Abstract
We review the hypotheses concerning the association between the paternal age at childbearing and childhood psychiatric disorders (autism spectrum‐ and attention deficit/hyperactive disorder) and adult disorders (schizophrenia, bipolar‐, obsessive–compulsive‐, and major depressive disorder) based on epidemiological studies. Several hypotheses have been proposed to explain the paternal age effect. We discuss the four main—not mutually exclusive—hypotheses. These are the de novo mutation hypothesis, the hypothesis concerning epigenetic alterations, the selection into late fatherhood hypothesis, and the environmental resource hypothesis. Advanced paternal age in relation to autism spectrum disorders and schizophrenia provided the most robust epidemiological evidence for an association, with some studies reporting a monotonic risk increase over age, and others reporting a marked increase at a given age threshold. Although there is evidence for the de novo mutation hypothesis and the selection into late fatherhood hypothesis, the mechanism(s) underlying the association between advanced paternal age and psychiatric illness in offspring remains to be further clarified. © 2016 The Authors. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics Published by Wiley Periodicals, Inc.
Keywords: paternal age effect, autism spectrum disorder, schizophrenia, bipolar disorder, epidemiological evidence
INTRODUCTION
The worldwide trend of postponing parenthood implies an increase in average parental age [Sobotka, 2010]. Notably maternal age increase is associated with heightened risks for the offspring, for instance, it is well established that increased maternal age at childbearing is a risk factor for errors of chromosome segregation [Penrose, 1933; Hassold and Hunt, 2009]. However, there is a growing realization that, independently of maternal age, advanced paternal age at childbearing is associated with offspring morbidity, including psychiatric disorders. The association between increased paternal age at childbearing and offspring morbidity is known as the “paternal age effect.” There is no general consensus about the mechanism underlying this effect. The following four—not necessarily mutually exclusive—hypotheses have been proposed.
The first and leading hypothesis is the “de novo mutation hypothesis.” De novo mutations occur spontaneously in the male germline during spermatogonial stem cell divisions and propagate in successive clones of spermatocytes [Penrose, 1955; Crow, 2000]. Such de novo mutations in the male germline occur more often with increasing paternal age and are hypothesized to increase offspring morbidity.
The second hypothesis concerns mechanisms related to epigenetic alterations, where impairments in epigenetic modifications result in altered chromatin structure and DNA‐methylation patterns, which lead to altered gene expressions [Perrin et al., 2007]. A genome‐wide DNA‐methylation screen comparing sperm from young and old mice revealed a significant loss of methylation in the older mice in regions associated with transcriptional regulation. The DNA‐methylation patterns of mice sperm decreased during life, and this decrease was reciprocal with brain gene expression alterations and behavioral problems [Milekic et al., 2015]. An altered methylation pattern in spermatozoa that was associated with paternal age was also seen in rats [Oakes et al., 2003]. A longitudinal study in human males with repeated sampling of sperm 9–19 years apart strongly suggested that 139 genomic regions in the male human germline become increasingly hypo‐methylated with aging [Jenkins et al., 2014]. Such age‐related epigenetic processes in sperm may contribute to the paternal age effect.
Thirdly, the “selection into late fatherhood” hypothesis states that delaying fatherhood (i.e., advanced age of father at the birth of his first child), rather than the biological correlates of advanced paternal age per se, is responsible for the paternal age effect [Petersen et al., 2011]. Factors associated with both a delayed fatherhood and the offspring's psychiatric risk are hypothesized to explain the relationship. One possible common factor is the father's genetic vulnerability to psychiatric disorders. Psychiatric disorders and predisposing traits in subclinical forms (e.g., poor social functioning) are markers of genetic vulnerability, and it is hypothesized that these markers limit cross‐sex interaction [Sipos et al., 2004; Petersen et al., 2011]. This in turn results in late fatherhood. Fathers with a genetic vulnerability to psychiatric illness are more likely to have offspring with a similar genetic liability. This likelihood is further increased by assortative mating occurs, where spouses resemble each other in their genetic vulnerability to the psychiatric illness.
The fourth hypothesis postulates that environmental characteristics are unequally distributed over the age of the fathers. These characteristics may have risk enhancing or protective effects on psychiatric development, which could account for paternal age effects [Miller et al., 2011b]. Such characteristics can be causal (e.g., assistive reproduction technologies) or non‐causal (e.g., socio‐economic status or higher risk of paternal death when the child is young). We refer to this hypothesis as the “environmental resource hypothesis.”
The past decades have seen an increased interest in the paternal age effect on offspring psychiatric morbidity, with the majority of the studies looking at Autism Spectrum Disorders (ASD), and Schizophrenia (SCZ). A much smaller number of studies looked at Attention Deficit/Hyperactive Disorder (ADHD), Bipolar Disorder (BPD), Major Depressive Disorder (MDD), and Obsessive‐Compulsive Disorder (OCD). The aim of this paper is to review epidemiological research into advanced paternal age at childbearing and the offspring's risk of the development of psychiatric disorders, and to discuss the epidemiological evidence for and against the four hypotheses concerning the causal pathway underlying the paternal age effect.
METHOD
A literature search was done in PubMed and PsycINFO, and search results were limited to English language papers published between January 1965 and July 2015 in academic journals. The search terms were (paternal OR parental) AND age in the title or keywords along with various combination of the following terms in the title or abstract: autism, Asperger, PDD‐NOS, attention, ADHD, bipolar, depression, depressive, obsessive, OCD, and schizophrenia. It was not necessary to add Autism Spectrum Disorders, ASD, or MDD to the search terms, because autism and depression and depressive were sufficient to identify the relevant studies. Next, the reference lists of the relevant articles were examined in order to identify additional significant articles. Studies were included if they (i) investigated the relationship between the age of the father at childbearing and offspring's risk for developing any of the aforementioned psychiatric disorders; (ii) included a comparison group; (iii) had no sample‐overlap with studies with the same index of morbidity as the outcome variable that was more recent or studied a larger cohort; and (iv) estimated risk. A flow diagram of the search results is given in Figure 1.
Figure 1.
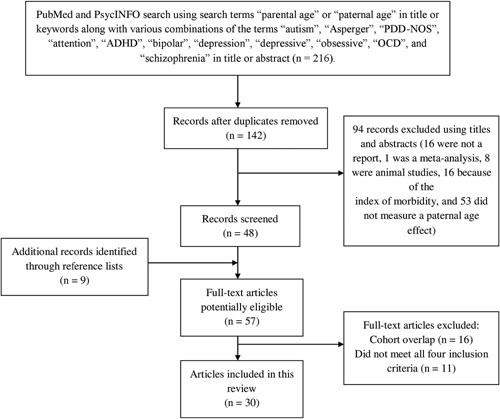
Flow diagram of the search strategy and results.
RESULTS
Characteristics of Studies of the Paternal Age Effect
Our review of the literature identified 30 epidemiological studies of the relationship between paternal age and offspring psychiatric morbidity. Most studies concerned ASD (16) and SCZ (11). Of the 16 ASD studies, 11 concerned risk of general ASD, 4 concerned ASD subphenotypes, and 1 concerned both. Of the 11 SCZ studies, 6 used the narrow diagnosis of SCZ. For ADHD, BPD, OCD, and MDD fewer studies were reviewed; the number of studies was three (ADHD), six (BPD), one (OCD), and one (MDD). One study of BPD distinguished between BPD with and without psychoses [Lehrer et al., 2016]. Additionally, several studies considered multiple indices of morbidity [Buizer‐Voskamp et al., 2011; Wu et al., 2012; McGrath et al., 2014; Lehrer et al., 2016].
There was sample overlap between Frans et al. [2013] and Idring et al. [2014], and Sipos et al. [2004] and Frans et al. [2011]. They were included because of the slight differences in index of morbidity. Sandin et al. [2016] analyzed multiple cohorts that overlapped with the samples of several other studies [Glasson et al., 2004; Reichenberg et al., 2006; Frans et al., 2013; Idring et al., 2014; McGrath et al., 2014]. This study is also included, however, because of the breadth of the investigated phenotype, and the additional cohort originating from Norway.
