Abstract
The risk of mental diseases is determined by both genetic and environmental factors, the latter of which may have an even greater impact. To assess individual risk and design efficient preventive measures will require phenotyping environmental risk factors.

Subject Categories: Neuroscience; S&S: Health & Disease; S&S: Politics, Policy & Law
“Doctor, as an expert on mental diseases, can you tell me what my chances are of being diagnosed with schizophrenia?” I am seeing a 23‐year‐old man, Albert, who came with his mother to my office. I learn that his monozygotic twin brother, Greg, who grew up with his father in Berlin, was recently diagnosed with schizophrenia. Albert has been living with his mother in a rural area near Hanover since his parents divorced, with each of them taking one of the twin babies along. There had obviously not been too much contact between them since.
Disease starts at a certain threshold, which is defined to some degree by societal and cultural standards.
What can I tell Albert? The concordance rate of schizophrenia in monozygotic twins is ~50%—indicating that, despite a 100% shared genome, there is a 50% chance that he will stay healthy, even though his twin brother is affected 1. It also means that there must be other factors at work, which significantly add to any genetic predisposition for behavioral abnormalities up to mental diseases.
When is a disease a disease?
What are these non‐genetic, environmental risk factors for behavioral abnormalities and mental disease? On the way to offering a satisfying answer based on state‐of‐the‐art research, a central, even philosophical question arises: How do behavioral abnormalities contribute to what we call a disease? And equally important, when does a disease start to be a disease?
Most of us can be depressed, lose our normal drive and pleasure at times, or be somewhat autistic when we intensely concentrate on a particularly interesting puzzle, or even become paranoid in certain situations. In other words, human (and animal) behavior can be regarded as consisting of quantifiable traits. Disease starts at a certain threshold, which is defined to some degree by societal and cultural standards 2. Reaching this threshold is the net result of the interaction between genetic and environmental risk and protective factors. Along these lines of thought, would it perhaps be better to talk about risk that lastingly shapes our behavior rather than only risk of mental illness?
Greg and Albert's story provides some insights to understand the importance of these questions. Greg is suffering from full‐blown paranoid schizophrenia: He feels hounded by aliens and hears voices that tell him how to protect himself from this extraterrestrial threat. Among others, these voices tell him to set fire in the house or to short‐circuit the power outlets in the kitchen. Albert is sometimes suspicious of some of his fellow physics students, whom he thinks spy on him to use his ideas or his experimental results, but he can easily ignore these thoughts when his classmates ask him, as a particularly talented colleague, for his help. Here we have two examples of paranoid behavior in individuals with an identical genetic background: one severe, non‐correctable, and even dangerous for self and others, the other in the frame of a still healthy personality. In Greg's case, it is likely that environmental factors pushed his genetic predisposition for paranoid behavior over the threshold to develop full‐blown schizophrenia. We should always keep in mind that the expression of a mental disease is never completely separable from the underlying inherent personality features of the affected subject. In mental disease, the original personality characteristics often emerge in an exaggerated (pathological) way.
We should always keep in mind that the expression of a mental disease is never completely separable from the underlying inherent personality features of the affected subject.
Another example from the story of the twins may further help to answer the central question of when a disease becomes a disease. Albert, the healthy twin, had gone through a phase which one could—in retrospect—interpret as an “abortive” disease prodrome. This so‐called prodrome is a period of 2–5 years before the onset of schizophrenia, which is variably characterized by dropping performance in school and learning problems, social withdrawal, depression, restlessness, and suicidal ideation or suicide attempts. A prodrome is often very hard to recognize as such, since puberty is an overall difficult time for almost anybody and symptoms may be non‐specific. During early puberty, Albert often thought about being worthless and about dying, and his performance in school clearly suffered. But his mother and her new partner, both teachers, unconditionally supported him during these difficult years. At the age of 17, he became the best student in his class. He is now finishing his examinations with excellence.
Greg, in turn, had a complete prodrome with all of the typical features. His father, a busy banker, found him unapproachable during these years. He hung out with problematic and partly criminal peers in Berlin, consumed cannabis and tried other drugs, frequently skipped school, and made his first suicide attempt at the age of 19. Three years later, he had not graduated when he had his first psychotic episode. This example tells us again that a genetic substrate can undergo different environmental shaping via risk and protective factors, which results in healthy coping versus mental disease.
