of cytochrome P450 genes between human embryonic stem cell-derived hepatocytes and primary hepatocytes. PLoS One. 2015;10(7):e0132992–e0132992. doi: 10.1371/journal.pone.0132992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim JH, Jang YJ, An SY, Son J, Lee J, Lee G, et al. Enhanced metabolizing activity of human ES cell-derived hepatocytes using a 3D culture system with repeated exposures to Xenobiotics. Toxicol Sci. 2015;147(1):190–206. doi: 10.1093/toxsci/kfv121. [DOI] [PubMed] [Google Scholar]
- 123.Sgodda M, Mobus S, Hoepfner J, Sharma AD, Schambach A, Greber B, et al. Improved hepatic differentiation strategies for human induced pluripotent stem cells. Curr Mol Med. 2013;13(5):842–855. doi: 10.2174/1566524011313050015. [DOI] [PubMed] [Google Scholar]
- 124.Xu F, Liu J, Deng J, Chen X, Wang Y, Xu P, et al. Rapid and high-efficiency generation of mature functional hepatocyte-like cells from adipose-derived stem cells by a three-step protocol. Stem Cell Res Ther. 2015;6:193–193. doi: 10.1186/s13287-015-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xue G, Han X, Ma X, Wu H, Qin Y, Liu J, et al. Effect of microenvironment on differentiation of human umbilical cord mesenchymal stem cells into hepatocytes in vitro and in vivo. Biomed Res Int. 2016;2016:8916534–8916534. doi: 10.1155/2016/8916534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ellis EC, Naugler WE, Parini P, Mork LM, Jorns C, Zemack H, et al. Mice with chimeric livers are an improved model for human lipoprotein metabolism. PLoS One. 2013;8(11):e78550–e78550. doi: 10.1371/journal.pone.0078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hickey RD, Mao SA, Glorioso J, Lillegard JB, Fisher JE, Amiot B, et al. Fumarylacetoacetate hydrolase deficient pigs are a novel large animal model of metabolic liver disease. Stem Cell Res. 2014;13(1):144–153. doi: 10.1016/j.scr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagamoto Y, Takayama K, Tashiro K, Tateno C, Sakurai F, Tachibana M, et al. Efficient engraftment of human induced pluripotent stem cell-derived hepatocyte-like cells in uPA/SCID mice by overexpression of FNK, a Bcl-xL mutant gene. Cell Transplant. 2015;24(6):1127–1138. doi: 10.3727/096368914X681702. [DOI] [PubMed] [Google Scholar]
- 129.Vosough M, Moslem M, Pournasr B, Baharvand H. Cellbased therapeutics for liver disorders. Br Med Bull. 2011;100:157–172. doi: 10.1093/bmb/ldr031. [DOI] [PubMed] [Google Scholar]
- 130.Ding Q, Strong A, Patel KM, Ng SL, Gosis BS, Regan SN, et al. Permanent alteration of PCSK9 with in vivo CRISPRCas9 genome editing. Circ Res. 2014;115(5):488–492. doi: 10.1161/CIRCRESAHA.115.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng R, Peng J, Yan Y, Cao P, Wang J, Qiu C, et al. Efficient gene editing in adult mouse livers via adenoviral delivery of CRISPR/Cas9. FEBS Lett. 2014;588(21):3954–3958. doi: 10.1016/j.febslet.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 132.Almutawaa W, Rohani L, Rancourt DE. Expansion of human induced pluripotent stem cells in stirred suspension bioreactors. Methods Mol Biol. 2016;1502:53–61. doi: 10.1007/7651_2015_311. [DOI] [PubMed] [Google Scholar]
- 133.Ng S, Schwartz RE, March S, Galstian A, Gural N, Shan J, et al. Human iPSC-derived hepatocyte-like cells support Plasmodium liver-stage infection in vitro. Stem Cell Reports. 2015;4(3):348–359. doi: 10.1016/j.stemcr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB, et al. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J Clin Invest. 2014;124(11):4953–4964. doi: 10.1172/JCI75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carpentier A, Jake Liang T. Transplantation of iPS-derived hepatocytes into a mouse liver: a new murine model of hepatitis C virus infection. Med Sci (Paris) 2015;31(3):256–259. doi: 10.1051/medsci/20153103010. [DOI] [PubMed] [Google Scholar]
- 136.Tang S, Xie M, Cao N, Ding S. Patient-specific induced pluripotent stem cells for disease Modeling and phenotypic drug discovery. J Med Chem. 2016;59(1):2–15. doi: 10.1021/acs.jmedchem.5b00789. [DOI] [PubMed] [Google Scholar]
- 137.Bale SS, Moore L, Yarmush M, Jindal R. Emerging in vitro liver technologies for drug metabolism and inter-organ interactions. Tissue Eng Part B Rev. 2016 doi: 10.1089/ten.teb.2016.0031. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grompe M, Strom S. Mice with human livers. Gastroenterology. 2013;145(6):1209–1214. doi: 10.1053/j.gastro.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 139.Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6(245):245sr2–245sr2. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]