Abstract
Electrical coupling in circuits can produce non‐intuitive circuit dynamics, as seen in both experimental work from the crustacean stomatogastric ganglion and in computational models inspired by the connectivity in this preparation. Ambiguities in interpreting the results of electrophysiological recordings can arise if sets of pre‐ or postsynaptic neurons are electrically coupled, or if the electrical coupling exhibits some specificity (e.g. rectifying, or voltage‐dependent). Even in small circuits, electrical coupling can produce parallel pathways that can allow information to travel by monosynaptic and/or polysynaptic pathways. Consequently, similar changes in circuit dynamics can arise from entirely different underlying mechanisms. When neurons are coupled both chemically and electrically, modifying the relative strengths of the two interactions provides a mechanism for flexibility in circuit outputs. This, together with neuromodulation of gap junctions and coupled neurons is important both in developing and adult circuits. © 2016 Wiley Periodicals, Inc. Develop Neurobiol 77: 597–609, 2017
Keywords: gap junctions, neuronal networks, stomatogastric, ganglion, inhibition, oscillations
Introduction
The first demonstrations of electrical coupling were made nearly sixty years ago (Furshpan and Potter, 1959), but the myriad potential roles of electrical coupling in circuit function are still often neglected or underestimated. In this paper we use the well‐studied circuits of the decapod crustacean stomatogastric nervous system (STNS) (Maynard, 1972; Marder and Bucher, 2007) to illustrate some of the potential for circuit flexibility that arises from combining electrical and chemical synapses within circuits. At the same time, we highlight some of the confounds in understanding circuit performance and interpreting connectomes that result directly from the presence of electrical coupling in circuits (Marder, 1984; Gutierrez and Marder, 2013; Gutierrez et al., 2013; Gutierrez and Marder, 2014).
The STNS consists of the single stomatogastric ganglion (STG), which has 26‐30 neurons depending on species (Kilman and Marder, 1996; Bucher et al., 2007), and three anterior ganglia from which modulatory projection neurons influence the STG circuits (Dando and Selverston, 1972; Nagy and Dickinson, 1983; Nagy et al., 1988; Nusbaum and Marder, 1989a; Nusbaum and Marder, 1989b; Nusbaum et al., 1992; Bal et al., 1994; Bartos and Nusbaum, 1997; Blitz and Nusbaum, 1997; Blitz et al., 1999; Beenhakker and Nusbaum, 2004; Beenhakker et al., 2007; Blitz et al., 2008; Blitz and Nusbaum, 2012).
The STG contains neurons that are components of the central pattern generating networks that produce several different feeding‐related rhythmic motor patterns. These include the distinct but interacting fast pyloric rhythm (pumping/filtering of chewed food; cycle period ∼1 s), and slower gastric mill rhythm (chewing; cycle period ∼10‐20 s). All STG neurons are physiologically identified, with many present as a single copy per STG (Kilman and Marder, 1996; Bucher et al., 2007).
Electrical Coupling Complicates Circuit Analysis
The earliest intracellular recordings from pyloric network neurons revealed electrical coupling between the Pyloric Dilator (PD) and Anterior Burster (AB) neurons (Maynard, 1972; Selverston and Miller, 1980; Eisen and Marder, 1982; Miller and Selverston, 1982b, a; Eisen and Marder, 1984; Marder and Eisen, 1984a, 1984b). Intracellular recordings also revealed that the Lateral Pyloric (LP) and Pyloric (PY) neurons (not shown in [Fig. 1(A)] are inhibited during each burst of the AB and PD neurons [Fig. 1(A)], and that LP neuron action potentials in turn evoke IPSPs in the AB and PD neurons [Fig. 1(A)]. If one simply looks at these recordings, one would assume that the connectivity is represented by the diagram shown in the top left panel of Fig. 1(B). However, Eisen and Marder (1982) realized that there were actually 9 different connectivity diagrams [Fig. 1(B)] that are consistent with the intracellular recordings seen in Figure 1(A). To disambiguate this circuit, Eisen and Marder (1982) used the then recently developed method of photoinactivation subsequent to dye‐filling (Miller and Selverston, 1979) to kill either the two PD neurons or the single AB neuron. Doing so revealed the actual circuit [Fig. 1(B), bottom left, black). Notice that the electrical coupling creates an ambiguity on both the presynaptic and postsynaptic side (Marder, 1984). In other words, it wasn't clear before the photoinactivation experiments whether the inhibition of the LP neuron came from the PD neurons, the AB neuron, or both. Likewise, it wasn't clear whether the LP inhibited the PD neurons, the AB neuron, or both. In the former case, it was both. In the latter case, the LP neuron only inhibits the PD neurons directly, and the IPSPs recorded in the AB neuron occur as a result of its electrical synapse with the PD neurons (Eisen and Marder, 1982). Disambiguating these circuit interactions is not simply useful for “dotting i's and crossing t's”, but is pivotal to understanding circuit function and its flexibility (Hooper and Marder, 1987). Any circuit with a combination of electrical and chemical synapses is likely to have similar ambiguities, whether on the presynaptic or postsynaptic side.
Figure 1.
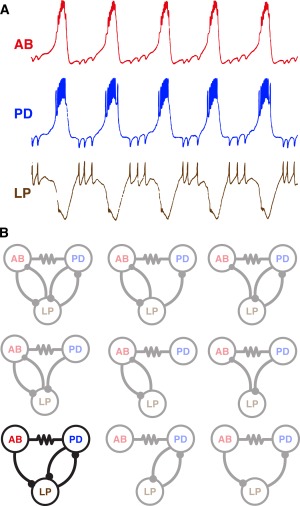
Ambiguous connectivity diagrams can result from electrophysiological recordings of electrically coupled neurons. A) Simultaneous intracellular recordings from three neurons of the STG of the lobster, Panulirus interruptus. Note that each action potential in the LP neuron evoked a unitary IPSP in both the PD and AB neurons. Also, the synchronous AB/PD neuron bursts evoked large IPSPs in the LP neuron. B) Nine connectivity diagrams that are all consistent with the recordings seen in (A). Photoinactivation experiments were done to determine the actual circuit (black circuit diagram, bottom left). Adapted from (Eisen and Marder, 1982). In this and later diagrams, chemical synapses are portrayed as balls on sticks (inhibitory connections) and bars (excitatory connections). Electrical coupling is indicated with resistor symbols between coupled neurons.