Sorting out environmental risk factors
We therefore need to specify potential or proven environmental risk factors for altered behavior or mental illness. The sociology or sociopsychology literature that analyzes externalizing behavior in childhood groups environmental risk factors into “child, sociocultural, parenting, and peer‐related”. Such externalizing behavior includes aggression and hostility, impulsivity and hyperactivity, and non‐compliance with limit‐setting. These behaviors have been linked to conduct disorders, attention‐deficit disorders, as well as personality disorders, early delinquency, criminality, and other forms of antisocial psychopathology in adulthood 3.
Not all environmental risk factors are founded on unequivocally or scientifically convincing data.
In the biomedical literature, environmental risk factors are much more heterogeneous and complex and go far beyond these sociopsychological risk factors. Not all of them are equally important and scientifically sound, and there appear to be what I like to call “shades of risk”. These shades are not simply black and white; their intensity depends on the position of the light source, the perspective of the observer, and they may even melt into each other. Several “shades of risk” can be distinguished.
Type 1 shades describe primary personal, intrinsic risks that are essentially unavoidable—what may also be called “fate”. They include, for instance, perinatal maternal infections, placental pathology, obstetric complications, low birth weight, advanced paternal age, number of siblings, season of birth, childhood infections, head injury, and adverse life events such as losing a close relative or enduring physical or sexual abuse.
Type 2 shades mark primary risk through society and surroundings that are also largely inevitable for the individual, but subject to political needs and actions. They include urban birth and upbringing, crowded living conditions, exposure to noise, air pollution, heavy metals, toxic organic compounds, and radioactivity (including natural radioactivity), famine, bullying among peers, migration, minority group status, and the parents' social status and socioeconomic position.
An extremely topical subject is migration as a risk factor of abnormal behavior and mental disease.
Type 3 shades signify clearly preventable, secondary risk factors that can act as detrimental add‐ons to preexisting factors. These are substance abuse—mainly cannabis and alcohol—but also nutrition factors such as vitamin D deficiency, or an unhealthy microbiome of the gut or skin.
Exemplifying some risk factors
Attributing neuropsychiatric diseases to environmental risk factors dates back to ancient times. Hippocrates (c. 460 – c. 370 BC) already associated mental disease with the constellation of the planets but also with nutrition and even developed treatment regimens based hereon. Not all environmental risk factors are founded on unequivocally or scientifically convincing data. They are often built on small numbers of individuals, are retrospective, and/or leave the “chicken or egg” question—whether they are the causes or consequences—unanswered. By way of example, the role of cannabis consumption as an inducer of schizophrenia, as self‐medication during psychotic episodes or disease prodrome, or as a side effect of a problematic peer situation has been intensely discussed. It is quite safe to say that, despite there being some truth in all these views, cannabis can induce schizophrenia in predisposed individuals, lead to earlier disease onset in a dose‐dependent manner, and trigger psychotic relapses 4. Depending on an individual's genetic make‐up and environmental risk profile, cannabis consumption can also result in amotivational behavior, social withdrawal, or cognitive deficits upon peripubertal use.
Traumatic brain injury has also been implicated as a risk factor for mental disease, but this possibility was only recently confirmed by a nationwide Danish study of 113,906 individuals who had suffered a neurotrauma. In fact, an injury to the head between 11 and 15 years of age is the strongest predictor for subsequent development of schizophrenia, depression, and bipolar disorder 5. In contrast, season of birth is a weak risk factor per se, but if we consider that influenza infection has clear seasonal peaks and poses a high risk during pregnancy for the unborn child to develop mental disease, season of birth may ultimately prove valid as a risk factor.
The influence of urbanicity on disease risk seems certain. But what are the exact reasons why the risk of abnormal behavior and mental disease increases if individuals grow up in an urban versus rural environment? The possible answers range from air pollution and noise to crowded living, problematic peers, and generally enhanced stress 6. In order to understand more about the discrete individual risk factors that belong to urban environments, we would need to separate these as much as possible, as not all cities are crowded, not all are heavily polluted, and not all are noisy in the center. This would require large international efforts with unrestricted data and information sharing to compare cities regarding living conditions, the number and status of minorities and migrants, smog, and mental disease prevalence. Quality of housing would have to be measured as much as rush hour traffic, commuting possibilities and public transportation, or the availability of leisure activities and ways to relax to name just a few contributing factors of “urbanicity”.
