Key Clinical Message
Subcutaneous fat necrosis (SFN) in infants producing severe hypercalcemia is a life‐threatening emergency. Pathophysiology may include enhanced gastrointestinal calcium absorption and bone resorption. We treated an infant with SFN and serum calcium of 15 mg/dL with prednisolone and low‐dose zoledronic acid. Serum calcium promptly normalized without rebound hypocalcemia, and redosing of zoledronic acid was not necessary.
Keywords: Failure to thrive, hypercalcemia, neonatal, subcutaneous fat necrosis, zoledronic acid
Neonatal subcutaneous fat necrosis (SFN) is a transient, generally benign panniculitis that presents within several weeks of life. The cause of SFN is not well understood, but the condition is believed to be a sequela of perinatal stress, including hypothermia or hypoxia 1, 2, 3, 4. Full‐term neonates classically develop firm, tender nodules with overlying purple erythematous plaques located on the cheeks, trunk, arms, thighs, and buttocks 1, 2. Plaques may be flesh colored or absent 1, 3. Neonates have relatively high concentrations of saturated fatty acids in adipose tissue 2. Insult to this tissue by way of ischemia or hypothermia may cause crystallization within granulomas (a hallmark of SFN). Some researchers have suggested that the relatively high melting point of the saturated fatty acids may predispose to its solidification and crystallization in cases of hypothermia 2. Further, the resulting inflammation leads to subsequent granuloma formation, believed to be related to the development of hypercalcemia 2. The majority of neonatal SFN cases occur in full‐term infants born via emergency C‐section for fetal distress, placental abruption, or reduced fetal movement 1, 2, 3, 4.
Subcutaneous fat necrosis is a clinical diagnosis. In cases of diagnostic uncertainty, biopsy is helpful and demonstrates subcutaneous granulomatous necrosis, histiocytes, and giant cells with crystals composed of triglycerides and necrotic adipocytes 1, 2, 4.
Most SFN cases are self‐limited and resolve spontaneously over weeks to months. In some cases, mild skin atrophy remains once subcutaneous lesions resolve. Hypercalcemia is the most serious complication of SFN; other complications include poor feeding, dehydration, seizures, and death. Prompt therapy is paramount and includes IV isotonic saline hydration, loop diuretics, corticosteroids, and/or bisphosphonates.
We describe a classic presentation of SFN in a five‐week‐old infant with hypercalcemia refractory to standard treatment. After administration of novel bisphosphonate therapy (zoledronic acid), there was a marked resolution of hypercalcemia. To our knowledge, this is the first report in which zoledronic acid is used for this purpose.
A five‐week‐old term male was admitted with failure to thrive, subcutaneous nodules, and hypercalcemia. For 3 weeks, firm, nontender, flesh‐colored nodules had been present on his arms, shoulders, and buttocks. Testing by his pediatrician revealed a calcium level of 15.1 mg/dL (9–11.5 mg/dL) and a PTH level of 2 pg/mL (15–65 pg/mL). His birth history was significant for fetal tachycardia, requiring an emergency C‐section, and neonatal hypoxemia and hypoglycemia.
Admission vital signs were normal. His weight was 3.97 kg (16th percentile) in comparison with his birthweight of 3.91 kg, length was 52 cm (14th percentile), and head circumference was 36.3 cm (35th percentile). His examination was significant for a I/VI systolic ejection murmur, and 12 nontender, flesh‐colored, mobile subcutaneous nodules ranging from several millimeters to approximately two centimeters were located over the arms, shoulders, thighs, and buttocks. Pertinent laboratory findings included the following: calcium 15 mg/dL (9–11.5 mg/dL), phosphorus 4.9 mg/dL (4–6.5 mg/dL), BUN 15 mg/dL (5–18 mg/dL), creatinine <0.2 mg/dL (0.2–0.4 mg/dL), albumin 3.8 g/dL (2.2–4.8 g/dL), 1, 25‐OH vitamin D 76 pg/mL (15–75 pg/mL) and 25‐OH vitamin D 15 ng/mL (30–100 ng/mL). Urinalysis revealed a urine calcium:creatinine ratio of 4.2 (normal <0.2).
Ultrasound of the nodules demonstrated homogenous, hyperechoic, lobulated foci up to 1 cm in depth, consistent with deposition of fat. A renal ultrasound revealed dense medullary nephrocalcinosis.
A diagnosis of subcutaneous fat necrosis was made based on the findings of firm subcutaneous nodules and hypercalcemia. The patient's hypoxemia and hypoglycemia at birth were known risk factors for the development of neonatal SFN.
Initial treatment included intravenous fluids (IVF) and furosemide. Methylprednisolone (initial dosing of 0.5 mg/kg IV q12 h) and low‐dose zoledronic acid (0.025 mg/kg IV) were then administered due to persistent hypercalcemia. For reference, zoledronic acid was administered 19 h and 49 min after initiation of IV fluids and 5 h and 34 min after IV furosemide. Zolderonic acid was given 73 min after methylprednisolone, but the purpose of these two medications was intended to be complementary and mutually aid in reducing the dangerously elevated calcium levels. Subsequently, the patient's serum calcium level normalized and IVF was stopped. Methylprednisolone was tapered over the next 7 weeks. During this time, he maintained normal calcium levels and experienced good weight gain (Fig. 1). The subcutaneous nodules progressively decreased in size and completely resolved by 11 weeks postdischarge. After stopping the prednisolone, his calcium levels remained normal over the next year.
Figure 1.