Photoinactivation studies also demonstrated that (1) the AB neuron is glutamatergic while the PD neurons are cholinergic (Marder and Eisen, 1984b), (2) the AB and PD neurons respond differently to modulators and modulatory inputs (Marder and Eisen, 1984a), and (3) their intrinsic membrane properties are different (Miller and Selverston, 1982a).
In the above example, different classes of identified neurons are electrically coupled. It is often assumed that electrical coupling primarily occurs within a population of cells of the same type, as occurs in many tissues in the body (Sherman and Rinzel, 1992). Nonetheless, it is important to remember that coupling between neurons with different intrinsic properties occurs routinely in the nervous system, and this can produce complex dynamics (Kepler et al., 1990; Coleman et al., 1995; Kopell et al., 1998; Soto‐Trevino et al., 2005).
Electrical Coupling can be Paradoxical
Electrical coupling often tends to synchronize coupled neurons (Manor et al., 1997; Mancilla et al., 2007; O'Brien, 2014). This can even occur when reciprocally inhibitory neurons are also electrically coupled (Lewis and Rinzel, 2003; Bem et al., 2005; Bem et al., 2008). Moreover, synchrony need not be the outcome of electrical coupling (Sherman and Rinzel, 1992). In the STG there are electrically‐coupled neurons that fire out of phase, including coupled neurons such as the AB and Ventricular Dilator (VD) neurons [Fig. 2(A)]. In Figure 2(B) the AB and VD neurons fire in alternation despite being electrically coupled, because the AB neuron also chemically inhibits the VD neuron [Fig. 2(A)]. When the chemical inhibition is blocked pharmacologically or the AB neuron is photoinactivated [Fig. 2(C)], the PD and VD neurons fire in phase with each other (Eisen and Marder, 1982). These parallel connections provide circuit flexibility. Specifically, depending on the relative strength of these two opposing factors, the relative synchrony of the neurons is altered (Eisen and Marder, 1982; Marder, 1984; Johnson et al., 1994).
Figure 2.
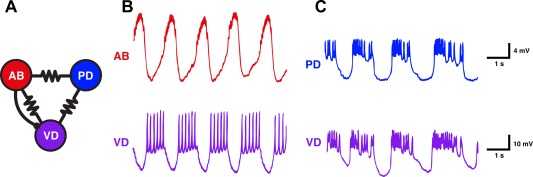
Cell kills produce phase shifts in electrically coupled neurons. A) Circuit diagram showing the connections between the AB, PD, and VD neurons. B) Intracellular recordings of the AB (top) and VD (bottom) neurons in P. interruptus. They burst in anti‐phase in the intact circuit. C) When AB was killed, the PD (top trace) and VD neurons became active in phase. Scale bars apply to the top and bottom rows, respectively, of B and C. Adapted from Eisen and Marder (1982).
Electrical Coupling and the Functional Connectivity Diagram of the Crab STG
Ensembles of electrically coupled neurons are found not only in the STG but in circuits in many (presumably all) other animals. One effective approach to demonstrate this coupling is to assess the extent of dye‐coupling, or tracer‐coupling, throughout a network (Tornqvist et al., 1988; McMahon et al., 1989; Peinado et al., 1993). In sets of such experiments involving identified circuit neurons, individual STG neuron somata were physiologically identified and localized, after which Neurobiotin tracer was injected in one soma in each STG and allowed to diffuse. Figure 3 shows the results of 4 such experiments, including injections into the single AB neuron [Fig. 3(A)], one of the two PD neurons [Fig. 3(B)], one of the two Lateral Posterior Gastric (LPG) neurons [Fig. 3(C)] and into the single VD neuron [Fig. 3(D)]. These four neuron types were determined previously to be electrically coupled. Note that the pattern of Neurobiotin‐spread is similar but not identical through this network. For example, when the AB and PD neurons were directly injected, the VD neuron filled, but when the VD neuron was directly filled, the tracer did not spread into the AB and PD neurons. What cannot be determined from these experiments is whether all of these neurons are directly coupled to each other, or whether some of the tracer‐coupling results from transit through an intermediary neuron. Examination of the full connectivity diagram of the STG circuit of the crab Cancer borealis (Fig. 4) shows that there are potentially multiple direct and indirect routes by which neurons might be electrically coupled, and this could contribute to the asymmetry in tracer‐coupling seen in Figure 3.
Figure 3.
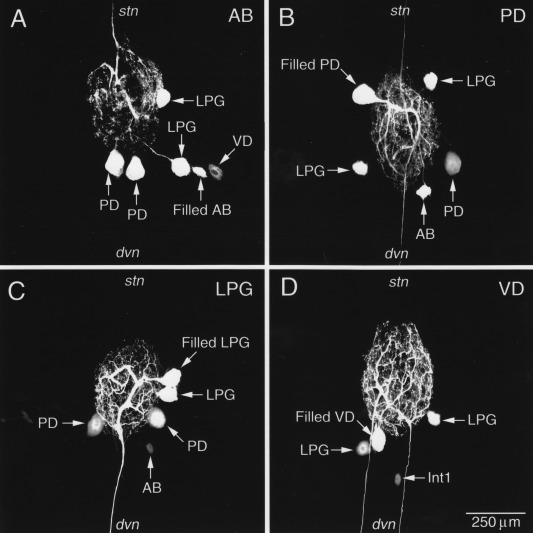
Neurobiotin fills reveal electrical synapses between neurons in the STG of the crab Cancer borealis. A) The AB neuron was intracellularly filled with Neurobiotin, which diffused into both LPG neurons and both PD neurons. Neurobiotin also crossed into VD, but to a lesser extent. B) The PD neuron was filled with Neurobiotin which crossed into AB, both LPGs, and the other PD neuron in the circuit. C) One LPG was injected with Neurobiotin and it filled the other LPG, both PDs, and the AB neuron. D) VD was filled with Neurobiotin and it traversed into both LPGs and Int1. Note that, despite the strong tracer‐coupling here between VD and the LPGs, Neurobiotin failed to cross into VD when the LPG was filled. Unpublished data from the Nusbaum laboratory.
