Abstract
Objective
Informal caregivers (ICs) of patients with cancer and cancer survivors report a number of psychological and physical complaints because of the burden associated with providing care. Given the documented effect of Cognitive Behavioral Therapy (CBT) on ICs' common psychological complaints, such as anxiety and depression, the objective was to conduct a meta‐analysis on the effect of CBTs for adult ICs.
Methods
A literature search was conducted in order to identify all intervention studies on adult ICs that employed at least one therapeutic component defined as a CBT component.
Results
Literature searches revealed 36 unique records with sufficient data. These studies were subjected to meta‐analyses using random effects models. A small, statistically significant effect of CBTs (Hedge's g = 0.08, p = 0.014) was revealed, which disappeared when randomized controlled trials were evaluated alone (g = 0.04, p = 0.200). A number of variables were explored as moderators. Only the percentage of female participants was positively associated with the effect size.
Conclusions
Based on the negligible effect of CBTs across outcomes, future studies should consider moving beyond traditional CBT methods as these do not appear efficacious. It is suggested that future interventions orient towards advances in the basic affective sciences and derived therapies in order to better understand and treat the emotional struggles experienced by ICs. © 2016 The Authors. Psycho‐Oncology published by John Wiley & Sons Ltd.
Keywords: caregivers, cancer, CBT, psychotherapy, psychological intervention, oncology
Background
There is growing recognition that informal caregivers (ICs) of chronically ill patients are themselves in need of care. Historically, research on caregiver burden has focused on ICs of patients with a variety of dementias, such as Alzheimer's and Parkinson's disease. More recently, the burden experienced by ICs of patients with cancer is receiving increased attention, which may in part be because of the rising incidence of cancer globally 1. Such caregivers face the concurrent stress of significant role transitions and the responsibilities of managing patient needs, in addition to existing responsibilities, which commonly results in caregiver burden. Given et al. 2 describe caregiver burden as a ‘multidimensional biopsychosocial reaction resulting from an imbalance of care demands relative to caregivers' personal time, social roles, physical and emotional states, financial resources, and formal care resources given the other multiple roles they fulfill’. Carrying this burden often comes with psychological and physical complaints 3, 4. ICs have been found to have high levels of psychological distress, and longitudinal studies have shown that caregiver burden is significantly associated with anxiety and depression over time [e.g. 5, 6]. Examples of specific complaints by ICs include feeling overwhelmed by taking on the patient's responsibilities, fear of losing the patient, and uncertainty about the future 7. Such complaints are likely to persist into survivorship, as 30–40% of caregivers continue to experience clinical levels of anxiety and depression if their loved one survives cancer 8, 9. Caregiver burden is also associated with a range of physical health complications, including sleep difficulties and fatigue 10, 11, cardiovascular disease 12, 13, poor immune functioning 14, 15, and increased mortality 16, 17. Together, the psychological and physical symptoms associated with providing care to a patient with cancer place caregivers at particular risk for experiencing negative outcomes and hence are in urgent need of effective interventions. Despite this fact, the state of science of intervention development for ICs of patients with cancer is in its infancy 18, 19.