The studies differed in terms of diagnostic criteria and populations. Scandinavian populations were the most commonly studied, followed by US, Japanese, Israeli, Australian, Dutch, Han Chinese, Aruban, UK, and Iranian populations. A more detailed description of the studied populations and diagnostic criteria used in the articles is provided in Supplementary Table SI.
In this section, we first discuss the evidence for a paternal age effect on each disorder. In the subsequent section, we discuss the evidence in support of each of the four hypotheses.
Autism Spectrum Disorders
A total of 16 epidemiological studies investigating the paternal age effect on ASD met our inclusion criteria. Supplementary Tables SII and SIII provide an overview of the results of these studies. The studies in Supplementary Table SII evaluated the risk within age groups that are dividable by five, each relative to a reference age group. The studies in Supplementary Table SIII used differently defined age categories. For each study, we present the risk estimates corrected for the co‐variates; we discard the raw (i.e., unadjusted) estimates. Most epidemiological studies of the paternal age effect take ASD as the outcome variable, represented by Supplementary Table SIII and the bottom 10 lines of Supplementary Table SII. Some studies looked at the effect on Autistic Disorder (AD) specifically or considered different subtypes of ASD (i.e., AD, Asperger's syndrome, and Pervasive Developmental Disorder‐Not Otherwise Specified [PDD‐NOS]). The risk estimates obtained in these studies are given in the upper part of Supplementary Table SII.
There was overlap between the cohorts used by some included studies of ASD [Frans et al., 2013; Idring et al., 2014; Sandin et al., 2016]. Sample sizes varied greatly with some of these overall sample sizes and/or case sample sizes being quite small. The risk estimates may be unstable when based on small sample sizes (small case sample sizes and small samples of fathers in the extremes of the paternal age distribution). Epidemiological results concerning the risk of all ASDs together are reasonably consistent: most studies suggested that the offspring's ASD risk increases with advancing paternal age at childbearing. A meta‐analytic study, which included 12 cohorts comprising over 1,035,000 individuals, confirmed this risk pattern [Hultman et al., 2011]. Compared with fathers who were 29 years of age or younger at birth of the affected child, the random effects pooled estimates of risk of ASD were 1.22 for offspring born to fathers aged 30–39 years (95% CI: 1.05–1.42); 1.78 for offspring born to fathers aged 40–49 years (95% CI: 1.52–2.07), and 2.46 for offspring born to fathers aged 50 years or older (95% CI: 2.20–2.76).
The studies of the risk of AD specifically showed a similar consistency: all reported an increasing risk with advancing paternal age at childbearing. This elevated risk was not consistently seen for the other ASD subtypes Asperger's syndrome and PDD‐NOS. However, the results collectively provide robust evidence in support of the hypothesis that older paternal age at childbearing is a risk factor for ASD in offspring.
Three studies suggested a linear relationship between increasing paternal age and the risk for their children to develop ASD [Reichenberg et al., 2006; Tsuchiya et al., 2008; Idring et al., 2014]. Grether et al. [2009] modeled paternal age as a continuous variable, and found that paternal age was linearly associated with the log(OR) over a paternal age range of 20–59 years. However, Lampi et al. [2013] rejected strict linearity of the relationship between paternal age and the subtypes of ASD, and Lundström et al. [2010] suggested a U‐shaped relationship.
The ASD prevalence in males is higher than in females [Glasson et al., 2004; Bilder et al., 2009; Grether et al., 2009; Sasanfar et al., 2010; Parner et al., 2012]. Results from studies that performed additional separate analyses of the paternal age effect for male and female offspring were inconsistent [Reichenberg et al., 2006; Grether et al., 2009; Sandin et al., 2016]. Sandin et al. [2016] performed sex‐specific analyses across multiple cohorts and found a similar effect for female and male offspring. Others found that the risk for ASD and AD increased less with older paternal age in male offspring than in female offspring [Reichenberg et al., 2006; Grether et al., 2009]. This sex difference in increase is in line with previous findings [Croen et al., 2007; Anello et al., 2009; Puleo et al., 2011; Parner et al., 2012].
Some studies took into account the parental history of psychiatric illness or a broader ASD phenotype (i.e., having a considerable number of ASD‐related traits within any two of the three main categories of characteristics, namely communication, stereotyped behavior, and social deficiencies [Tsuchiya et al., 2008]). Tsuchiya et al. [2008] found that mean paternal age for ASD‐affected children did not differ between fathers with or without a broader ASD phenotype. The studies that took psychiatric familial history into account [Frans et al., 2013; Lampi et al., 2013; Idring et al., 2014; McGrath et al., 2014] still revealed a significant paternal age effect, except for Lampi et al. [2013] on PDD‐NOS and Asperger's syndrome.
ASD‐affected children are likely to have a low birth order. Therefore, the majority of the studies took birth order or parity into account [Glasson et al., 2004; Durkin et al., 2008; Tsuchiya et al., 2008; Grether et al., 2009; Sasanfar et al., 2010; Shimada et al., 2012; Lampi et al., 2013; Idring et al., 2014]. The risk of developing an ASD tended to be highest for firstborn children, and the risk was inversely associated with birth order of the child [Glasson et al., 2004; Durkin et al., 2008; Grether et al., 2009; Sasanfar et al., 2010; Rahbar et al., 2012; Shimada et al., 2012]. Durkin et al. [2008] examined the birth‐order effect in parents of “younger” ages (i.e., mother aged between 20 and 34 and the father younger than 40 at childbearing), “older” ages (i.e., mother older than 34 and father 40 years or older during childbearing), and in couples for whom either the mother or the father was older (i.e., either mother older than 34 or father 40 years or older during childbearing). In all three subgroups, a birth‐order effect was observed, with the strongest effect when both parents had an “older” reproductive age and the weakest effect when both parents had a “younger” reproductive age. D'Onofrio et al. [2014] (this study is not included earlier because of sample overlap with Frans et al. [2013]) performed an analysis in which only second‐ or later‐born children were included. Although smaller, a paternal age effect on AD risk was still seen. Bilder et al. [2009] did not observe an association between parity and ASD risk, possibly due to a lack of statistical power. However, the within‐family study of Hultman et al. [2011] on birth order reported that, relative to father's age at childbearing of their first child, the father's risk to have a subsequent child diagnosed with autism increased monotonically with advancing age. There was one exception: a monotonic decrease in offspring's autism risk with aging for fathers who were 40 years or older at childbearing of their first child. Thus, this within‐family design confirms the paternal age effect on ASD for first‐time fathers aged 39 years of age or younger.
Three studies took intellectual disability into account [Tsuchiya et al., 2008; Lampi et al., 2013; Idring et al., 2014]. Tsuchiya et al. [2008] only included high‐functioning ASD cases and detected a relationship with paternal age. The findings of Idring et al. [2014] suggested that the paternal age effect on the risk of ASD with intellectual disability was stronger than the effect on ASD risk without intellectual disability. Lampi et al. [2013] adjusted for child's intellectual disability, which may result in biased estimates of risk, but for AD a significant risk of advanced paternal age was still observed. However, this was not the case for PDD‐NOS or Asperger's syndrome.
Schizophrenia
A total of 11 epidemiological studies that investigated the paternal age effect on SCZ and related disorders are summarized in Supplementary Tables SIV and SV. These provide an overview of the results of the models that adjusted for co‐variates. The studies in Supplementary Table SIV used age categories split into 5‐, 10‐ or 15‐year age segments, and the single study in Supplementary Table SV used otherwise defined age categories. Two studies had overlap in cohorts, but both were still included because of the differences in breadth of diagnosis [Sipos et al., 2004; Frans et al., 2011].
As can be seen from Supplementary Tables SIV and SV, a substantial number of studies reported that paternal age at childbearing was a risk factor for offspring SCZ or related disorders. The two studies that did not report significant risks had a low number of cases, which might have led to insufficient power to detect the effect. All studies that reported significant associations between a paternal age category and SCZ risk in offspring have documented that the greatest increase in risk was seen in the oldest age category. The tables show that some studies reported a steady and monotonic increase in risk over the paternal age categories, whereas, others reported significantly elevated SCZ risk only in the oldest age categories. Linear trend tests in three studies suggested that a linear relationship between paternal age and offspring SCZ risk [Malaspina et al., 2001; Zammit et al., 2003; Tsuchiya et al., 2005]. McGrath et al. [2014] found a U‐shaped relationship. Overall, the findings point toward an increase of SCZ risk with advancing paternal age, but the exact shape of the relationship remains to be clarified.