Figure 4.
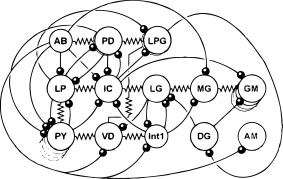
Crab STG connectivity diagram. The neurons of the crab stomatogastric ganglion are schematized along with their connections. Modified from Marder and Bucher (2007).
Distinguishing between direct coupling and coupling through an intermediate would require a systematic set of photoinactivation experiments. Even in a small nervous system as intensively studied as the STG, extensive electrical coupling and the fact that many synapses are highly modulated (Dickinson et al., 1990; Johnson and Harris‐Warrick, 1990; Johnson et al., 1994; Thirumalai et al., 2006; Zhao et al., 2011) can make it difficult to unambiguously determine a connectivity diagram using electrophysiology alone. For example, there are some synaptic potentials that are virtually silent in control saline, but are strong in the presence of a modulator (Thirumalai et al., 2006). These synapses are presumably present anatomically, but require either neuromodulation of the presynaptic terminal to allow transmitter release and/or modulation on the postsynaptic side to increase the number of available receptors. This kind of ambiguity illustrates the advantage of having a high‐quality electron microscope‐determined connectome (Briggman et al., 2011; Helmstaedter et al., 2013; Kasthuri et al., 2015; Mikula and Denk, 2015), although those high‐density connectomes were done with methods that didn't have sufficient resolution to reveal the electrical synapses.
The connectivity diagram of the C. borealis STG in Figure 4 includes several of the connections that have been recorded in some but not all preparations. What is not clear is whether these connections are always anatomically present but might be physiologically silent in some preparations under some conditions, or whether there could be real animal‐to‐animal variability in some of the connections. If the latter is the case, it would be fascinating to ask whether there are correlated circuit configurations, such that a missing synapse in one animal might be compensated by other changes in the circuit.
Coupling between Circuit Inputs and Circuit Elements
While Figure 4 is a connectivity diagram describing the interactions among STG neurons themselves, there are approximately 25 pairs of descending modulatory input neurons whose terminals interact both chemically and electrically with STG neurons in the neuropil of the STG (Coleman et al., 1992; Nusbaum et al., 1992; Coleman and Nusbaum, 1994; Coleman et al., 1995). One of the most striking features of the interactions between the modulatory inputs to the STG and their target neurons are electrical synapses between the STG neurons and the terminals of the projection neurons in the STG neuropil. These can be revealed with tracer‐fills (Fig. 5) and with direct electrophysiological recordings (Fig. 6) (Nusbaum et al., 1992; Coleman et al., 1995; Blitz and Nusbaum, 1997; Bartos et al., 1999; Blitz and Nusbaum, 2012). An example of the tracer‐coupling that supports the presence of electrical coupling between the terminals of identified modulatory projection neurons and specific STG neurons is shown in Figure 5. Filling the descending modulatory neuron MCN1 with Neurobiotin reveals extensive coupling among the gastric mill neurons in the STG [Fig. 5(A)]. Another descending modulatory neuron, CPN2, is similarly tracer‐coupled to many of the same gastric STG neurons [Fig. 5(B)].
Figure 5.
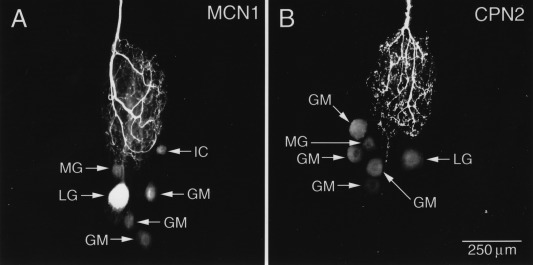
Descending modulatory neurons are electrically coupled to STG neurons. A) The terminals of MCN1, a descending modulatory neuron, were injected with Neurobiotin. The tracer secondarily filled the MG, LG, IC, and three of the four GM neurons. B) When CPN2, another descending neuromodulatory neuron, was injected with Neurobiotin the tracer crossed into a similar ensemble of gastric mill neurons. Unpublished data from the Nusbaum lab.
Figure 6.
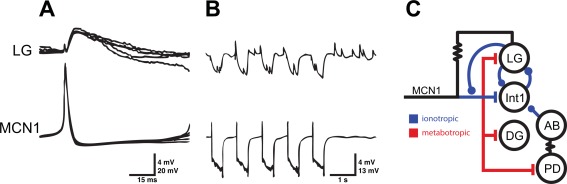
MCN1 and LG are electrically coupled. A) MCN1 action potentials (bottom superimposed traces) evoked an EPSP in LG (top superimposed traces) preceded by a small, rapid depolarization. B) Hyperpolarizing MCN1 (bottom trace) also hyperpolarized LG (top trace), indicating the electrical synapse between the two neurons. Periodic current pulses were delivered to MCN1. C) Schematic of a portion of the STG circuit illustrating how the electrical coupling between MCN1 and LG acts in parallel with the neuromodulatory effects of MCN1 on the rest of the circuit. Traces from A and B adapted from Nusbaum et al (1992). Circuit in C from Bartos et al (1999).
Figures 6(A,B) shows simultaneous intracellular recordings from the axon of the MCN1 neuron where it enters the STG and from the soma of the LG neuron. Figure 6(A) shows that a MCN1 action potential first evokes a small, rapid depolarization in LG followed by a larger, slower EPSP. Figure 6(B) shows that hyperpolarization of the MCN1 terminal evokes a smaller but quite noticeable hyperpolarization of the LG neuron. Figure 6(C) shows a connectivity diagram that highlights the interactions of the MCN1 terminals with STG neurons that underlie gastric mill rhythm generation (Coleman et al., 1995; Bartos and Nusbaum, 1997; Nadim et al., 1998; Bartos et al., 1999). Connectivity diagrams rarely provide sufficient information to explain how a circuit works. For example, one pivotal event that is not discernable from this connectivity diagram is the fact that the indicated electrical coupling between the MCN1 terminals and the LG neuron is voltage‐dependent such that it contributes significantly during one phase of the gastric mill rhythm and is relatively ineffective during the other phase (Coleman et al., 1995). As this example portrays, the extensive interactions between the terminals of the descending modulatory neurons and STG neurons are crucial for understanding the dynamics of the STG motor patterns (Nusbaum and Beenhakker, 2002; Blitz and Nusbaum, 2008; DeLong et al., 2009a; DeLong et al., 2009b; Blitz and Nusbaum, 2011; Rodriguez et al., 2013) but these interactions mean that the connectivity diagram in Figure 4 is missing all of the circuitry that involves the terminals of the modulatory neurons in the STG, with their often complex array of co‐transmitter actions (Blitz and Nusbaum, 1999; Stein et al., 2007; Marder, 2012). Consequently, it is not surprising that despite universal consensus about most of the connectivity in the C. borealis STG, there are several connections that are weak and/or state‐dependent and so are not recorded in every electrophysiological experiment.