Cognitive behavioral therapies (CBTs) have been found to be effective in treating individuals presenting with symptoms of anxiety and depression 20, 21 – common complaints of ICs – and hence it is likely an appropriate first choice treatment for ICs. A number of systematic reviews, including one meta‐analysis, of psychological interventions for ICs have been conducted. Most reviews have evaluated intervention feasibility and quality of design, and only a few have attempted to evaluate intervention efficacy and effectiveness 22, 23, 24, 25. Two reviews have specifically evaluated the effect of CBTs on a number of different outcomes in ICs such as quality of life and burden 19, 26. In one meta‐analysis 27, 29 RCTs for ICs were evaluated. Although studies were categorized according to intervention framework, that is, their self‐claimed main treatment orientation, the specific effect of the 7 studies categorized as employing CBTs was not evaluated. In a later narrative, systematic review of 49 existing psychological intervention studies for ICs of patients with cancer, the three interventions categorized as CBTs all had a positive effect 19. Across these reviews, the manner in which interventions were categorized should be highlighted. Northouse et al. 27 categorized interventions according to the ‘primary framework’ as stated by the authors. However, a ‘stress and coping' framework may not look much different than a ‘cognitive‐behavioral’ framework in terms of the therapeutic methods used. Likewise, categorizing interventions according to their ‘primary focus’ 19 may instill arbitrary differences between interventions that use similar techniques. In contrast, a narrative systematic review by O'Toole et al. 26 employed a definition of CBT according to the intervention strategies actually employed (i.e. cognitive restructuring, imaginal or in vivo exposure, coping skills training, problem‐solving, behavior activation, behavioral experiments, structured homework, acceptance‐based strategies, stress and anxiety management through relaxation, or mindfulness [cf. 28]). Thirty‐nine studies belonging to this umbrella of CBTs were evaluated, and results showed that about half of the studies produced at least one positive outcome, whereas 33% did not detect any effect of the intervention, and 15% did not report any inferential statistics because of a small sample size, or did not report the relevant statistical analyses. The review concluded that meta‐analytic efforts would be an important next step in evaluating the effect of CBTs for ICs.
The primary aim of the present study was therefore to evaluate the overall effect of interventions using CBT components, which has not previously been meta‐analytically evaluated. The goal was to evaluate the effect of CBTs, tested in both randomized and open designs, on a number of outcomes, including mastery and well‐being (i.e. psychological, physical and social well‐being) in ICs of patients with cancer. We hypothesized that CBTs would be effective across outcomes. A secondary aim was to explore possible moderators of this effect, including trial design, outcomes evaluated, demographic variables, intervention duration and modality of delivery, illness‐related variables among patients, and study quality.
Methods
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) recommendations 29.
Selection criteria
Included studies were peer‐reviewed and (a) investigated the effect of a CBT for ICs of patients with cancer or cancer survivors, (b) employed at least one quantitative measure of psychological, physical, or interpersonal functioning/well‐being of the IC both pre‐ and post‐intervention, (c) enrolled adult samples (age ≥ 18 years), (d) reported results that could be converted into an effect size, and (e) were written in English. An intervention was considered a CBT if it included at least one of the following components: cognitive restructuring, imaginal or in vivo exposure, coping skills training, problem‐solving, behavior activation, behavioral experiments, structured homework, acceptance‐based strategies, stress and anxiety management through relaxation, or mindfulness [cf. 28]. All papers were evaluated independently by authors MSO and MR, and disagreement on the inclusion/exclusion of a study was resolved by consensus.
Search strategy
A keyword‐based search in the electronic databases of PsychINFO, Cochrane, CINAHL, and Embase was conducted. Keywords related to oncology (cancer OR neoplasm OR oncology OR palliative care OR palliative medicine OR malignancy) were combined with keywords related to the population (caregiver* OR carer* OR caregiving OR spouse OR relative OR partner OR family) and the intervention (intervention OR coping skills OR psychosocial OR problem‐solving OR iCBT OR CBT OR cognitive therapy OR behavioral intervention OR cognitive intervention OR home practice OR e‐Health OR cognitive restructuring OR exposure OR mindfulness, OR meditation OR relaxation training OR cognitive behavior therapy OR cognitive behavioral therapy OR online therapy OR online treatment OR internet treatment, internet‐based therapy OR psychotherapy). Filters limiting the search to peer‐reviewed studies on the adult, human population written in English were employed.
Two independent searches were conducted by MSO and MR for the period from the earliest time available through January 2014. In addition, a backward search (snowballing) was conducted of reference lists of identified articles and earlier systematic reviews together with a forward search (citation tracking) until no additional relevant articles were found.
Data extraction
Studies were coded and rated for type of outcome (mastery, psychological well‐being, interpersonal well‐being, physical well‐being, and generic quality of life), caregiver characteristics (mean age, percent women), intervention characteristics (explicit CBT framework [yes, no], treatment recipient [IC only or couple/dyad], treatment format [individual or group], treatment modality [face‐to‐face, web/phone‐based, combination], treatment duration [weeks from pre to post therapy], number of treatment sessions, number of cognitive‐behavioral treatment components), patient characteristics (disease stage [early (i.e. I or II), late (i.e. III or IV), survivors], time since diagnosis), and study quality characteristics (trial type [RCT, open trial; OT]), control type [active control group vs. non‐active], and quality (Jadad score; 30).