The results obtained by a meta‐analysis, including over 3,000,000 individuals originating from 12 studies, reported an association between advanced paternal age and offspring's risk of developing SCZ [Miller et al., 2011a]. However, these results were not adjusted for factors that were associated with SCZ risk in offspring [McGrath et al., 2008].
Several studies adjusted for prior history of psychiatric morbidity in parents and sometimes siblings [Sipos et al., 2004; Tsuchiya et al., 2005; Frans et al., 2011; McGrath et al., 2014], and some only adjusted for maternal morbidity [Dalman and Allebeck, 2002; Miller et al., 2011b]. The epidemiological data of Dalman and Allebeck [2002] are not included in Supplementary Table SIV because of overlap in samples with Frans et al. [2011]. Dalman and Allebeck [2002] adjusted for maternal psychosis and found a significantly elevated risk of offspring SCZ for the oldest paternal age group (45 years and older). Of note, a model without adjustment for maternal psychosis morbidity revealed that the oldest and second oldest paternal age groups had significantly greater risk of offspring SCZ. Miller et al. [2011b] found that paternal age was not associated with non‐affective psychosis in offspring, after adjustment for maternal SCZ. Note that the morbidity investigated was non‐affective psychosis in general rather than SCZ per se, and note that the sample size might have been too low to detect an association. Frans et al. [2011] performed a posthoc analysis in which cases with a parental bipolar or psychotic disorder diagnosis were excluded, but arrived at the same conclusions. Sipos et al. [2004] and Tsuchiya et al. [2005], following a correction for familial psychiatric history, still observed a significant association. McGrath et al. [2014] adjusted for familial psychiatric illness, resulting in a slightly attenuated risk. The overall effect remained significant, however, and the risks of all age categories upwards from 30 to 34 were similar. Interestingly, the significantly greater risk in the 20–24‐year age category was rendered insignificant by the adjustment for family history of psychiatric disorders. Lehrer et al. [2016] did not find any significant relationship when calculating the odds of “no psychiatric family history” in each paternal age group.
Some studies carried out sex‐specific analyses [Byrne et al., 2003; Zammit et al., 2003; Frans et al., 2011; Wu et al., 2012]. Frans et al. [2011] demonstrated that the age of the maternal grandfather was associated with offspring SCZ risk, while the age of the paternal grandfather was not. Byrne et al. [2003] used a cohort overlapping with the cohort of McGrath et al. [2014], and observed a somewhat stronger paternal age effect on SCZ risk in females than for males. A meta‐analysis revealed sex similarities in SCZ risk of offspring born to fathers of 30 years of age or older. However, younger paternal age (i.e., younger than 25 years of age) was significantly associated with increased SCZ risk in males but not in females [Miller et al., 2011a]. Several studies with sex‐specific analyses did not support sexual differences in offspring's SCZ risk with advancing paternal age. Zammit et al. [2003] only included male individuals, and the results do not appreciably vary from results of other studies that included individuals of both sexes. The results of Wu et al. [2012] suggested that the paternal age effect on SCZ risk is equal in both sexes. However, the sample size might have been too low to detect differences in risk. In brief, results concerning sex differences in the paternal age effect on offspring SCZ are inconsistent.
Bipolar Disorder
Seven analyses of BPD risk were found for inclusion in this review. The results, which included a correction for co‐variates, are displayed in the upper part of Supplementary Table SVI. Lehrer et al. [2016] found a significantly decreased risk of BPD without psychosis for offspring born to fathers younger than 20 years of age. Two analyses found no association between advanced paternal age and BPD risk, one analysis found a U‐shaped relationship, one analysis showed a monotonic increase in BPD risk with advancing categories of paternal age, and two analyses showed an elevated risk associated only with the oldest age category. In the analysis of McGrath et al. [2014], the overall effect of paternal age was not significant. Of note, additional analyses, which adjusted for psychiatric history of the siblings and parents, did not find a significantly greater risk in the oldest paternal age group. However, without this adjustment, the oldest age group was significantly associated with BPD risk (see Supplementary Table SVI). In summary, the most consistent paternal age effect on the risk of BPD development was seen in the oldest age group.
Attention Deficit/Hyperactive Disorder, Obsessive–Compulsive Disorder, and Major Depressive Disorder
The results of the epidemiological studies of ADHD, MDD, and OCD are also displayed in Supplementary Table SVI. D'Onofrio et al. [2014] examined the paternal age effect on ADHD risk in offspring, using a proportional hazards regression. The authors fitted three models: (i) a baseline model (i.e., estimation of uncorrected risks); (ii) an adjusted model (i.e., including potential confounders); and (iii) a sibling fixed‐effect model (i.e., including potential confounders and all factors shared by siblings to control for unmeasured cluster‐level co‐variates). The results of the second model, given in Supplementary Table SVI, showed a lack of significant association between the age of fathering children and offspring's risk of developing ADHD. We note that a critic commented that the actual ADHD prevalence in Sweden must be higher than the prevalence reported in the article (see http://archpsyc.jamanetwork.com/article.aspx?articleid=1833092) [Kadesjö and Gillberg, 2001; Giacobini et al., 2014]. The other two studies of ADHD indicated that paternal age is associated with offspring's risk of having ADHD. The results suggested that both younger and older fathers had an increased risk of having an ADHD‐affected child. Remarkably, the increased risk was greater in younger fathers. Correcting for prior history of mental illness in the family attenuated the results of McGrath et al. [2014] displayed in Supplementary Table SVI. The results of these three analyses suggest a U‐shaped pattern in which young paternal age is a stronger risk factor for ADHD in offspring than advanced paternal age.
The one study of OCD revealed a monotonic increase over advancing categories of paternal age. The one study of MDD reported that the risk of MDD in offspring was significantly greater for the two youngest as well as two oldest paternal age groups. Since studies of MDD or OCD are rare, no firm conclusions can be drawn regarding paternal age effects.
DISCUSSION
We reviewed literature on the association between advanced paternal age at childbearing and the offspring's risk of ASD, SCZ, BPD, ADHD, MDD, or OCD. We found that the parental age effect on ASD and SCZ‐related disorders is well established. Associations with BPD and ADHD are less clear, and no conclusions could be drawn regarding associations with MDD and OCD.
Despite the strong evidence that paternal age at childbearing is associated with the risk of offspring SCZ and ASD, the exact functional relationship between paternal age and the risk is unknown. The evidence for a gradual increase in risk with advancing paternal age is inconsistent. Knowledge concerning the exact functional relationship and sensible cut‐off ages (if any) is important in counseling and in determining public health policy. Therefore, this remains an important question for further research.
The paternal age effect may vary over the distinct but related disorders. The results of Sandin et al. [2016] suggest that the parental age effect on AD is greater than the effect on ASDs together. However, the risk estimates in epidemiological studies of AD are not consistently greater than the estimates in studies of ASD. Based on the results of Lampi et al. [2013], the associations found in the studies that examined ASD risk may be mainly attributable to AD. AD is the most severe form of ASD [Idring et al., 2014], and as such might be diagnosed earlier in life than less severe forms. This may influence the prevalence of the less severe forms of ASDs, as the controls may include false negatives. Alternatively, the paternal age effect might be more prominently present on AD risk than on the risk of Asperger's syndrome and PDD‐NOS. This is consistent with the idea that ASD is etiologically heterogeneous [Grafodatskaya et al., 2010; Kalkbrenner et al., 2014].
Studies employing a narrow definition of SCZ reported risks comparable to those reported by studies of SCZ and related disorders. Two studies distinguished between a narrow diagnosis of SCZ and a broad diagnosis of schizophrenia spectrum disorder or non‐affective psychosis and found different risks. Specifically, Sipos et al. [2004] investigated the paternal age effect on non‐affective, non‐schizophrenic psychosis as well as on SCZ. The risk of non‐affective, non‐schizophrenic psychosis was remarkably lower. McGrath et al. [2014] performed separate analyses for SCZ, schizoaffective disorder, and for SCZ and related disorders. Whereas the paternal age effect on the risk of SCZ and related disorders was clear, an effect on schizoaffective disorder was not observed.