Molecular Substrates of Coupling in the STG: Innexin Expression
In invertebrates, electrical‐coupling and dye‐coupling are mediated by gap junction proteins encoded by innexin genes (Phelan et al., 1998; Phelan, 2005; Ducret et al., 2006; Phelan et al., 2008). There are six innexin genes in the transcriptomes of C. borealis and H. americanus (Shruti et al., 2014). Innexins 1‐6 are expressed in the C. borealis STG, but single neuron analyses showed animal‐to‐animal variability in some of the innexin expression, consistent with the possibility that this variability could cause some animal‐to‐animal variations in the presence and/or strength of some electrical synapses (Shruti et al., 2014).
Electrical Coupling Creates Parallel Pathways
The extent of electrical coupling in the connectivity diagram of the STG (Fig. 4) produces the potential for information to flow through multiple parallel pathways. In other words, there are many examples of two neurons connected by both monosynaptic and polysynaptic pathways. The existence of these parallel pathways creates potential degenerate circuit mechanisms: multiple changes in circuit parameters that can elicit the same or similar changes in circuit behavior.
Figure 7 shows an example illustrating this general principle: a circuit of 5 model neurons is coupled with a mixture of chemical and electrical synapses, according to the diagram shown (Gutierrez et al., 2013). Note that there are parallel pathways connecting the f1 and hn neurons and the s1 and hn neurons, including both monosynaptic inhibitory connections and those mediated via the electrical synapses. What this model demonstrates is that three different changes in circuit parameters can produce essentially the same change in circuit output! In this case, the degeneracy is a direct consequence of the electrical coupling in the circuit. Similarly, two different pathways, including the projection neuron MCN1 and the bath‐applied neuropeptide CabPK, elicit the same gastric mill rhythm by configuring different circuits (Saideman et al., 2007) The aforementioned coupling between MCN1 and the LG neuron is pivotal to MCN1‐gastric mill rhythm generation, but not during CabPK‐gastric mill rhythm generation because MCN1 is silent at that time (Saideman et al., 2007; Rodriguez et al., 2013).
Figure 7.
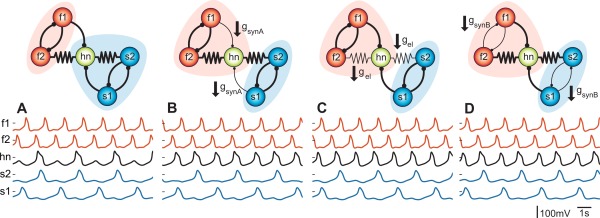
The neuromodulation of different synaptic connections can produce the same modification in circuit behavior. Panel A) is the control in this computational simulation of a 5‐cell circuit. Voltage traces corresponding to the five neurons are displayed below each circuit and colored according to cell type (orange, fast oscillators; black, intermediate; blue, slow). B) Inhibitory synapse strengths from f1 and s1 are reduced resulting in a switch of hn (hub neuron) from the slow to the fast rhythm. C) Electrical coupling is reduced while all chemical synapses are left intact resulting in the same switch of the hn. D) Mutual inhibition is reduced for both half centers and the same switch is achieved. From (Gutierrez et al., 2013).
The strength of electrical coupling can influence the extent to which modulation of a single neuron alters the activity of an entire group of neurons. In the simulation shown in Figure 8 (Gutierrez and Marder, 2014) the intrinsic properties of the hn neuron were altered so that the isolated hn neuron showed different wave‐forms, although its frequency was maintained [Fig. 8(A)]. Then these modulated neurons were embedded into two circuits. The circuit shown in Figure 8(B) has relatively weak electrical synapses but strong chemical synapses and is thus dominated by the inhibitory interactions through the chemical synapses. In this case, although the hn's waveform was altered, it continues to oscillate at approximately the same frequency in time with the intrinsically slow oscillators while the other 4 neurons in the circuit are relatively unaffected. In contrast, in Figure 8(C) we see the opposite case. Here the electrical synapses are strong and the chemical synapses are relatively weak. In this regime, electrical‐coupling dominates and modulation of the hn's intrinsic properties alters the output of all of the neurons in the circuit, entirely changing the circuit dynamics (Gutierrez and Marder, 2014). Compare the first two panels of traces in Figure 8(C). The fast and slow oscillators maintain distinct oscillatory rhythms while neuromodulation of the hn is able to switch it from being active with the slow oscillators to being active with the fast part of the circuit. Neuromodulation of the hub neuron in this model circuit thus changes the network‐wide activity as illustrated in the last two panels of traces in Figure 8(C). In the third panel, all of the neurons oscillate at the same frequency and the distinct sub‐rhythms are no longer present. This work shows that the effect of modulation of a single circuit element can indirectly influence neurons that are not themselves the direct targets of a modulatory input.
Figure 8.