An extremely topical subject is migration as a risk factor of abnormal behavior and mental disease. But again, why is it? To understand its role, we must first ask what the driving forces are for people to migrate, and which problems they have to face in their new country. How much does the culture of their country of origin differ from their new homeland? Ultimately, we must analyze why second‐generation migrants are similarly or even more at risk. All these questions have high political and practical relevance; they are at present predominantly “answered” with some “logical assumptions”, but urgently need to be addressed in a systematic scientific approach parallel to efforts toward optimizing integration for migrants.
… phenotyping of environment and individual environmental risk accumulation will be crucial to identify the root cause of behavioral abnormality and mental disease.
In the case of Albert and Greg, Albert's rural upbringing may have had a protective effect, just as Greg's urban life may have been a critical risk factor. Parental care was obviously different for the twins, too. Greg started to consume cannabis around puberty, as did many of his classmates and peers in Berlin, whereas Albert has never tried it. In addition, Greg fell from his bike and hit his head while in elementary school. He was diagnosed with a concussion and had to stay in the hospital for 2 weeks. Thus, traumatic brain injury is also on his list of obvious risk factors.
Protective factors and risk accumulation
Again, genes obviously play only a limited role—if at all—and it is the balance of environmental risk and protective factors that ultimately determines the outbreak of a mental disease in a genetically predisposed individual (Fig 1). This brings us to the next question, namely, which environmental factors can neutralize adverse effects or protect against mental disease? Another important point is the composition of risk factors. Which combinations are more or less deleterious? Back to the sociopsychological literature and to Albert's story, a warm home with caring and loving parents, a good education, school or professional rewards, trusting relationships, and a healthy lifestyle can likely absorb some of the negative impact of environmental risks. These considerations should more prominently inform preventive or therapeutic strategies for children at risk.
Figure 1. Environmental risk factors of mental disease.
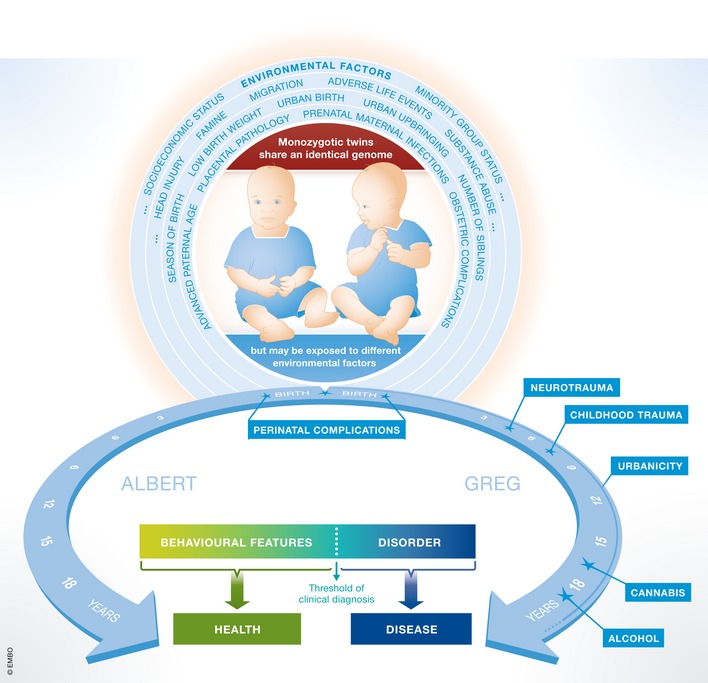
On the other hand, accumulation of risk seems to have the most severe detrimental effects. Recently, we assessed, for the first time, in more than 750 schizophrenic men environmental risk factors experienced before the age of 18 years—well before the disease started. Strikingly, we found that patients who experienced more than four risk factors had an onset of schizophrenia ~10 years earlier 4. We note that it does make a big difference in the life of a young man whether schizophrenia starts at the age of 21 or 30. In these critical in‐between years, people finish their education, get settled in a profession and in society, and often start a family—which is seriously hampered by the disease. Importantly, the same study showed no measurable influence of genetic risk factors as derived from the latest, large‐scale, genome‐wide association study on schizophrenia, including 37,000 cases and 113,000 healthy controls. There was no significant evidence of additional “genes X environment” interaction, which emphasizes the enormous impact of environmental risk per se 4.