Studies of BPD, ADHD, and MDD subtypes are scarce. Lehrer et al. [2016] distinguished between SCZ, BPD with and BPD without psychosis, and found different associated risks. This suggests that the paternal age effect was mainly present in offspring with psychotic symptoms, but was absent in BPD without psychotic symptoms. Psychosis could be the core aspect of these disorders, with advancing paternal age specifically increasing the risk of psychosis. This is in line with evidence that BPD and SCZ share susceptibility genes [Craddock et al., 2006].
Based on the association between advanced paternal age and offspring risk of ASD and SCZ, one might expect that the risk of ASD or SCZ among children from the same parents increases with paternal age. Contrary to this expectation, the results regarding ASD suggest that ASD‐affected children are likely to have a low birth order. If the paternal age effect mainly pertains to firstborn children, it is unlikely that this paternal age effect is attributable to age‐related mutagenesis and epigenetic alterations in the male germline.
The results regarding SCZ are somewhat inconsistent. Some studies that examined the effect of parity or birth order did not detect any significant relationship with SCZ risk [Malama et al., 1988; Westergaard et al., 1999; Tsuchiya et al., 2005]. On the other hand, a study of a Danish population [Petersen et al., 2011] (this study was not reviewed because of a sample overlap with McGrath et al. [2014]) investigated the paternal age effect, and the interference with birth order. Cox regression was carried out on a subsample consisting of individuals who had an older sibling of the same father. The age of the father at birth of the second‐ or later‐born did not have an effect on offspring's SCZ risk when adjusted for age at birth of the firstborn child. Advancing age at childbearing of the firstborn child had an increasing effect on the SCZ risk of second‐ or later‐born children.
Several explanations have been proposed for the birth‐order effect. Firstly, the “hygiene hypothesis” states that firstborn children are often less exposed to infections, eliciting an autoimmune response from the immune system [Durkin et al., 2008]. This could, in turn, play a role in developing disorders such as ASD and SCZ [Becker, 2007]. Secondly, parents who delay their parenthood tend to have fewer children because the range of reproductive age is smaller. Parents who delay their first parenthood as a result of psychologically reproduction‐impeding traits or psychiatric hospitalization might have fewer children. In other words, if parents’ genetic predisposition to a psychiatric disorder is associated with a delayed parenthood and fewer children, this could explain the highest paternal age effect among firstborn children. Thirdly, the “stoppage” effect might explain the occurrence of a low birth order of children diagnosed with an ASD [Hoffmann et al., 2014]. After recognizing ASD or related traits in offspring, parents might decide to stop having children. If so, stoppage would increase the likelihood that an ASD‐affected child from an older father has a low birth order.
An aspect of the paternal age effect on ASD risk involves the possibility of a paternal age effect showing a sexual dimorphism, with paternal age contributing differently to ASD‐like traits in male and female offspring. Several studies reported a greater risk of ASD in female than in male offspring [Reichenberg et al., 2006; Puleo et al., 2011; Parner et al., 2012], whereas others did not [Sandin et al., 2016]. A sexual dimorphism seems consistent with the results of two sequencing studies [Neale et al., 2012; Sanders et al., 2012], which detected a higher de novo mutation rate in female children with ASD than in male children with ASD.
We conclude that most studies have found that children of older fathers are more likely to develop an ASD or a SCZ‐related disorder. Several hypotheses can explain these associations, that is, de novo mutations, epigenetic alterations, selection into late fatherhood, and other environmental factors. The empirical support for the hypotheses is discussed next.
Sequencing studies have provided some support for the de novo mutation hypothesis related to psychiatric morbidity [Girard et al., 2011; Xu et al., 2011; Iossifov et al., 2012; Kong et al., 2012; Michaelson et al., 2012; Neale et al., 2012; O'Roak et al., 2012; Sanders et al., 2012; Iossifov et al., 2014; McCarthy et al., 2014]. In general, the number of single nucleotide polymorphisms with a paternal origin increased with two mutations per year [Kong et al., 2012; Francioli et al., 2015]. The number of de novo mutations observed in a child is largely determined by the age of the father at conception. These mutations are predominantly of paternal origin.
The results of sequencing studies of ASD provide evidence for the de novo mutation hypothesis [Iossifov et al., 2012; Kong et al., 2012; Michaelson et al., 2012; Neale et al., 2012; O'Roak et al., 2012; Sanders et al., 2012; Iossifov et al., 2014]. O'Roak et al. [2012] and Sanders et al. [2012] did not observe a difference in overall mutation rate between ASD cases and their unaffected siblings, but observed a trend toward a higher non‐synonymous mutation rate in ASD cases. Similarly, Iossifov et al. [2014] found no difference in synonymous mutation rate between cases and their unaffected siblings, whereas there was a difference between cases and their unaffected siblings regarding the mutation rate of probable gene‐disruptive mutations. Michaelson et al. [2012] reported that genes associated with ASD are more mutable than others. Michaelson et al. [2012] suggested the existence of single nucleotide‐substitution hotspots, in which de novo mutations all occurred at once during cell division in the male germline. If the hypermutable ASD genes contain such hotspots, a threshold effect, that is, a large difference in risk estimates in two adjacent age categories, is likely. Several studies support this [Reichenberg et al., 2006; Durkin et al., 2008; Sasanfar et al., 2010; Buizer‐Voskamp et al., 2011; Frans et al., 2013; McGrath et al., 2014; Sandin et al., 2016]. Note that risk estimates in the older age groups might be unstable in some studies because of a low case sample size. Still, dramatic increase in ASD risk beyond a given reproductive age was not consistently observed. Rather the risk estimates rather gradually increase with age, which renders a mutational threshold effect less likely.
Sequencing studies of SCZ and related disorders also support the de novo mutation hypothesis. Cases with SCZ or a related disorder without a family history of SCZ carried a higher number of de novo mutations than cases with a family history of SCZ and unaffected individuals [Girard et al., 2011; Xu et al., 2011; McCarthy et al., 2014]. However, the number and type of de novo mutations in the SCZ‐affected offspring are likely to differ across chromosomes and genes [Xu et al., 2011; Kong et al., 2012; McCarthy et al., 2014]. McCarthy et al. [2014] found no correlation between paternal age and the number of de novo mutations. They did find de novo nonsense mutations to be more numerous. It could be that genes involved in vulnerability to SCZ are less conserved, and the mutability is partly controlled by the father's age [McCarthy et al., 2014]. This would explain the association between increased paternal age at childbearing and SCZ in offspring.
Based on sequencing studies, the association between advanced paternal age and offspring ASD and SCZ may be explained by de novo mutations related to the father's age. Some of the detected de novo events are located in loci that have previously been associated with ASD, intellectual disability, or SCZ, suggesting a genetic overlap [Iossifov et al., 2014; McCarthy et al., 2014]. Another interesting similarity between ASD and SCZ is that the increased rate is most prominent in mutations that are likely to be gene‐disruptive. Awadalla et al. [2010] observed significantly more potentially deleterious de novo mutations in individuals with ASD and SCZ than in unaffected controls. Sequencing studies thus, consistently support the role of de novo mutations in offspring's liability to an ASD or SCZ, but other hypotheses cannot be ruled out.
Epigenetic disregulation can give rise to impairments in brain functioning and cognitive development, and is involved in the etiology of several psychiatric disorders [Schanen, 2006; Dempster et al., 2011; Elia et al., 2012; Akbarian, 2014]. In mice, age‐related methylation patterns of genes implicated in ASD and SCZ were suggested to increase offspring's risk of behavioral problems [Milekic et al., 2015]. It is also suggested that advanced paternal age is a marker for epigenetic disregulation in the human germline [Jenkins et al., 2014], and that an altered DNA methylation pattern in human males contributes to the association of increased paternal age and psychiatric morbidity. One human study suggested that a paternally altered methylation patterns in the germline is associated with the early signs of ASD risk in offspring [Feinberg et al., 2015]. However, the empirical evidence for paternally derived epigenetic errors is currently limited.