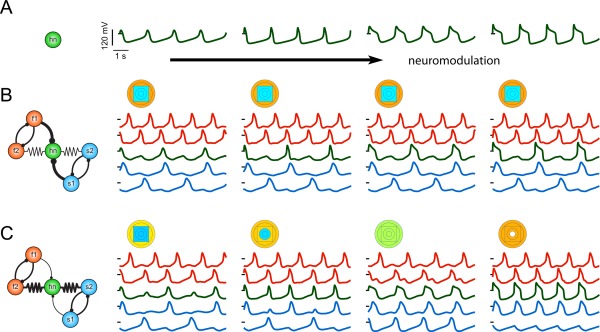
The circuit‐wide effects of local neuromodulation depend on the parameters of the circuit connectivity. A) Example voltage traces from an isolated hub neuron (hn) that were taken from a frequency‐invariant manifold in intrinsic conductance space. The changes in these voltage waveforms are due to changes in the intrinsic conductances, which simulates the effects of neuromodulation. B) The output pattern of a circuit dominated by inhibition is robust to neuromodulation of hn, as schematized in the circuit diagram to the left. In this case, the circuit only produces one pattern in which the hn oscillates with the slow part of the circuit regardless of the neuromodulation of the hn. This is shown in the circuit traces (right) which correspond to the neuromodulatory changes in hn in the traces in A (traces are colored according to cell type (orange, fast oscillators; green, intermediate; blue, slow). The circuit output pattern is also summarized by the “parameterscape” points that accompany each set of traces. Each concentric shape corresponds to a neuron in the circuit (outer circles, f1 and f2; square, hn; inner circles, s1 and s2) and is color‐coded for the neuron's oscillation frequency (red, high frequencies; to blue, low frequencies). C) A circuit dominated by electrical coupling transitions between several different circuit patterns as the hn is modulated. From (Gutierrez and Marder, 2014).
Rectifying and non‐Rectifying Synapses
Some electrical synapses show rectification, defined as current flowing preferentially in one direction (Furshpan and Potter, 1959; Phelan et al., 2008; Shruti et al., 2014). Rectification may arise as a consequence of different innexins expressed by different cell types that are electrically coupled (Phelan, 2005; Phelan et al., 2008). The electrical connections in the STG show a range of rectification properties (Shruti et al., 2014), but as each STG neuron expresses multiple innexins and is electrically coupled to many different neurons, it is difficult to know the extent to which different innexin genes are involved in specific STG electrical connections.
Rectification is illustrated in Figure 9. The impact of rectification on the synchronization of two neurons is shown (Gutierrez and Marder, 2013). The maximal conductance through the electrical synapse is a function of the junctional potential [Fig. 9(A)] which permits current flow when neuron 1 is more depolarized than neuron 2 but not when neuron 1 is more hyperpolarized than neuron 2. For example, if one of the neurons is an oscillator while the second is not oscillatory, the direction of the current flow completely changes the output of this two‐neuron circuit [Figs. 9 (B,C,D)]. With a rectifying electrical synapse that allows hyperpolarizing current to flow from the oscillator to the non‐oscillator [Fig. 9(C)], the non‐oscillating neuron is hyperpolarized during the troughs of its partner's oscillations, but does not depolarize with it at the peaks. In Figure 9(D), the polarity of the rectifying electrical synapse is reversed and both neurons depolarize together at the peaks of the oscillatory neuron's trajectory, however the formerly non‐oscillatory neuron also hyperpolarizes with its partner due to the activation of its own intrinsic hyperpolarizing conductances. Even with a two‐cell circuit, the unintuitive effects that can result from the interactions between the electrical coupling and the intrinsic neuron properties are evident. If three neurons with different intrinsic oscillating frequencies are electrically coupled, their ability to synchronize could depend on the type and strength of electrical coupling [Fig. 9(E)]. The coupling configurations in Case 1 and Case 4 effectively behave as Case 0, where all electrical synapses are non‐rectifying. There, only a small amount of coupling conductance is required to fully synchronize the three neurons, while stronger electrical coupling is required for synchrony in Cases 2 and 3.
Figure 9.
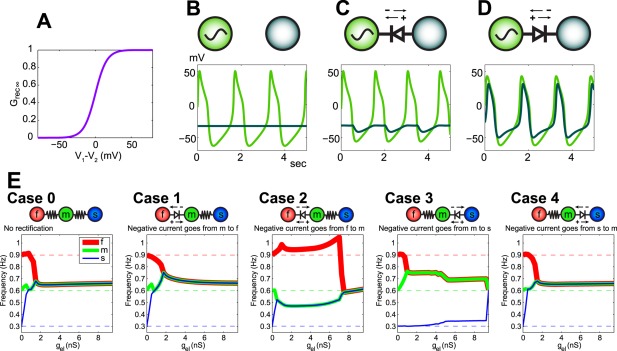
Rectifying electrical synapses complicate the functional outcome of electrical coupling. A) Plot of junctional conductance for one of the neurons as a function of the junctional potential between it and its coupled partner (V1 – V2). When the junctional potential is negative (neuron 1 is more hyperpolarized than neuron 2), there is little conductance across the gap junction. When neuron 1 is more depolarized than neuron 2, electrical synapse conductance is increased. B) The voltage traces of two neurons (one is an intrinsic oscillator and the other is intrinsically quiescent) plotted when the neurons are not coupled. C) A rectifying electrical synapse is placed between the neurons such that hyperpolarizing current is able to flow from the oscillator to the quiescent neuron while the opposite current flow is restricted. The quiescent neuron hyperpolarizes with the oscillator but does not depolarize with it. D) When the polarity of the rectifying synapse between the neurons is reversed, depolarizing current flows from the oscillator to the quiescent neuron while current flow of the opposite sign is restricted. The quiescent neuron is entrained by the intrinsic oscillator. The quiescent neuron traverses above and below its baseline voltage because its intrinsic hyperpolarizing currents are activated by the upswing. E) In a circuit with 3 oscillator neurons with different intrinsic frequencies, the properties of the electrical synapses connecting them govern the resulting circuit behavior. When both synapses are symmetrical, the three neurons synchronize their oscillation frequencies with a moderately small maximal coupling conductance (Case 0). Likewise when the electrical synapse between the intrinsically fast and medium neurons is rectifying such that depolarizing current passes easily from the fast to the intermediate neuron (2nd panel from the left; Case 1) and when a rectifying synapse between the medium and the slow oscillators permits hyperpolarizing current to pass easily from the slow to the medium neuron (right panel; Case 4). The reversed polarity of those same rectifying synapses requires a higher maximal coupling conductance to synchronize all 3 neurons (Cases 2 and 3). From (Gutierrez and Marder, 2013).