In an ongoing follow‐up study, we detected that accumulated environmental risk was significantly associated with violent aggression and a five times higher likelihood of being convicted of bodily injury, sexual assault, manslaughter or murder, or a history of forensic hospitalization. This unexpected finding was confirmed in six independent replication samples, including general population (publication in process). It shows the severe societal consequences of risk accumulation and should make the need for preventive measures more than obvious.
What do we know about mechanisms?
How can risk factors as different as perinatal maternal infection, migration, and urbanicity act together to shape personality and co‐determine the likelihood of mental disease? Importantly, they act long before adulthood and can impact the vulnerable, developing brain whether they occur once, as in cases of neurotrauma, or repeatedly, as in continuous sexual abuse. On top of this lies the transgenerational risk: Imprinting before conception has to be taken into account, even if it is not yet well understood. Taken together, how much do we really know about mechanisms? Numerous reactions to environmental exposure have been described, including changes in neuroendocrine and neurotransmitter systems or neuronal/synaptic plasticity, but also changes to the adaptive immune system, for instance pro‐inflammatory cytokine secretion 7. Another interesting phenomenon of still unclear significance is the large number of circulating autoantibodies directed against brain antigens in healthy individuals that increases with age. Do these autoantibodies also represent adaptive changes in response to environmental risks 8? Importantly, risk‐mediated alterations in brain dimensions, for instance in white matter tracts—as well as early interference with developmental myelination—affect brain connectivity and network function and lay the foundation for behavioral abnormalities and neuropsychiatric disease 9.
We need more considerate, large international efforts to systematically quantify environmental risk factors analogous to genome sequencing and GWAS projects.
Epigenetic alterations of the genome involving histone modifications, DNA methylation or DNA hydroxymethylation, or non‐coding RNAs may also underlie some of these changes. These alterations cause changes of gene expression and have become a highly topical field of research. Here, certain risk factors—alone or in combination—can be modeled and their consequences for brain function, morphology, and biochemistry can be studied. Potential epigenetic therapy approaches are also being discussed 10. Unfortunately, translation to the extremely heterogeneous human population is less reliable. Instead of studying the brain as an adequate target tissue, only blood cells are accessible for epigenetic analysis for appreciable numbers of individuals. But even here replications are often missing, and reproducibility remains limited. In the future, human neurons or other brain cells derived from inducible pluripotent stem cells might be helpful to study at least some translational aspects.
The need to phenotype the environment
This all points to the need to systematically and quantitatively study the influence of environmental factors on behavioral alterations and the onset of mental illness. We need more considerate, large international efforts to systematically quantify environmental risk factors analogous to genome sequencing and GWAS projects. We also need more systems biology approaches to understand the mechanisms of how environmental factors drive disease. All of these endeavors will be extremely labor‐intense and expensive. Thus, and most importantly, we need to convince politicians and funding agencies to grant adequate funding for research that addresses these fundamental societal questions.
Given the enormous influence of the environment, which far outlasts any general genetic effects on mental disease, the impact that such studies will ultimately have on our understanding of psychiatric conditions, prophylaxis, and treatment options is huge. In addition, environmental risk is ubiquitous and “unspecific”; it inflicts its share of damage on any individual, and may cause anything from mild behavioral consequences, perhaps in the presence of strong protective factors, to personality changes or, given a genetic predisposition, severe mental disease.
So, how can we assess an individual's environmental risk of developing a mental disease? Are there dichotomous, or even better continuous, measures that quantify these risks? Depending on the specific risk factor, we would need to conduct patient interviews (urbanicity, migration), study charts (birth complications, neurotrauma), and directly measure environmental conditions (air pollution, exposure to toxins). Ultimately, it may be possible to estimate the accumulated personal risk, but controlled and independently replicated studies are needed to analyze the relative impact of individual factors and their combinations. These studies should be comparable to large, international GWAS efforts. Even then, we will never be able to measure and understand all possible risks. Nevertheless, owing to its gigantic impact, phenotyping of environment and individual environmental risk accumulation will be crucial to identify the root cause of behavioral abnormality and mental disease.