If fathers with a genetic vulnerability to develop psychiatric illness tend to postpone parenthood, this would result in a greater genetic vulnerability in older fathers. These fathers will transmit their risk‐increasing genes to their offspring, giving rise to an increased offspring risk. Assortative mating between parents sharing a genetic vulnerability would further increase the association between advanced paternal age and offspring psychiatric illness. A Norwegian population‐based study suggested that first‐time fathers of advanced ages had more sleeping problems and previous depressive symptoms compared to younger first‐time fathers [Nilsen et al., 2013]. A recent study using population genetic modeling to investigate the mechanisms underlying the association between advanced paternal age and common psychiatric disorders in offspring supported the hypothesis of selection into late fatherhood [Gratten et al., 2016]. The authors suggested that delaying fatherhood due to an increased liability to common psychiatric disorders, such as ASD and SCZ, accounts for more of the risk than age‐related de novo mutations.
Based on the empirical data reviewed here, it is not clear if the selection into late fatherhood hypothesis is applicable to ASD. Several studies of ASD took into account the potential confounding factor of a familial history of psychiatric disorder. Hultman et al. [2011] concluded that both maternal and paternal psychiatric hospitalization increased the odds of having an autistic child. Therefore, the authors included psychiatric hospitalization as a co‐variate in their analyses. However, they still saw a significant risk pattern for paternal age. The same is true for the studies by Frans et al. [2013], Lampi et al. [2013], Idring et al. [2014], and McGrath et al. [2014], who also included family history as a co‐variate but still found a significant risk pattern. However, Lampi et al. [2013] did not find a robust association between father's age at childbearing and risk of PDD‐NOS or Asperger's syndrome. The significant risk pattern suggests involvement of biological mechanisms, such as age‐related mutagenesis or epigenetic alterations in father's germline rather than a selection into late fatherhood.
The selection into late fatherhood hypothesis has been examined in different ways. Tsuchiya et al. [2008] tested whether the mean paternal age differed between fathers of ASD‐affected children with or without a broader ASD phenotype. The mean paternal age did not differ significantly between these groups. Parner et al. [2012] (sample overlap with McGrath et al. [2014]) analyzed a sibling cohort that included families with at least two full siblings, of whom at least one sibling was diagnosed with ASD. Among the families, in which the first sibling was born when the parents were young, and the second sibling was born when one or both parents were of advanced age, the second sibling had a higher risk of ASD. This finding is inconsistent with the selection into late fatherhood hypothesis.
The hypothesis of selection into late fatherhood might be more applicable to the association between father's age and SCZ in offspring. The results of two studies in which the analysis was only adjusted for maternal morbidity, point toward an association between maternal psychiatric morbidity and advanced paternal age [Dalman and Allebeck, 2002; Miller et al., 2011b]. The genetic predisposition of the father would only account for the paternal age effect if there is assortative mating, as the paternal age effect could then be explained by the psychiatric history of the mother. An epidemiological study that corrects for maternal and paternal history of psychiatric illness both separately and together may provide insights regarding the role of assortative mating. Based on current findings, genetic predisposition for SCZ does not seem to account for the relationship between paternal age at childbearing and SCZ occurrence in offspring [Tsuchiya et al., 2005; Frans et al., 2011; McGrath et al., 2014]. Furthermore, the results of Lehrer et al. [2016] are contrary to the assumption that parents with a psychiatric family history tend to postpone parenthood. However, it is still possible that parents with such a genetic vulnerability do not have a family history of SCZ, but rather carry genetic predisposing traits that contribute to a late childbearing pattern. However, Petersen et al. [2011] and Ek et al. [2014] provided strong evidence for the selection into late fatherhood hypothesis in relation to SCZ. Ek et al. [2014] demonstrated an association between age at fatherhood and offspring SCZ risk among second‐ and third‐born children, but found no association between advanced paternal age and SCZ after controlling for the age of first paternity. Petersen et al. [2011] suggested that only postponement of fathering the firstborn child affected the SCZ risk of the first‐ as well as later‐born children.
There are three studies of BPD that adjusted for family history of psychiatric illness. The results of Frans et al. [2008] and Chudal et al. [2014] revealed a significant risk pattern after controlling for this possible confounder, suggesting that the paternal age effect is not attributable to a selection into late fatherhood. The oldest age group was the only group in which an increased risk of BPD was seen. The results of McGrath et al. [2014] pointed toward parental history of psychiatric illness being a confounder, and supported the selection into late fatherhood hypothesis. This association disappeared after adjustment for a family history of psychiatric illness in a secondary analysis.
A remarkable finding of two studies of ADHD risk was that children born to older fathers and children born to younger fathers were more likely to develop ADHD. The association with younger fathers is contrary to the selection into late fatherhood hypothesis. Patients with ADHD have, on average, more children compared to unaffected individuals [Williams and Taylor, 2006]. The age of having the first child might therefore, be lower, so a genetic vulnerability of the father to ADHD could result in a selection into early, rather than late, fatherhood. The increased risk associated with younger paternal age is consistent with the suggestion that teenage pregnancy is a risk factor for ADHD [Chang et al., 2014].
It is possible that there is no causal link between the father's age at conception and the risk for children to develop ASD or SCZ. The association may be due to a distinct confounding risk factor. If so, paternal age would be a risk indicator rather than a risk factor. The environmental circumstances and characteristics of older fathers may differ from those of younger fathers. For instance, assisted reproductive technologies, which are more frequently used in older fathers, may increase the offspring ASD risk. However, a large study of the Netherlands Twin Register did not find differences in growth, attainment of motor milestones, or behavioral development between IVF and matched non‐IVF children [van Beijsterveldt et al., 2011].
Although parental death is more common among older parents, Sipos et al. [2004] suggested that this does not explain the association between old paternal age and offspring risk. Furthermore, a study of the age of the adoptive father and the risk of non‐affective psychosis in adoptees did not provide evidence for psychosocial or environmental factors explaining the paternal age effect [Ek et al., 2012].
It is always possible that there are unknown, and therefore, unmeasured confounders. The matched control group as used in some studies [Bilder et al., 2009; Buizer‐Voskamp et al., 2011; Frans et al., 2011; van Balkom et al., 2012; Lampi et al., 2013] may provide additional correction for unmeasured confounders, in so far as the unmeasured confounders correlate with the variables on which the matching was based. With exception of van Balkom et al. [2012], the studies of ASD demonstrated an attenuated risk pattern compared to the unmatched analyses. This attenuation is not seen in studies of SCZ. All epidemiological studies that used a matched control group matched cases to controls based on features of the children. Some features may reflect characteristics of their fathers, such as place of birth of the child. Matched analyses based on environmental features of the father himself may provide additional information.
The several mechanisms underlying the paternal age effect may work together or even act synergistically. For example, the de novo mutation hypothesis does not rule out the hypothesis concerning epigenetic disregulation and vice versa. An altered methylation pattern in the male germline could be a consequence of an increased mutation rate, cause an increased mutation rate or be independent of de novo mutations. Furthermore, there could be an interaction between selection into late fatherhood and environmental resources; a factor that is both associated with a higher risk of developing a psychiatric disorder and delayed fatherhood as a consequence of genetic liability could facilitate such an interaction.
The paternal age effect requires further study. One promising line would be to perform polygenetic risk score profiling. With genetic data, polygenetic risk scores can be determined and used to investigate whether men with higher polygenetic risk have children at later ages. Polygenetic risk scoring has already provided evidence for an overlap between genetic factors associated with SCZ risk and genetic factors associated with the age of first maternity [Mehta et al., 2016].
Another promising line would be to investigate symptoms rather than disorders [Weiser et al., 2008]. Some symptoms are associated with several disorders, and some symptoms are not always seen in patients diagnosed with the same disorder. Attention should also be paid to learning abilities and disabilities. A reduced ability to learn and cognitive impairments are frequently observed among ASD‐ and SCZ‐affected individuals [Freitag, 2006; Newschaffer et al., 2007; Koenen et al., 2009; Coe et al., 2012]. A genetic overlap between ASD, SCZ, and intellectual disability has been suggested [McCarthy et al., 2014]. In addition, the age of the father at childbearing is suggested to be a risk factor for intellectual disability [Malaspina et al., 2005; Saha et al., 2009; Hehir‐Kwa et al., 2011]. Offspring of older mice displayed transcriptional disregulation of developmental genes that are implicated in not only ASD and SCZ, but also in mental retardation [Milekic et al., 2015]. Note that there are practical issues regarding investigation of SCZ and intellectual disability. For instance, the IQ of SCZ patients is more subjected to changes over time compared to the IQ of unaffected individuals [Hedman et al., 2013].