Modulation of Coupling
In many physiological systems the strength of electrical coupling is modulated by hormones and neurotransmitters (Piccolino et al., 1984; Spray and Bennett, 1985; Neyton and Trautmann, 1986a, 1986b; Tornqvist et al., 1988; McMahon et al., 1989; Connors and Long, 2004; O'Brien, 2014). In all cases, it is necessary to distinguish between changes in apparent coupling due to modulation of the impedance of one or both of the coupled neurons, versus changes in coupling caused by direct actions on the gap junctions themselves. It is worth noting that, even when modulation directly alters gap junctional conductance, there are additional physiological consequences for the coupled neurons because the event will alter the impedance of each neuron either locally (near the gap junctions) or globally (throughout the neuron). Although in the STG there are numerous indications that neuromodulators can alter coupling coefficients (Johnson et al., 1993; Johnson et al., 1994), the direct demonstration that the gap junctional conductance is the target of neuromodulation has not been accomplished.
Electrical Coupling in Development
Early in development it is common to find extensive electrical coupling (e.g. Peinado et al., 1993). Neurobiotin‐fills of individual embryonic and larval neurons from the lobster Homarus americanus result in labelling of 10‐15 STG neurons (Rehm, 2007); many more than are seen in the adult preparations. This is consistent with either a developmental decrease in electrical coupling, or a consequence of the need to inject much more dye into the significantly larger adult neurons for it to travel into other neurons (Rehm, 2007). Nonetheless, it has been argued that changes in electrical coupling early in development are critical for the maturation of adult motor patterns (Ducret et al., 2006; Ducret et al., 2007).
The embryonic STG generates relatively irregular motor patterns (Richards et al., 1999) in which the neurons that will eventually be part of the separate pyloric and gastric mill rhythms fire together (Casasnovas and Meyrand, 1995). The modulatory inputs to the STG develop sequentially during the embryonic and larval stages (Fénelon et al., 1999; Kilman et al., 1999; Pulver and Marder, 2002; Pulver et al., 2003) and responses to modulators are present quite early (Le Feuvre et al., 1999; Richards and Marder, 2000; Le Feuvre et al., 2001; Rehm et al., 2008a; Rehm et al., 2008b). Ducret et al. (2007) argue that a GABAergic input is responsible for controlling the strength of the electrical coupling that allows the emergence of adult rhythms. While this may be part of the story, the responses of the embryonic and larval preparations to neuromodulators are not fully mature (Rehm et al., 2008a), so modulatory control of the electrical coupling may be important but not the only determinant of the transition from embryonic to adult rhythms.
Conclusions
Even with the expectation that there is more to be learned about the roles of electrical coupling in neural circuits, it is already clear that such coupling provides a number of additional degrees of freedom to circuit operation. So far, electrical coupling is best known for its synchronizing actions in circuits, but it can also provide non‐intuitive actions and it is likely that the establishment of parallel pathways by electrical coupling may be as important, or more important, for how signals propagate through circuits under different modulatory conditions.
We thank Drs. Brian J. Norris and Andrew E. Christie for their contributions to Figures 3 and 5.
References
- Bal T, Nagy F, Moulins M. 1994. Muscarinic modulation of a pattern‐generating network: Control of neuronal properties. J Neurosci 14:3019–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Nusbaum MP. 1997. Intercircuit control of motor pattern modulation by presynaptic inhibition. J Neurosci 17:2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. 1999. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci 19:6650–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Nusbaum MP. 2004. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci 24:6741–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Kirby MS, Nusbaum MP. 2007. Mechanosensory gating of proprioceptor input to modulatory projection neurons. J Neurosci 27:14308–14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem T, Le Feuvre Y, Rinzel J, Meyrand P. 2005. Electrical coupling induces bistability of rhythms in networks of inhibitory spiking neurons. Eur J Neurosci 22:2661–2668. [DOI] [PubMed] [Google Scholar]
- Bem T, Meyrand P, Branchereau P, Hallam J. 2008. Multi‐stability and pattern‐selection in oscillatory networks with fast inhibition and electrical synapses. PLoS ONE 3:e3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. 1997. Motor pattern selection via inhibition of parallel pathways. J Neurosci 17:4965–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. 1999. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci 19:6774–6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. 2008. State‐dependent presynaptic inhibition regulates central pattern generator feedback to descending inputs. J Neurosci 28:9564–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. 2011. Neural circuit flexibility in a small sensorimotor system. Curr Opin Neurobiol 2011;21:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. 2012. Modulation of circuit feedback specifies motor circuit output. J Neurosci 32:9182–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. 1999. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci 19:5449–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP. 2008. A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol 211:1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. 2011. Wiring specificity in the direction‐selectivity circuit of the retina. Nature 471:183–188. [DOI] [PubMed] [Google Scholar]
- Bucher D, Johnson CD, Marder E. 2007. Neuronal morphology and neuropil structure in the stomatogastric ganglion of the lobster, Homarus americanus . J Comp Neurol 501:185–205. [DOI] [PubMed] [Google Scholar]
- Casasnovas B, Meyrand P. 1995. Functional differentiation of adult neural circuits from a single embryonic network. J Neurosci 15:5703–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Nusbaum MP. 1994. Functional consequences of compartmentalization of synaptic input. J Neurosci 14:6544–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Meyrand P, Nusbaum MP. 1995. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378:502–505. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Nusbaum MP, Cournil I, Claiborne BJ. 1992. Distribution of modulatory inputs to the stomatogastric ganglion of the crab, Cancer borealis . J Comp Neurol 325:581–594. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. 2004. Electrical synapses in the mammalian brain. Annu Rev Neurosci 27:393–418. [DOI] [PubMed] [Google Scholar]