All outcomes were categorized according to type as follows: Mastery refers to appraisal efforts, self‐efficacy, coping skills, knowledge about cancer, and ability to perform caregiver related tasks of assisting the patient. Psychological well‐being refers to mood, distress (e.g. anxiety and depression), and overall mental quality of life. Interpersonal well‐being concerns social support, quality of communication with family and cancer patient, intimacy, sexual satisfaction, and overall quality of relationship. Physical well‐being refers to the presence of physical symptoms, exercise habits, physical aspects of sexual performance, and overall physical quality of life. Finally, generic quality of life concerns global measures of quality of life that could not be categorized as either psychological, interpersonal, or physical well‐being. Regarding control type, a control condition was considered active if participants received psychoeducation but not one of the methods described as defining CBT, or if they received other planned, non‐specific psychosocial support.
To determine the quality score, the original 11 Jadad criteria were used 30. Five criteria relevant for the type of studies reviewed in the present paper were added: (a) Was an active control condition included (other than waitlist)? (b) Was there a clear description of the control (comparison) group(s)? (c) Was there a clear description of therapist/interventionist background and level of competency? (d) Were the statistical methods clearly described? (e) Are study reports free of suggestion of selective outcome reporting [cf. 31])? These five criteria address methodological clarity regarding the intervention, the degree of the findings' specificity to CBT, and potential biases in reporting. Together, the quality ratings yielded a total modified Jadad score ranging between 0 and 16. Quality scores were not used as weights when calculating effect sizes, as this is not recommended 32.
Fifteen studies did not report an effect size or means and standard deviations. Therefore, the authors of those studies were contacted with a request for the relevant information or data. Twelve authors responded out of which five were able to provide data. For studies where it was possible, an effect size was computed based on statistics other than a mean and standard deviation, for instance a t and p‐value. It was not possible to calculate an effect size for four studies, in which cases it was set to 0.
All codings and ratings were provided by the first (MO) and third (MR) author. Disagreements were discussed and solved by consensus. Literature search and data extraction protocols are available upon request.
Analytic overview
Meta‐analyses were performed to determine both the pooled effects size for the effect of the CBTs on the combined and individual outcomes based on random‐effects models. Effects were averaged within and across outcomes so that any given study in any given analysis was only represented once in order to satisfy the assumption of independence between observations 33. Given the large number of participants, resulting in small effect sizes being significant, results were mainly interpreted with regard to the produced effect size.
A number of moderation analyses were conducted with meta‐regression analyses, based on random‐effects models and estimated with the Maximum Likelihood method. All moderators were analyzed both individually (unadjusted models) and together in models combining moderators concerning caregiver, intervention, or patient characteristics (adjusted models). Regarding study characteristics, these moderators were not evaluated in a combined, adjusted model. This would not be meaningful because of overlap between variables, where most OTs had no control group, and because design and control characteristics were included in the modified Jadad‐score.
Effect sizes were expressed as Hedge's g instead of Cohen's d, given the former (and not the latter) adjusts for a potential bias to overestimate the effect size in small samples 34, and a p‐value < 0.05 was considered significant. Positive effect sizes indicate an effect of CBTs in the expected direction. Each effect size was weighted by its precision (inverse variance). Attrition was large in several of the studies, and when available, the N used in the calculation was the N in the final analysis for each outcome.
Heterogeneity was explored using Q and I2 statistics. Q‐tests concern the probability that results reflect systematic between‐study differences. Because of the generally low statistical power of heterogeneity tests, a p‐value ≤0.10 was used to determine significant heterogeneity 35. The I2 statistic is an estimate of the degree of observed heterogeneity unexplained by sampling error and is unaffected by the number of studies. I2 values of 0%, 25%, 50%, and 75% are considered negligible, low, moderate, and high, respectively 36.