Although this review strongly suggests evidence for an increased risk related to advanced paternal age, having an older father might not be exclusively risk‐increasing. For example, offspring of older men might have advantages from a social point of view. The initial results of Byrne et al. [2003] showed a U‐shaped relationship, but after controlling for familial psychiatric history and social factors, this U‐shaped pattern was attenuated. Additionally, some analyses exposed age categories that were not associated with risk or had a lower risk than the adjacent age categories. This suggests that having an older father during birth may be protective, which is not compatible with the selection into late fatherhood hypothesis. Offspring of older men are more likely to have fathers with a higher educational attainment, a higher income, and financial security [Bray, 2006; Sobotka, 2010]. However, there is no general consensus regarding social class and the risk of SCZ or ASD. Previous research has shown that there is a modest increase in SCZ risk for children born to fathers in the lowest social class at their birth, but there is no gradient over social classes [Corcoran et al., 2008].
CONCLUSION
The epidemiological results to date suggest that the father's age at childbearing is associated with adverse psychological health consequences in offspring with the strongest evidence for ASD and SCZ. The extent to which the four hypotheses about the underlying mechanisms apply to the separate disorder might differ between one another. Evidence for the de novo mutation hypothesis is most plentifully provided for ASD and SCZ, suggesting that age‐related mutagenesis plays a role. There is currently more evidence for the selection into late fatherhood hypothesis to be applicable to SCZ development than to other psychiatric disorders. Whether the environmental resource hypothesis and the hypothesis about epigenetic disregulations are more valid for certain psychiatric conditions than others is not yet possible to decide.
Supporting information
Additional supporting information may be found in the online version of this article.
Supplementary Table I
Supplementary Table II
Supplementary Table III
Supplementary Table IV
Supplementary Table V
Supplementary Table VI
ACKNOWLEDGMENTS
This study was supported by Royal Netherlands Academy of Science Professor Award (PAH/6635) to DIB; Consortium on Individual Development (CID) which is funded through the Gravitation program of the Dutch Ministry of Education, Culture, and Science and The Netherlands Organization for Scientific Research (NWO grant number 024.001.003); and Biobanking and Biomolecular Resources Research Infrastructure (BBMRI −NL, 184.021.007).
de Kluiver H, Buizer‐Voskamp JE, Dolan CV, Boomsma DI. 2016. Paternal Age and Psychiatric Disorders: A Review. Am J Med Genet Part B 174B: 202–213.
Conflicts of interest: None.
REFERENCES
- Akbarian S. 2014. Epigenetic mechanisms in schizophrenia. Dialogues Clin Neurosci 16:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anello A, Reichenberg A, Luo X, Schmeidler J, Hollander E, Smith CJ, Puleo CM, Kryzak LA, Silverman JM. 2009. Brief report: Parental age and the sex ratio in autism. J Autism Dev Disord 39:1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awadalla P, Gauthier J, Myers RA, Casals F, Hamdan FF, Griffing AR, Cote M, Henrion E, Spiegelman D, Tarabeux J, Piton A, Yang Y, Boyko A, Bustamante C, Xiong L, Rapoport JL, Addington AM, DeLisi JL, Krebs MO, Joober R, Millet B, Fombonne E, Mottron L, Zilversmit M, Keebler J, Daoud H, Marineau C, Roy‐Gagnon MH, Dube MP, Eyre‐Walker A, Drapeau P, Stone EA, Lafreniere RG, Rouleau GA. 2010. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am J Hum Genet 87:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KG. 2007. Autism, asthma, inflammation, and the hygiene hypothesis. Med Hypotheses 69:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D, Pinborough‐Zimmerman J, Miller J, McMahon W. 2009. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics 123:1293–1300. [DOI] [PubMed] [Google Scholar]
- Bray I. 2006. Advanced paternal age: How old is too old? J Epidemiol Community Health 60:851–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buizer‐Voskamp JE, Laan W, Staal WG, Hennekam EAM, Aukes MF, Termorshuizen F, Kahn RS, Boks MPM, Ophoff RA. 2011. Paternal age and psychiatric disorders: Findings from a Dutch population registry. Schizophr Res 129:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. 2003. Parental age and risk of Schizophrenia: A case‐control study. Arch Gen Psychiatry 60:673–678. [DOI] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, D'Onofrio BM, Almqvist C, Kuja‐Halkola R, Sjolander A, Larsson H. 2014. Maternal age at childbirth and risk for ADHD in offspring: A population‐based cohort study. Int J Epidemiol 43:1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudal R, Gissler M, Sucksdorff D, Lehti V, Suominen A, Hinkka‐Yli‐Salomäki S, Brown AS, Sourander A. 2014. Parental age and the risk of bipolar disorders. Bipolar Disord 16:624–632. [DOI] [PubMed] [Google Scholar]
- Chudal R, Joelsson P, Gyllenberg D, Lehti V, Leivonen S, Hinkka‐Yli‐Salomäki S, Gissler M, Sourander A. 2015. Parental age and the risk of attention‐deficit/hyperactivity disorder: A nationwide, population‐based cohort study. J Am Acad Child Adolesc Psychiatry 54:487–494. [DOI] [PubMed] [Google Scholar]
- Coe BP, Girirajan S, Eichler EE. 2012. The genetic variability and commonality of neurodevelopmental disease. Am J Med Genet C Semin Med Genet Part C 160C:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Perrin M, Harlap S, Deutsch L, Fennig S, Manor O, Nahon D, Kimhy D, Malaspina D, Susser E. 2008. Effect of socioeconomic status and parents’ education at birth on risk of schizophrenia in offspring. Soc Psychiatry Psychiatr Epidemiol 44:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, O'Donovan M, Owen MJ. 2006. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull 32:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Najjar DV, Fireman B, Grether JK. 2007. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Med 161:334–340. [DOI] [PubMed] [Google Scholar]
- Crow JF. 2000. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 1:40–47. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Rickert ME, Frans E, Kuja‐Halkola R, Almqvist C, Sjölander A, Larsson H, Lichtenstein P. 2014. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry 71:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman C, Allebeck P. 2002. Paternal age and schizophrenia: Further support for an association. Am J Psychiatry 159:1591–1592. [DOI] [PubMed] [Google Scholar]
- Dempster EL, Pidsley R, Schalkwyk LC, Owens S, Georgiades A, Kane F, Kalidindi S, Picchioni M, Kravariti E, Toulopoulou T, Murray RM, Mill J. 2011. Disease‐associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet 20:4786–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, Kirby RS, Leavitt L, Miller L, Zahorodny W, Schieve LA. 2008. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol 168:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek M, Wicks S, Magnusson C, Dalman C. 2012. Adoptive paternal age and risk of psychosis in adoptees: A register based cohort study. PLoS ONE 7:e47334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek M, Wicks S, Svensson AC, Idring S, Dalman C. 2014. Advancing paternal age and schizophrenia: The impact of delayed fatherhood. Schizophr Bull 41:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Sackett J, Turner T, Schardt M, Tang SC, Kurtz N, Dunfey M, McFarlane NA, Susi A, Danish D, Li A, Nissley‐Tsiopinis J, Borgmann‐Winter K. 2012. Attention‐deficit/hyperactivity disorder genomics: Update for clinicians. Curr Psychiatry Rep 14:579–589. [DOI] [PubMed] [Google Scholar]