- Dando MR, Selverston AI. 1972. Command fibres from the supra‐oesophageal ganglion to the stomatogastric ganglion in Panulirus argus . J Comp Physiol 78:138–175. [Google Scholar]
- DeLong ND, Beenhakker MP, Nusbaum MP. 2009a. Presynaptic inhibition selectively weakens peptidergic cotransmission in a small motor system. J Neurophysiol 102:3492–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ND, Kirby MS, Blitz DM, Nusbaum MP. 2009b. Parallel regulation of a modulator‐activated current via distinct dynamics underlies comodulation of motor circuit output. J Neurosci 29:12355–12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson PS, Mecsas C, Marder E. 1990. Neuropeptide fusion of two motor pattern generator circuits. Nature 344:155–158. [DOI] [PubMed] [Google Scholar]
- Ducret E, Le Feuvre Y, Meyrand P, Fenelon VS. 2007. Removal of GABA within adult modulatory systems alters electrical coupling and allows expression of an embryonic‐like network. J Neurosci 27:3626–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Alexopoulos H, Le Feuvre Y, Davies JA, Meyrand P, Bacon JP, Fenelon VS. 2006. Innexins in the lobster stomatogastric nervous system: Cloning, phylogenetic analysis, developmental changes and expression within adult identified dye and electrically coupled neurons. Eur J Neurosci 24:3119–3133. [DOI] [PubMed] [Google Scholar]
- Eisen JS, Marder E. 1982. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J Neurophysiol 48:1392–1415. [DOI] [PubMed] [Google Scholar]
- Eisen JS, Marder E. 1984. A mechanism for production of phase shifts in a pattern generator. J Neurophysiol 51:1375–1393. [DOI] [PubMed] [Google Scholar]
- Fénelon VS, Kilman V, Meyrand P, Marder E. 1999. Sequential developmental acquisition of neuromodulatory inputs to a central pattern‐generating network. J Comp Neurol 408:335–351. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Potter DD. 1959. Transmission at the giant motor synapses of the crayfish. J Physiol 145:289–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez GJ, Marder E. 2013. Rectifying electrical synapses can affect the influence of synaptic modulation on output pattern robustness. J Neurosci 33:13238–13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez GJ, Marder E. 2014. Modulation of a single neuron has state‐dependent actions on circuit dynamics. eNeuro 1:ENEURO.0009‐14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez GJ, O'Leary T, Marder E. 2013. Multiple mechanisms switch an electrically coupled, synaptically inhibited neuron between competing rhythmic oscillators. Neuron 77:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. 2013. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500:168–174. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Marder E. 1987. Modulation of the lobster pyloric rhythm by the peptide proctolin. J Neurosci 7:2097–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Harris‐Warrick RM. 1990. Aminergic modulation of graded synaptic transmission in the lobster stomatogastric ganglion. J Neurosci 10:2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris‐Warrick RM. 1993. Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol A 172:715–732. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris‐Warrick RM. 1994. Differential modulation of chemical and electrical components of mixed synapses in the lobster stomatogastric ganglion. J Comp Physiol A 175:233–249. [DOI] [PubMed] [Google Scholar]
- Kasthuri N, et al. 2015. Saturated reconstruction of a volume of neocortex. Cell 162:648–661. [DOI] [PubMed] [Google Scholar]
- Kepler TB, Marder E, Abbott LF. 1990. The effect of electrical coupling on the frequency of model neuronal oscillators. Science 248:83–85. [DOI] [PubMed] [Google Scholar]
- Kilman VL, Marder E. 1996. Ultrastructure of the stomatogastric ganglion neuropil of the crab, Cancer borealis . J Comp Neurol 374:362–375. [DOI] [PubMed] [Google Scholar]
- Kilman VL, Fénelon V, Richards KS, Thirumalai V, Meyrand P, Marder E. 1999. Sequential developmental acquisition of cotransmitters in identified sensory neurons of the stomatogastric nervous system of the lobsters, Homarus americanus and Homarus gammarus . J Comp Neurol 408:318–334. [DOI] [PubMed] [Google Scholar]
- Kopell N, Abbott LF, Soto‐Trevino C. 1998. On the behavior of a neural oscillator electrically coupled to a bistable element. Physica D 121:367–395. [Google Scholar]
- Le Feuvre Y, Fénelon VS, Meyrand P. 1999. Unmasking of multiple adult neural networks from a single embryonic circuit by removal of neuromodulatory inputs. Nature 402:660–664. [DOI] [PubMed] [Google Scholar]
- Le Feuvre Y, Fénelon VS, Meyrand P. 2001. Ontogeny of modulatory inputs to motor networks: Early established projection and progressive neurotransmitter acquisition. J Neurosci 21:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TJ, Rinzel J. 2003. Dynamics of spiking neurons connected by both inhibitory and electrical coupling. J Comput Neurosci 14:283–309. [DOI] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. 2007. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci 27:2058–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y, Rinzel J, Segev I, Yarom Y. 1997. Low‐amplitude oscillations in the inferior olive: A model based on electrical coupling of neurons with heterogeneous channel densities. J Neurophysiol 77:2736–2752. [DOI] [PubMed] [Google Scholar]
- Marder E. 1984. Roles for electrical coupling in neural circuits as revealed by selective neuronal deletions. J Exp Biol 112:147–167. [DOI] [PubMed] [Google Scholar]
- Marder E. 2012. Neuromodulation of neuronal circuits: back to the future. Neuron 76:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Eisen JS. 1984a. Electrically coupled pacemaker neurons respond differently to the same physiological inputs and neurotransmitters. J Neurophysiol 51:1362–1374. [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS. 1984b. Transmitter identification of pyloric neurons: Electrically coupled neurons use different neurotransmitters. J Neurophysiol 51:1345–1361. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. 2007. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69:291–316. [DOI] [PubMed] [Google Scholar]
- Maynard DM. 1972. Simpler networks. Ann NY Acad Sci 193:59–72. [DOI] [PubMed] [Google Scholar]
- McMahon DG, Knapp AG, Dowling JE. 1989. Horizontal cell gap junctions: Single‐channel conductance and modulation by dopamine. Proc Natl Acad Sci USA 86:7639–7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikula S, Denk W. 2015. High‐resolution whole‐brain staining for electron microscopic circuit reconstruction. Nat Methods 12:541–546. [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston A. 1979. Rapid killing of single neurons by irradiation of intracellularly injected dye. Science 206:702–704. [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston AI. 1982a. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. II. Oscillatory properties of pyloric neurons. J Neurophysiol 48:1378–1391. [DOI] [PubMed] [Google Scholar]