Societal task: avoiding risks
But what can we do once we know? Not all risk factors are avoidable, but some—such as cannabis—are. In the case of cannabis, clinicians and the general public need to become aware of its risks. Legalizing cannabis for instance may not send the right message to the public regarding its health risks. The impact of migration and urbanicity could be alleviated with political and social measures, such as better integration of migrants or humane city planning. Other factors, such as perinatal complications, traumatic brain injury, or traumatic life events, might not be easily avoidable. Yet even for these factors, introducing prophylactic measures such as better management of at‐risk pregnancies, wearing a helmet when cycling, early therapeutic intervention after trauma, and better awareness of signals that indicate physical or sexual abuse—along with reducing false shame and building trust—might be beneficial 4. Again, only controlled studies will help to estimate the benefits and efficiency of these potential prophylactic measures and interventions.
Unfortunately, primary preventive measures are too late for Greg. He will need secondary prevention after remission. He will have to learn, in long‐term psychotherapeutic sessions, that he is suffering from schizophrenia, a fact that is often difficult for patients and their relatives to accept, and that cannabis use will lead to psychotic relapses and negatively affect his long‐term prognosis. Albert has a reasonable chance of remaining healthy. He should be made aware of protective factors and potential risks, as well as how to avoid the latter. At present, without any efficient possibilities of phenotyping his environment for risk estimation, any recommendations will remain rather vague.
Conflict of interest
The author declares that she has no conflict of interest.
Acknowledgements
This work was supported by the Max Planck Society, the Max Planck Förderstiftung, the DFG (CNMPB), EXTRABRAIN EU‐FP7, and the Niedersachsen‐Research Network on Neuroinfectiology (N‐RENNT).
References
- 1. Cardno AG, Gottesman II (2000) Twin studies of schizophrenia: from bow‐and‐arrow concordances to star wars Mx and functional genomics. Am J Med Genet 97: 12–17 [PubMed] [Google Scholar]
- 2. Ehrenreich H, Mitjans M, Van der Auwera S, Centeno TP, Begemann M, Grabe HJ, Bonn S, Nave KA (2016) OTTO: a new strategy to extract mental disease‐relevant combinations of GWAS hits from individuals. Mol Psychiatry doi: 10.1038/mp.2016.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deater‐Deckard K, Dodge KA, Bates JE, Pettit GS (1998) Multiple risk factors in the development of externalizing behavior problems: group and individual differences. Dev Psychopathol 10: 469–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stepniak B, Papiol S, Hammer C, Ramin A, Everts S, Hennig L, Begemann M, Ehrenreich H (2014) Accumulated environmental risk determining age at schizophrenia onset: a deep phenotyping‐based study. Lancet Psychiat 1: 444–453 [DOI] [PubMed] [Google Scholar]
- 5. Orlovska S, Pedersen MS, Benros ME, Mortensen PB, Agerbo E, Nordentoft M (2014) Head injury as risk factor for psychiatric disorders: a nationwide register‐based follow‐up study of 113,906 persons with head injury. Am J Psychiat 171: 463–469 [DOI] [PubMed] [Google Scholar]
- 6. Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wust S, Pruessner JC, Rietschel M, Deuschle M et al (2011) City living and urban upbringing affect neural social stress processing in humans. Nature 474: 498–501 [DOI] [PubMed] [Google Scholar]
- 7. Nemeroff CB (2016) Paradise lost: the neurobiological and clinical consequences of child abuse and neglect. Neuron 89: 892–909 [DOI] [PubMed] [Google Scholar]
- 8. Dahm L, Ott C, Steiner J, Stepniak B, Teegen B, Saschenbrecker S, Hammer C, Borowski K, Begemann M, Lemke S et al (2014) Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol 76: 82–94 [DOI] [PubMed] [Google Scholar]
- 9. Nave KA, Ehrenreich H (2014) Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry 71: 582–584 [DOI] [PubMed] [Google Scholar]
- 10. Fischer A (2014) Epigenetic memory: the Lamarckian brain. EMBO J 33: 945–967 [DOI] [PMC free article] [PubMed] [Google Scholar]