Positive and negative findings may not be equally likely to get published, thereby introducing risk of publication bias. The distribution of effect sizes was visually inspected by means of funnel plots 37, and tested with Egger's test 38. When a possible publication bias was indicated, an adjusted effect size was estimated using Duval and Tweedie's 39 trim‐and‐fill method, which imputes missing results and recalculates the effect size. The fail‐safe number refers to the number of unidentified or unpublished studies with null findings that will reduce the pooled result to statistical non‐significance 40. If the fail‐safe number exceeded 5K + 10, with K being the number of studies included in the meta‐analysis, the results were considered sufficiently robust in the face of possible publication bias 41.
All analyses were conducted using the Comprehensive Meta‐Analysis program, version 3.3.070 42.
Results
Search results and study characteristics
Information flow of study selection with reasons for exclusion is presented in Figure 1. The literature searches yielded 1131 unique records of which 36 independent studies were included and subjected to meta‐analytic evaluation.
Figure 1.
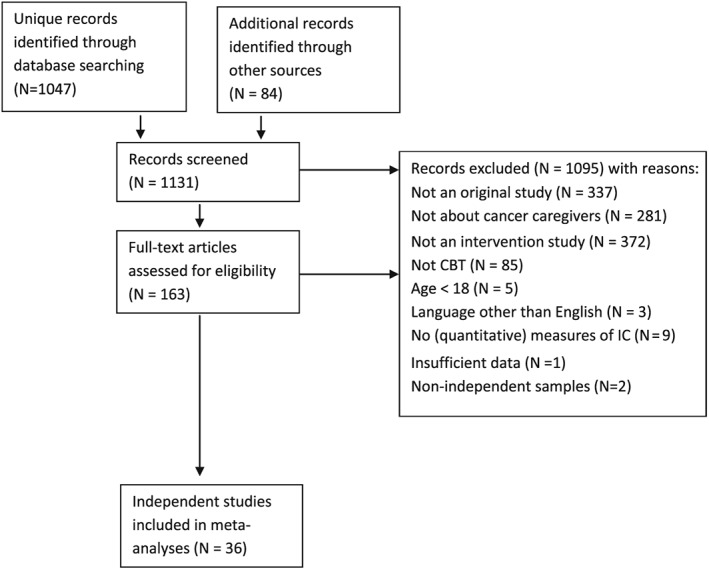
Flow chart of study selection
Study characteristics
The studies reviewed included a total of 4746 ICs with a mean sample size of 131. Final data were analyzed for 3820 ICs with a mean sample size of 106. For study characteristics see Supplemental Appendix 1. Most studies were RCTs (K = 27) comparing CBTs with a non‐active control condition (K = 21). The most common type of treatment was individual (as opposed to group) (K = 28) therapy for couples/dyads (K = 28), and delivered face‐to‐face (K = 22). Most ICs were providing care to patients with mixed stages of cancer (K = 14). Twelve studies explicitly stated that the intervention adhered to a cognitive behavioral framework. The most commonly employed treatment components (see definition above) were coping skills training (K = 24), problem‐solving (K = 15), cognitive restructuring (K = 14), structured homework (K = 11), and relaxation (K = 10). Regarding quality ratings, the two raters showed good inter‐rater agreements, agreeing between 80 and 100% on the individual quality criteria. Each disagreement was solved by consensus. The final mean quality rating was 10.1 (SD = 2.3; range: 5–14 on the 0–16 scale). The lowest scores were found for the criteria of masking (i.e. masking the condition to the participants; K = 0), blinding (i.e. concealing group allocation to the researchers; K = 6), and a priori power calculations (K = 8).