- Miller JP, Selverston AI. 1982b. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. IV. Network properties of pyloric system. J Neurophysiol 48:1416–1432. [DOI] [PubMed] [Google Scholar]
- Nadim F, Manor Y, Nusbaum MP, Marder E. 1998. Frequency regulation of a slow rhythm by a fast periodic input. J Neurosci 18:5053–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F, Dickinson PS. 1983. Control of a central pattern generator by an identified modulatory interneurone in crustacea. I. Modulation of the pyloric motor output. J Exp Biol 105:33–58. [DOI] [PubMed] [Google Scholar]
- Nagy F, Dickinson PS, Moulins M. 1988. Control by an identified modulatory neuron of the sequential expression of plateau properties of, and synaptic inputs to, a neuron in a central pattern generator. J Neurosci 8:2875–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. 1986a. Physiological modulation of gap junction permeability. J Exp Biol 124:993–114. [PubMed] [Google Scholar]
- Neyton J, Trautmann A. 1986b. Acetylcholine modulation of the conductance of intercellular junctions between rat lacrimal cells. J Physiol 377:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Marder E. 1989a. A modulatory proctolin‐containing neuron (MPN). I. Identification and characterization. J Neurosci 9:1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Marder E. 1989b. A modulatory proctolin‐containing neuron (MPN). II. State‐dependent modulation of rhythmic motor activity. J Neurosci 9:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. 2002. A small‐systems approach to motor pattern generation. Nature 417:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Weimann JM, Golowasch J, Marder E. 1992. Presynaptic control of modulatory fibers by their neural network targets. J Neurosci 12:2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. 2014. The ever‐changing electrical synapse. Curr Opin Neurobiol 29:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. 1993. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 10:103–114. [DOI] [PubMed] [Google Scholar]
- Phelan P. 2005. Innexins: Members of an evolutionarily conserved family of gap‐junction proteins. Biochimica et biophysica Acta 1711:225–245. [DOI] [PubMed] [Google Scholar]
- Phelan P, Goulding LA, Tam JLY, Allen MJ, Dawber RJ, Davies JA, Bacon JP. 2008. Molecular mechanism of rectification at identified electrical synapses in the Drosophila giant fiber system. Curr Biol 18:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan P, Bacon JP, Davies JA, Stebbings LA, Todman MG, Avery L, Baines RA, et al. 1998. Innexins: A family of invertebrate gap‐junction proteins. Trends Genet 14:348–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M, Neyton J, Gerschenfeld HM. 1984. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'‐monophosphate in horizontal cells of turtle retina. J Neurosci 4:2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Marder E. 2002. Neuromodulatory complement of the pericardial organs in the embryonic lobster, Homarus americanus . J Comp Neurol 451:79–90. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Thirumalai V, Richards KS, Marder E. 2003. Dopamine and histamine in the developing stomatogastric system of the lobster Homarus americanus . J Comp Neurol 462:400–414. [DOI] [PubMed] [Google Scholar]
- Rehm KJ (2007) Neuromodulation and the maturation of the stomatogastric mootor patterns of the lobster, Homarus americanus. In: Ph.D. Thesis Neuroscience, p 163: Brandeis University.
- Rehm KJ, Deeg KE, Marder E. 2008a. Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus . J Neurosci 28:9828–9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm KJ, Taylor AL, Pulver SR, Marder E. 2008b. Spectral analyses reveal the presence of adult‐like activity in the embryonic stomatogastric motor patterns of the lobster, Homarus americanus. J Neurophysiol 99:3104–3122. [DOI] [PubMed] [Google Scholar]
- Richards KS, Marder E. 2000. The actions of crustacean cardioactive peptide on adult and developing stomatogastric ganglion motor patterns. J Neurobiol 44:31–44. [PubMed] [Google Scholar]
- Richards KS, Miller WL, Marder E. 1999. Maturation of the rhythmic activity produced by the stomatogastric ganglion of the lobster, Homarus americanus . J Neurophysiol 82:2006–2009. [DOI] [PubMed] [Google Scholar]
- Rodriguez JC, Blitz DM, Nusbaum MP. 2013. Convergent rhythm generation from divergent cellular mechanisms. J Neurosci 33:18047–18064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saideman SR, Blitz DM, Nusbaum MP. 2007. Convergent motor patterns from divergent circuits. J Neurosci 27:6664–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston AI, Miller JP. 1980. Mechanisms underlying pattern generation in the lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. I. Pyloric neurons. J Neurophysiol 44:1102–1121. [DOI] [PubMed] [Google Scholar]
- Sherman A, Rinzel J. 1992. Rhythmogenic effects of weak electrotonic coupling in neuronal models. Proc Natl Acad Sci USA 89:2471–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruti S, Schulz DJ, Lett KM, Marder E. 2014. Electrical coupling and innexin expression in the stomatogastric ganglion of the crab Cancer borealis . J Neurophysiol 112:2946–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto‐Trevino C, Rabbah P, Marder E, Nadim F. 2005. Computational model of electrically coupled, intrinsically distinct pacemaker neurons. J Neurophysiol 94:590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Bennett MV. 1985. Physiology and pharmacology of gap junctions. Annu Rev Physiol 47:281–303. [DOI] [PubMed] [Google Scholar]
- Stein W, DeLong ND, Wood DE, Nusbaum MP. 2007. Divergent co‐transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci 26:1148–1165. [DOI] [PubMed] [Google Scholar]
- Thirumalai V, Prinz AA, Johnson CD, Marder E. 2006. Red pigment concentrating hormone strongly enhances the strength of the feedback to the pyloric rhythm oscillator but has little effect on pyloric rhythm period. J Neurophysiol 95:1762–1770. [DOI] [PubMed] [Google Scholar]
- Tornqvist K, Yang XL, Dowling JE. 1988. Modulation of cone horizontal cell activity in the teleost fish retina. III. Effects of prolonged darkness and dopamine on electrical coupling between horizontal cells. J Neurosci 8:2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Sheibanie AF, Oh M, Rabbah P, Nadim F. 2011. Peptide neuromodulation of synaptic dynamics in an oscillatory network. J Neurosci 31:13991–14004. [DOI] [PMC free article] [PubMed] [Google Scholar]


