The potential superior benefits of adaptive deep brain stimulation (aDBS) approaches1 compared to classical, constant‐parameters DBS were already proven by scientific evidence from different research groups.2, 3, 4 aDBS provides better symptoms control in Parkinson's disease patients by adapting the stimulation parameters to the patient's clinical state estimated through the analysis of subthalamic neuronal oscillations (ie, local field potentials) in the beta band (13‐30 Hz).5
Because aDBS administration was never systematically assessed during prolonged stimulation sessions in more ecologic conditions, we tested unilateral aDBS delivered for 2 hours, with specific focus on the concurrent administration of levodopa treatment, in freely moving parkinsonian patients.
We therefore randomly administered aDBS and cDBS through an external wearable prototype6 in 10 PD patients with DBS electrode implant in 2 different experimental sessions taking place the 5th and the 6th day after surgery (Fig. 1A). Each experimental session lasted 2 hours, during which the patient, after a baseline assessment (OFF DBS and OFF medication, stimOFF/medOFF), received both levodopa and stimulation (aDBS or cDBS), thus allowing one to study the interaction between electrical and pharmacological stimulation (ON DBS and ON medication, stimON/medON). The patient was blind to the type of DBS received during the session. The clinical effects were blindly evaluated through the UPDRS III (motor part) and the Unified Dyskinesia Rating Scale (UDysRS). According to the gold standard, the clinical assessment was performed by a blinded video rater (rigidity scores were excluded from the analysis). The total electrical energy delivered (TEED) was used for energy efficiency assessment and adverse events were collected for safety assessment.
Figure 1.
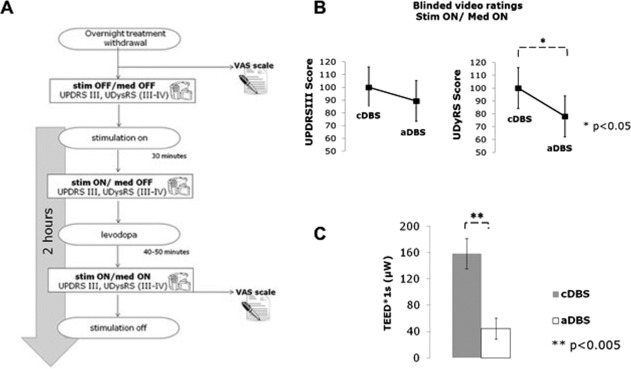
(A) Experimental design of each experimental session. Clinical effects were evaluated using the motor part of the Unified PD Rating Scale (UPDRS III) and the Unified Dyskinesia Rating Scale (UDysRS III and IV) during the concurrent administration of DBS (adaptive deep brain stimulation [aDBS] or conventional DBS [cDBS]) and levodopa. (B) The UPDRS III and UDysRS scores during aDBS and cDBS, normalized for the maximum score between aDBS and cDBS. (C) Total electrical energy delivered (TEED) per unit of time (μW) for aDBS (white color) and cDBS (gray color). Error bars represent the standard error (SE). med, medication; stim, stimulation.
The clinical scores were not significantly different between the 2 experimental sessions at baseline (stimOFF/medOFF UPDRS III, aDBS vs cDBS: 37.0 ± 16.8 vs 36.6 ± 16.2; F 1,9 = 0.2, P > .05). When the patient was under the effect of both levodopa and DBS (stimON/medON), we observed a similar improvement on global motor symptoms regardless to the type of DBS (UPDRS III percent change from baseline, aDBS vs cDBS: −46.1% ± 10.5% vs −40.1% ± 17.5%; F 1,9 = 0.6, P > .05; Fig. 1B). Conversely, in this condition, aDBS was more effective on dyskinesias than cDBS (UDysRS score, aDBS vs cDBS: 11.7 ± 67 vs 15.0 ± 8.7; F 1,9 = 6.1, P = .02; Fig. 1C). These results were obtained with an average power saving of 73.6% ± 22.9% in aDBS compared with cDBS (mean TEED aDBS vs cDBS: 44.6 ± 47.9 μW vs 158.7 ± 69.7 μW; F 1,8 = 30.4, P = .0005). Throughout the entire experiment, we did not observe any serious adverse event specifically linked to DBS.