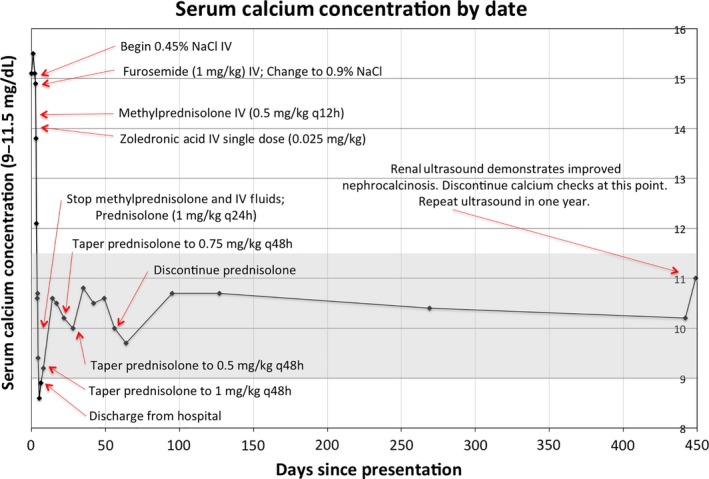
Serum calcium levels over time with interventions noted.
Hypercalcemia is a rare complication of SFN, but may be serious and fatal when present 2. Hypercalcemia can significantly reduce urinary concentrating capacity, producing polyuria; dehydration may occur, in addition to vomiting, hypotonia, seizures, and intellectual impairment 2, 3, 4. These hypercalcemic infants typically have low PTH along with elevated 1,25‐OH vitamin D levels 1, 2, 3, 4. 1,25‐OH vitamin D levels may also be inappropriately normal 1. Granulomatous lesions, similar to sarcoidosis, can demonstrate increased 1‐hydroxylase activity, with increased extrarenal production of 1,25‐OH vitamin D 1, 2. 1,25‐OH vitamin D increases gastrointestinal absorption of calcium, but also can increase bone resorption. In addition, local inflammation may increase production of bone‐resorbing prostaglandin E, which may in turn lead to osteoclast activation 1, 2, 3, 4. Other theories for the source of hypercalcemia include calcium release from the subcutaneous nodules secondary to necrotic adipocytes (which is less plausible because most cases do not have calcification to the SNF lesions or subsequently develop hypercalcemia) and elevated PTH (however, most cases have low PTH levels) 2.
Classic treatment for hypercalcemia includes intravenous isotonic fluids, loop diuretics, and corticosteroids 4. Corticosteroids likely reduce 1‐hydroxylase activity in granulomatous lesions. Ideally, prednisolone would be initiated in the context of elevated 1,25‐OH vitamin D levels, although these levels are rarely available promptly, necessitating empiric therapy. In addition, given the proposed role of enhanced bone resorption in SFN and the severity of hypercalcemia, the decision in this case was made to add bisphosphonate to empiric treatment. Bisphosphonates can rapidly reduce osteoclast activity. Zoledronic acid was chosen in this circumstance as it acts rapidly, can be given intravenously over 15–30 min, and has relatively long duration of action. Hypercalcemia may persist for several months after SFN, and reports of pamidronate treatment in this condition demonstrate that repeated dosing is often necessary for several weeks to maintain normocalcemia 4. Selection of this zoledronic acid dose used weight‐based recommendations from protocol that minimized rebound hypocalcemia when used in children with other disturbances of mineral metabolism 5. Ultimately, this case illustrates that low‐dose zoledronic acid was effective in treating the patient's hypercalcemia and minimizing the side effect of hypocalcemia.
Subcutaneous fat necrosis should be considered in newborns and infants with firm subcutaneous fat nodules in conjunction with an emergent or difficult delivery, hypoxia, hypoglycemia, or cooling/hypothermia. Uncomplicated cases usually resolve spontaneously, but these cases require careful monitoring for the development of hypercalcemia. In particular, hypercalcemia may develop up to 6 months after the initial development of skin lesions. Thus, frequent monitoring of calcium levels is recommended. Hypercalcemia, if present, should be treated with intravenous isotonic saline hydration and loop diuretics. If hypercalcemia remains significantly elevated, prednisolone should be started; if PTH is suppressed, we recommend low‐dose zoledronic acid therapy be considered.
Conflict of Interest
None declared.
Authorship
JADB: assigned (as a medical student) to patient during hospitalization and participated in review of the literature, drafting, and editing of case report. JAN: attending assigned to patient during hospitalization and performed editing of case report and final approval. SJS: provided nephrology consult during hospitalization and performed editing of case report and final approval.
References
- 1. Burden, A. D. , and Krafchik B. R.. 1999. Subcutaneous fat necrosis of the newborn: a review of 11 cases. Pediatr. Dermatol. 16:384–387. [DOI] [PubMed] [Google Scholar]
- 2. Tran, J. T. , and Sheth A. P.. 2003. Complications of subcutaneous fat necrosis of the newborn: a case report and review of the literature. Pediatr. Dermatol. 20:257–261. [DOI] [PubMed] [Google Scholar]
- 3. Lombardi, G. , Cabano R., Bollani L., Delforno C., and Stronati M.. 2009. Effectiveness of pamidronate in severe neonatal hypercalcemia caused by subcutaneous fat necrosis: a case report. Eur. J. Pediatr. 168:625–627. [DOI] [PubMed] [Google Scholar]
- 4. Alos, N. , Eugène D., Fillion M., Powell J., Kokta V., and Chabot G.. 2006. Pamidronate: treatment for severe hypercalcemia in neonatal subcutaneous fat necrosis. Horm. Res. Paediatr. 65:289–294. [DOI] [PubMed] [Google Scholar]
- 5. Munns, C. F. , Rajab M. H., Hong J., Briody J., Högler W., McQuade M., et al. 2007. Acute phase response and mineral status following low dose intravenous zoledronic acid in children. Bone 41:366–370. [DOI] [PubMed] [Google Scholar]