Pooled effect sizes and between‐study differences
The overall combined effect across studies was negligible (Hedge's g = 0.08, 95% CI [0.02–0.14]), also when adjusted for publication bias (0.01). See results below and in Table 1. The largest statistically significant effect was found for psychological well‐being (g = 0.16; K = 31, p < 0.001). The effect sizes for interpersonal well‐being (g = 0.13; K = 16, p = 0.006) and physical well‐being (g = 0.13; K = 18, p = 0.012) also reached statistical significance. Effect sizes for generic quality of life (g = 0.04; K = 10, p = 0.868) and mastery (g = 0.07; K = 20, p = 0.138) were non‐significant. The results of the heterogeneity tests indicated no statistically significant systematic differences between effects on interpersonal (p = 0.139, I2 = 0.0) or physical well‐being (p = 0.139, I2 = 27.2). However, there was statistically significant heterogeneity of the effects on psychological well‐being (p = 0.050, I2 = 31.4), mastery outcomes (p = 0.075, I2 = 33.3), and the generic quality of life outcomes (p = 0.001, I2 = 70.4).
Table 1.
Pooled effect sizes across outcomes and levels of moderator variables
| Sample size | Heterogeneity | Global effect sizes | Failsafe N d | Criterion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomea | K | N | Q b | df | p | I 2 | Hedge's gc | 95 % CI | p | ||
| Overall combined effect | 36 | 3820 | 36.1 | 35 | 0.416 | 0.3 | 0.08 | 0.02 – 0.14 | 0.014 | 65 | 190 |
| Adjusted for publication bias | (49) | — | — | — | — | — | 0.01 | −0.05 – 0.08 | — | — | — |
| Psychological well‐being | 31 | 3044 | 43.7 | 30 | 0.050 | 31.4 | 0.16 | 0.07 – 0.24 | <0.001 | 131 | 165 |
| Adjusted for publication bias | (34) | — | — | — | — | — | 0.12 | 0.06 – 0.18 | — | — | — |
| Interpersonal well‐being | 16 | 1664 | 18.4 | 15 | 0.907 | 0.0 | 0.13 | 0.04 – 0.22 | 0.006 | 22 | 90 |
| Adjusted for publication bias | (21) | — | — | — | — | — | 0.08 | −0.01 – 0.17 | — | — | — |
| Physical well‐being | 18 | 1812 | 23.3 | 17 | 0.139 | 27.2 | 0.13 | 0.03 – 0.24 | 0.012 | 38 | 100 |
| Adjusted for publication bias | (24) | — | — | — | — | — | 0.04 | −0.08 – 0.17 | — | — | — |
| Generic QoL | 10 | 1292 | 30.4 | 9 | <0.001 | 70.4 | 0.02 | −0.20 – 0.24 | 0.868 | — | — |
| Mastery | 20 | 2616 | 28.5 | 19 | 0.075 | 33.3 | 0.07 | −0.02 – 0.16 | 0.138 | — | — |
| Proposed moderators | |||||||||||
| Study design | |||||||||||
| Open trials | 9 | 334 | 4.5 | 8 | 0.813 | 0.0 | 0.21 | 0.07 – 0.34 | 0.002 | 9 | 55 |
| RCTs | 27 | 3486 | 26.8 | 26 | 0.418 | 3.1 | 0.04 | −0.02 – 0.11 | 0.200 | — | — |
| Control condition | |||||||||||
| Active control | 9 | 1213 | 14.3 | 8 | 0.075 | 43.9 | 0.03 | −0.15 – 0.21 | 0.725 | — | — |
| Non‐active control | 21 | 2508 | 6.7 | 20 | 0.998 | 0.0 | 0.07 | −0.01 – 0.15 | 0.068 | — | — |
| Therapeutic framework | |||||||||||
| CBT | 12 | 1100 | 8.24 | 11 | 0.692 | 0.0 | 0.09 | −0.02 – 0.20 | 0.125 | ||
| Other | 24 | 2720 | 27.8 | 23 | 0.225 | 17.2 | 0.08 | 0.00 – 0.16 | 0.045 | 21 | 130 |
| Adjusted for publication bias | (33) | — | — | — | — | — | 0.01 | −0.06 – 0.07 | — | ||
| Intervention Modality | |||||||||||
| Face‐to‐face | 22 | 1886 | 27.4 | 21 | 0.157 | 23.5 | 0.11 | 0.01 – 0.22 | 0.037 | 20 | 120 |
| Adjusted for publication bias | (30) | — | — | — | — | — | <0.01 | −0.07 – 0.08 | — | — | — |
| Web/phone | 7 | 789 | 2.4 | 6 | 0.884 | 0.0 | 0.03 | −0.10 – 0.16 | 0.637 | — | — |