These results support the idea that aDBS, being effective, efficient, and safe, when administered concomitantly to levodopa could help clinicians limit the severity of side effects induced by the transient summation of DBS stimulation and pharmacological therapy. However, the acute experimental setting, characterized by a microlesional effect and by the presence of edema, is a major limitation for the generalizability of our results that need to be confirmed by other studies conducted in a more chronic condition, possibly with implantable devices.
Manuela Rosa, PhD,1 Mattia Arlotti, MS,1,2 Sara Marceglia, PhD,1,3 Filippo Cogiamanian, MD,4 Gianluca Ardolino, MD,4 Alessio Di Fonzo, PhD,5 Leonardo Lopiano, PhD,6 Emma Scelzo, MD,1 Aristide Merola, MD,6 Marco Locatelli, MD,7 Paolo M. Rampini, MD,7 and Alberto Priori, PhD1,8* 1Clinical Center for Neurostimulation, Neurotechnology, and Movement Disorders, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy 2Department of Electronics, Computer Science and Systems, University of Bologna, Cesena, Italy 3Department of Engineering and Architecture, University of Trieste, Trieste, Italy 4Unit of Clinical Neurophysiology, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy 5Dino Ferrari Center, Neuroscience Section, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy 6Department of Neuroscience “Rita Levi Montalcini,” University of Turin, Turin, Italy 7Unit of Stereotactic Functional and Neuroendoscopic Neurosurgery, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy 8Department of Health Sciences, University of Milan & Ospedale San Paolo, Milan, Italy
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique.
M.R.: 1B, 1C, 2B, 3A
M.A.: 1B, 1C, 2C, 3B
SM: 1B, 2A, 2C, 3A
F.C.: 1B
G.A.: 1B
A.D.F.: 1C
L.L.: 3B
E.S.: 3A
A.M.: 2C
M.L.: 1C
P.M.R.: 1C, 3B
A.P.: 1B
Full financial disclosures for the previous 12 months
AP and SM were consultant for Newronika srl in the last 12 months
Acknowledgments
The data reported are part of the PhD thesis of Dr. Manuela Rosa. The work was supported by Grant GR‐2011‐02352807 from the Italian Ministry of Health.
This study was supported by a the ERA‐ NET NEURON Grant JTC 2013 (RD‐aDBS project), by the Ministry of Health Young Researcher Grant (GR‐2011‐0235287), and the by Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico and Università degli Studi di Milano (Italy). The University of Milan was partly supported by donation in memory of Aldo Ravelli for research on Parkinson's disease and other neuropsychiatric disorders.
Relevant conflicts of interests/financial disclosures: S.M., F.C., M.L., P.M.R., and A.P. are shareholders of Newronika Srl, a spin‐off company of the Fondazione IRCCS Ca'Granda Ospedale Maggiore Policlinico and of the Università degli Studi di Milano. All the other authors declare no conflict of interest.
References
- 1. Priori A, Foffani G, Rossi L, et al. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol 2013;245:77–86. doi:10.1016/j.expneurol.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 2. Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013;74:449–457. doi:10.1002/ana.23951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson's disease. J Neurol Neurosurg Psychiatry 2016;87:717–721. doi:10.1136/jnnp-2015-310972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosa M, Arlotti M, Ardolino G, et al. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov Disord 2015;30:1003–1005. doi:10.1002/mds.26241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arlotti M, Rosa M, Marceglia S, et al. The adaptive deep brain stimulation challenge. Parkinsonism Relat Disord 2016;28:12–17. doi:10.1016/j.parkreldis.2016.03.020 [DOI] [PubMed] [Google Scholar]
- 6. Arlotti M, Rossi L, Rosa M, et al. An external portable device for adaptive deep brain stimulation (aDBS) clinical research in advanced Parkinson's disease. Med Eng Phys 2016;38:498–505. doi:10.1016/j.medengphy.2016.02.007 [DOI] [PubMed] [Google Scholar]