- Feinberg JI, Bakulski KM, Jaffe AE, Tryggvadottir R, Brown SC, Goldman LR, Croen LA, Hertz‐Picciotto I, Newschaffer CJ, Fallin MD, Feinberg AP. 2015. Paternal sperm DNA methylation associated with early signs of autism risk in an autism‐enriched cohort. Int J Epidemiol 44:1199–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francioli LC, Polak PP, Koren A, Menelaou A, Chun S, Renkens I, van Duijn CM, Swertz M, Wijmenga C, van Ommen G, Slagboom PE, Boomsma DI, Ye K, Guryev V, Arndt PF, Kloosterman WP, de Bakker PIW, Sunyaev SR. 2015. Genome‐wide patterns and properties of de novo mutations in humans. Nat Genet 47:822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Långström N, Hultman CM. 2008. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry 65:1034–1040. [DOI] [PubMed] [Google Scholar]
- Frans EM, McGrath JJ, Sandin S, Lichtenstein P, Reichenberg A, Långström N, Hultman CM. 2011. Advanced paternal and grandpaternal age and schizophrenia: A three‐generation perspective. Schizophr Res 133:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frans EM, Sandin S, Reichenberg A, Långström N, Lichtenstein P, McGrath JJ, Hultman CM. 2013. Autism risk across generations. JAMA Psychiatry 70:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM. 2006. The genetics of autistic disorders and its clinical relevance: A review of the literature. Mol Psychiatry 12:2–22. [DOI] [PubMed] [Google Scholar]
- Giacobini M, Medin E, Ahnemark E, Russo LJ, Carlqvist P. 2014. Prevalence, patient characteristics, and pharmacological treatment of children, adolescents, and adults diagnosed with ADHD in Sweden. J Atten Disord DOI: 10.1177/1087054714554617 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Girard SL, Gauthier J, Noreau A, Xiong L, Zhou S, Jouan L, Dionne‐Laporte A, Spiegelman D, Henrion E, Diallo O, Thibodeau P, Bachand I, Bao JYJ, Tong AHY, Lin C‐H, Millet B, Jaafari N, Joober R, Dion PA, Lok S, Krebs M‐O, Rouleau GA. 2011. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet 43:860–863. [DOI] [PubMed] [Google Scholar]
- Glasson EJ, Bower C, Petterson B, de Klerk N, Chany G, Hallmayer JF. 2004. Perinatal factors and the development of autism: A population study. Arch Gen Psychiatry 61:618–627. [DOI] [PubMed] [Google Scholar]
- Grafodatskaya D, Chung B, Szatmari P, Weksberg R. 2010. Autism spectrum disorders and epigenetics. J Am Acad Child Adolesc 49:794–809. [DOI] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Peyrot WJ, McGrath JJ, Visscher PM, Goddard ME. 2016. Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nat Genet 48:718–724. [DOI] [PubMed] [Google Scholar]
- Grether JK, Anderson MC, Croen LA, Smith D, Windham GC. 2009. Risk of autism and increasing maternal and paternal age in a large north american population. Am J Epidemiol 170:1118–1126. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. 2009. Maternal age and chromosomally abnormal pregnancies: What we know and what we wish we knew. Curr Opin Pediatr 21:703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman AM, van Haren NE, van Baal CG, Kahn RS, Hulshoff Pol HE. 2013. IQ change over time in schizophrenia and healthy individuals: A meta‐analysis. Schizophr Res 146:201–208. [DOI] [PubMed] [Google Scholar]
- Hehir‐Kwa JY, Rodriguez‐Santiago B, Vissers LE, de Leeuw N, Pfundt R, Buitelaar JK, Perez‐Jurado LA, Veltman JA. 2011. De novo copy number variants associated with intellectual disability have a paternal origin and age bias. J Med Genet 48:776–778. [DOI] [PubMed] [Google Scholar]
- Hoffmann TJ, Windham GC, Anderson M, Croen LA, Grether JK, Risch N. 2014. Evidence of reproductive stoppage in families with autism spectrum disorder. JAMA Psychiatry 71:943–951. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. 2011. Advancing paternal age and risk of autism: New evidence from a population‐based study and a meta‐analysis of epidemiological studies. Mol Psychiatry 16:1203–1212. [DOI] [PubMed] [Google Scholar]
- Idring S, Magnusson C, Lundberg M, Ek M, Rai D, Svensson AC, Dalman C, Karlsson H, Lee BK. 2014. Parental age and the risk of autism spectrum disorders: Findings from a Swedish population‐based cohort. Int J Epidemiol 43:107–115. [DOI] [PubMed] [Google Scholar]
- Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee Y‐h, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. 2014. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Y‐h Lee, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton Lucinda L, Fulton Robert S, Magrini Vincent J, Ye K, Darnell Jennifer C, Darnell Robert B, Mardis Elaine R, Wilson Richard K, Schatz Michael C, McCombie WR, Wigler M. 2012. De novo gene disruptions in children on the autistic spectrum. Neuron 74:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TG, Aston KI, Pflueger C, Cairns BR, Carrell DT. 2014. Age‐associated sperm DNA methylation alterations: Possible implications in offspring disease susceptibility. PLoS Genet 10:e1004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesjö B, Gillberg C. 2001. The comorbidity of ADHD in the general population of Swedish school‐age children. J Child Psychol Psychiatry 42:487–492. [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, Penlesky AC. 2014. Environmental chemical exposures and autism spectrum disorders: A review of the epidemiological evidence. Curr Probl Pediatr Adolesc Health Care 44:277–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, Poulton R, Caspi A. 2009. Childhood IQ and adult mental disorders: A test of the cognitive research hypothesis. Am J Psychiatry 166:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Jonasdottir A, Wong WSW, Sigurdsson G, Walters GB, Steinberg S, Helgason H, Thorleifsson G, Gudbjartsson DF, Helgason A, Magnusson OT, Thorsteinsdottir U, Stefansson K. 2012. Rate of de novo mutations and the importance of father's age to disease risk. Nature 488:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KM, Hinkka‐Yli‐Salomäki S, Lehti V, Helenius H, Gissler M, Brown AS, Sourander A. 2013. Parental age and risk of autism spectrum disorders in a finnish national birth cohort. J Autism Dev Disord 43:2526–2535. [DOI] [PubMed] [Google Scholar]
- Lehrer DS, Pato MT, Nahhas RW, Miller BR, Malaspina D, Buckley PF, Sobell JL, Walsh‐Messinger J, Genomic Psychiatry Cohort Consortium, Pato CN. 2016. Paternal age effect: Replication in schizophrenia with intriguing dissociation between bipolar with and without psychosis. Am J Med Genet Part B 171B:495–505. [DOI] [PubMed] [Google Scholar]
- Lundström S, Haworth CMA, Carlström E, Gillberg C, Mill J, Råstam M, Hultman CM, Ronald A, Anckarsäter H, Plomin R, Lichtenstein P, Reichenberg A. 2010. Trajectories leading to autism spectrum disorders are affected by paternal age: Findings from two nationally representative twin studies. J Child Psychol Psychiatry 51:850–856. [DOI] [PubMed] [Google Scholar]
- Malama IM, Papaioannou DJ, Kaklamani EP, Katsouyanni KM, Koumantaki IG, Trichopoulos DV. 1988. Birth order sibship size and socio‐economic factors in risk of schizophrenia in Greece. Br J Psychiatry 152:482–486. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. 2001. Advanced paternal age and the risk of schizophrenia. Arch Gen Psychiatry 58:361–367. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Reichenberg A, Weiser M, Fennig S, Davidson M, Harlap S, Wolitzky R, Rabinowitz J, Susser E, Knobler HY. 2005. Paternal age and intelligence: Implications for age‐related genomic changes in male germ cells. Psychiatr Genet 15:117–125. [DOI] [PubMed] [Google Scholar]
- McCarthy SE, Gillis J, Kramer M, Lihm J, Yoon S, Berstein Y, Mistry M, Pavlidis P, Solomon R, Ghiban E, Antoniou E, Kelleher E, O'Brien C, Donohoe G, Gill M, Morris DW, McCombie WR, Corvin A. 2014. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry 19:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ, Saha S, Chant D, Welham J. 2008. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. 2014. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry 71:301–309. [DOI] [PubMed] [Google Scholar]