| Combined | 9 | 1356 | 6.0 | 8 | 0.645 | 0.0 | 0.09 | −0.01 – 0.19 | 0.063 | — | — |
| Intervention recipient | |||||||||||
| Caregiver only | 8 | 676 | 2.3 | 7 | 0.942 | 0.0 | 0.13 | −0.01 – 0.26 | 0.064 | ||
| Dyad/group | 28 | 3144 | 33.0 | 27 | 0.197 | 18.2 | 0.08 | 0.00 – 0.15 | 0.050 | 32 | 150 |
| Adjusted for publication bias | (39) | — | — | — | — | — | −0.01 | −0.01 – −0.09 | — | ||
| Intervention format | |||||||||||
| Individual | 28 | 3315 | 30.5 | 27 | 0.291 | 11.5 | 0.07 | −0.00 – 0.14 | 0.059 | ||
| Group | 8 | 505 | 4.2 | 7 | 0.760 | 0.0 | 0.16 | 0.01 – 0.32 | 0.045 | 3 | 50 |
| Adjusted for publication bias | (9) | — | — | — | — | — | 0.14 | −0.01 – 0.29 | — | ||
| Patient disease stage | |||||||||||
| Early stage | 6 | 255 | 2.7 | 5 | 0.745 | 0.0 | 0.08 | −0.1 – 0.27 | 0.396 | — | — |
| Late stage | 9 | 1364 | 18.5 | 8 | 0.018 | 56.8 | 0.05 | −0.10 – 0.20 | 0.509 | — | — |
| Mixed | 14 | 1888 | 7.9 | 13 | 0.849 | 0.0 | 0.09 | −0.01 – 0.18 | 0.046 | 10 | 80 |
| Adjusted for publication bias | (18) | — | — | — | — | — | 0.07 | −0.02 – 0.15 | — | — | — |
| Survivors | 3 | 128 | 1.7 | 2 | 0.435 | 0.0 | 0.31 | 0.05 – 0.56 | 0.021 | 1 | 25 |
Note. K and N do not necessarily add up because of exclusion of non‐independent samples/studies from the comparison analyses. Statistically significant results are highlighted in bold.
Possible publication bias was examined with funnel plots and Egger's test, followed by imputation of missing studies 39. (K) = K + number of imputed studies.
Q‐statistic: p‐values < 0.1 taken to suggest heterogeneity. I2 statistic: 0% (no heterogeneity), 25% (low heterogeneity), 50% (moderate heterogeneity), and 75% (high heterogeneity).
Effect size = Hedge's g. Standardized mean difference, adjusting for small sample bias. A positive value indicates an effect size in the hypothesized direction, i.e. improvement following CBT. To ensure independency, if a study reported results for more than one measure, effect sizes were combined (mean), ensuring that only one ES per study was used in the calculation. Conventions: small (<0.3); medium (0.5); large (>0.8).
In case of statistically significant effect sizes, it was planned to examine the robustness of findings by calculating the Failsafe N (number of non‐significant studies that would bring the p‐value to non‐significant (p > 0.05)) 40.
Setting the effect size to 0 in the four instances of a missing effect size may be too conservative. We therefore calculated the mean effect size excluding the four studies (g = 0.09) and repeated the main analysis imputing this effect size. This did not change the overall effect (g = 0.08). Furthermore, in order to evaluate the influence of possible outliers, the standard deviation for Hedge's g across outcomes was first estimated (SD = 0.20). A search for outliers above or below two standard deviations from the pooled effect size (range: −0.32–0.40) was conducted. Only one study fell outside of this range (g = 0.82; 43). The effect size for this study was winsorized by replacing it with the upper value of the range (0.40), thereby retaining the study with an attenuated influence 44. Re‐analyzing the pooled effect size across outcomes with the winsorized effect size revealed an effect of similar magnitude (g = 0.07, 95% CI [0.12–0.13]). Consequently, this effect size was not adjusted in the following analyses.