- Mehta D, Tropf FC, Gratten J, Bakshi A, Zhu Z, Bacanu S, Hemani G, Magnusson PKE, Barban N, Esko T, Metspalu A, Snieder H, Mowry BJ, Kendler KS, Yang J, Visscher PM, McGrath JJ, Mills MC, Wray NR, Lee SH. 2016. Evidence for genetic overlap between schizophrenia and age at first birth in women. JAMA Psychiatry 73:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson JJ, Shi Y, Gujral M, Zheng H, Malhotra D, Jin X, Jian M, Liu G, Greer D, Bhandari A, Wu W, Corominas R, Peoples Á, Koren A, Gore A, Kang S, Lin Guan N, Estabillo J, Gadomski T, Singh B, Zhang K, Akshoomoff N, Corsello C, McCarroll S, Iakoucheva LM, Li Y, Wang J, Sebat J. 2012. Whole‐genome sequencing in autism identifies hot spots for de novo germline mutation. Cell 151:1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milekic MH, Xin Y, O'Donnell A, Kumar KK, Bradley‐Moore M, Malaspina D, Moore H, Brunner D, Ge Y, Edwards J, Paul S, Haghighi FG, Gingrich JA. 2015. Age‐related sperm DNA methylation changes are transmitted to offspring and associated with abnormal behavior and dysregulated gene expression. Mol Psychiatry 20:995–1001. [DOI] [PubMed] [Google Scholar]
- Miller B, Messias E, Miettunen J, Alaraisanen A, Jarvelin MR, Koponen H, Rasanen P, Isohanni M, Kirkpatrick B. 2011a. Meta‐analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull 37:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Suvisaari J, Miettunen J, Järvelin M, Haukka J, Tanskanen A, Lönnqvist J, Isohanni M, Kirkpatrick B. 2011b. Advanced paternal age and parental history of schizophrenia. Schizophr Res 133:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, Lin C‐F, Stevens C, Wang L‐S, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, DePristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH, Jr , Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ. 2012. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485:242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto‐Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. 2007. The epidemiology of autism spectrum disorders. Annu Rev Publ Health 28:235–258. [DOI] [PubMed] [Google Scholar]
- Nilsen ABV, Waldenström U, Rasmussen S, Hjelmstedt A, Schytt E. 2013. Characteristics of first‐time fathers of advanced age: A Norwegian population‐based study. BMC Pregnancy Childbirth 13:29 DOI: 10.1186/1471‐2393‐13‐29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. 2012. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes CC, Smiraglia DJ, Plass C, Trasler JM, Robaire B. 2003. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc Natl Acad Sci USA 100:1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parner ET, Baron‐Cohen S, Lauritsen MB, Jørgensen M, Schieve LA, Yeargin‐Allsopp M, Obel C. 2012. Parental age and autism spectrum disorders. Ann Epidemiol 22:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose LS. 1933. The relative effects of paternal and maternal age in mongolism. J Genet 27:219–224. [DOI] [PubMed] [Google Scholar]
- Penrose LS. 1955. Parental age and mutation. Lancet 269:312–313. [DOI] [PubMed] [Google Scholar]
- Perrin MC, Brown AS, Malaspina D. 2007. Aberrant epigenetic regulation could explain the relationship of paternal age to schizophrenia. Schizophr Bull 33:1270–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Mortensen PB, Pedersen CB. 2011. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry 168:82–88. [DOI] [PubMed] [Google Scholar]
- Puleo CM, Schmeidler J, Reichenberg A, Kolevzon A, Soorya LV, Buxbaum JD, Silverman JM. 2011. Advancing paternal age and simplex autism. Autism 16:367–380. [DOI] [PubMed] [Google Scholar]
- Rahbar MH, Samms‐Vaughan M, Loveland KA, Pearson DA, Bressler J, Chen Z, Ardjomand‐Hessabi M, Shakespeare‐Pellington S, Grove ML, Beecher C, Bloom K, Boerwinkle E. 2012. Maternal and paternal age are jointly associated with childhood autism in Jamaica. J Autism Dev Disord 42:1928–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman JM, Harlap M, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler Y, Davidson M, Susser E. 2006. Advancing paternal age and autism. Arch Gen Psychiatry 63:1026–1032. [DOI] [PubMed] [Google Scholar]
- Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, Buka SL, McGrath JJ. 2009. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med 6:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan‐Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El‐Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Šestan N, Lifton RP, Günel M, Roeder K, Geschwind DH, Devlin B, State MW. 2012. De novo mutations revealed by whole‐exome sequencing are strongly associated with autism. Nature 485:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Schendel D, Magnusson P, Hultman C, Surén P, Susser E, Grønborg T, Gissler M, Gunnes N, Gross R, Henning M, Bresnahan M, Sourander A, Hornig M, Carter K, Francis R, Parner E, Leonard H, Rosanoff M, Stoltenberg C, Reichenberg A. 2016. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry 21:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasanfar R, Haddad SA, Tolouei A, Ghadami M, Yu D, Santangelo SL. 2010. Paternal age increases the risk for autism in an Iranian population sample. Molecular Autism 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanen NC. 2006. Epigenetics of autism spectrum disorders. Hum Mol Genet 15:R138–R150. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kitamoto A, Todokoro A, Ishii‐Takahashi A, Kuwabara H, Kim S‐Y, Watanabe K‐I, Minowa I, Someya T, Ohtsu H, Osuga Y, Kano Y, Kasai K, Kato N, Sasaki T. 2012. Parental age and assisted reproductive technology in autism spectrum disorders, attention deficit hyperactivity disorder, and Tourette syndrome in a Japanese population. Res Autism Spectr Disord 6:500–507. [Google Scholar]
- Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, Gunnell D. 2004. Paternal age and schizophrenia: A population based cohort study. BMJ 329:1070–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotka T. 2010. Shifting parenthood to advanced reproductive ages: Trends, causes and consequences In: Tremmel JC, editor. A young generation under pressure? London: Springer; pp 129–154. [Google Scholar]
- Tsuchiya KJ, Matsumoto K, Miyachi T, Tsujii M, Nakamura K, Takagai S, Kawai M, Yagi A, Iwaki K, Suda S, Sugihara G, Iwata Y, Matsuzaki H, Sekine Y, Suzuki K, Sugiyama T, Mori N, Takei N. 2008. Paternal age at birth and high‐functioning autistic‐spectrum disorder in offspring. Br J Psychiatry 193:316–321. [DOI] [PubMed] [Google Scholar]
- Tsuchiya KJ, Takagai S, Kawai M, Matsumoto H, Nakamura K, Minabe Y, Mori N, Takei N. 2005. Advanced paternal age associated with an elevated risk for schizophrenia in offspring in a Japanese population. Schizophr Res 76:337–342. [DOI] [PubMed] [Google Scholar]
- van Balkom IDC, Bresnahan M, Vuijk PJ, Hubert J, Susser E, Hoek HW. 2012. Paternal age and risk of autism in an ethnically diverse, non‐industrialized setting: Aruba. PLoS ONE 7:e45090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Bartels M, Boomsma DI. 2011. Comparison of naturally conceived and IVF‐DZ twins in the netherlands twin registry: A developmental study. J Pregnancy 2011:517614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Reichenberg A, Werbeloff N, Kleinhaus K, Lubin G, Shmushkevitch M, Caspi A, Malaspina D, Davidson M. 2008. Advanced parental age at birth is associated with poorer social functioning in adolescent males: Shedding light on a core symptom of schizophrenia and autism. Schizophr Bull 34:1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard T, Mortensen PB, Pedersen CB, Wohlfahrt J, Melbye M. 1999. Exposure to prenatal and childhood infections and the risk of schizophrenia. Arch Gen Psychiatry 56:993–998. [DOI] [PubMed] [Google Scholar]
- Williams J, Taylor E. 2006. The evolution of hyperactivity, impulsivity and cognitive diversity. J R Soc Interface 3:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu X, Luo H, Deng W, Zhao G, Wang Q, Zhang L, Ma X, Liu X, Murray RA, Collier DA, Li T. 2012. Advanced paternal age increases the risk of schizophrenia and obsessive‐compulsive disorder in a Chinese Han population. Psychiatry Res 198:353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Roos JL, Dexheimer P, Boone B, Plummer B, Levy S, Gogos JA, Karayiorgou M. 2011. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet 43:864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingson T, Owen MJ, Lewis G. 2003. Paternal age and risk for schizophrenia. Br J Psychiatry 183:405–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supplementary Table I
Supplementary Table II
Supplementary Table III
Supplementary Table IV
Supplementary Table V
Supplementary Table VI