Moderator analyses
Pooled effect sizes can be found in Table 1, and results from meta‐regression‐based moderation analyses are displayed in Table 2. The association between continuous moderators and the magnitude of the effect is expressed in unstandardized regression coefficients (B).
Table 2.
Results from meta‐regression‐based moderation analyses
| Variable | Unadjusted modela | Adjusted modelb | |||||
|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | K | |
| Study quality characteristics c | |||||||
| Design (RCT vs. OT) | −0.17 | 0.08 | 0.028 | ||||
| Control type (Active vs. non‐active) | −0.15 | 0.08 | 0.059 | ||||
| JADAD | −0.00 | 0.02 | 0.874 | ||||
| Caregiver characteristics | 29 | ||||||
| Age | −0.01 | 0.01 | 0.468 | −0.02 | 0.01 | 0.046 | |
| % women | <0.01 | <0.01 | 0.002 | 0.01 | <0.01 | 0.001 | |
| Interactiond | −0.00 | <0.01 | 0.731 | ||||
| Intervention characteristics | 24 | ||||||
| # sessions | −0.01 | 0.01 | 0.484 | 0.01 | 0.05 | 0.809 | |
| Treatment duration | −0.01 | <0.01 | 0.154 | −0.02 | 0.02 | 0.310 | |
| # components | 0.02 | 0.02 | 0.454 | −0.06 | 0.08 | 0.451 | |
| CBT (CBT vs. other) | 0.01 | 0.08 | 0.855 | 0.11 | 0.18 | 0.541 | |
| Recipient (IC vs. group/dyad) | 0.05 | 0.09 | 0.541 | 0.06 | 0.12 | 0.578 | |
| Modality (face‐to‐face vs. web/phone) | 0.04 | 0.08 | 0.639 | −0.03 | 0.17 | 0.869 | |
| Format (individual vs. group) | −0.09 | 0.09 | 0.313 | −0.01 | 0.20 | 0.953 | |
| Patient characteristics | 13 | ||||||
| Stage | |||||||
| Mixed (vs. early) | 0.01 | 0.12 | 0.939 | −0.14 | 0.21 | 0.503 | |
| Late (vs. early) | −0.05 | 0.12 | 0.648 | −0.23 | 0.20 | 0.252 | |
| Survivor (vs. early) | 0.21 | 0.18 | 0.247 | −0.25 | 0.33 | 0.450 | |
| Time since diagnosis | 0.02 | 0.04 | 0.610 | 0.04 | 0.04 | 0.431 | |
Note. Statistically significant p‐values are in bold. K = number of studies in adjusted model.
Variables were explored individually in unadjusted models.
Variables within the same group of characteristics were explored together in adjusted models.
The three variables concerning study quality were not explored in a combined model due an overlap between variables.
Two models were tested concerning caregiver characteristics, one with and one without the interaction term. Results for age and number of women refer to the model without the interaction term.
Concerning study quality characteristics, the difference in effect size magnitude depended on study design (p = 0.028), showing that the effect size was larger for OTs (g = 0.21) than for RCTs (g = 0.04). Of the RCTs, two studies had two different control conditions and were therefore excluded from the analyses concerning possible difference in effect size magnitude between active and non‐active control groups. Results revealed a non‐significant difference (p = 0.059) between active (g = 0.03) and non‐active (g = 0.07) control groups. The modified Jadad‐score was not associated with the magnitude of the effect (B < −0.01, p = 0.874).
Exploring the role of caregiver characteristics showed that only the percentage of women was a significant moderator of the effect size when evaluated separately. However, when evaluated together, both age (B = −0.02, p = 0.046) and percentage of women (B = 0.01, p = 0.001) were significantly associated with the magnitude of the effects, with younger age and more female participants both being associated with larger effects. Because age became significant when evaluated together with the percentage of women, the age × women interaction term was explored post hoc, which did not reach statistical significance (B < −0.01, p = 0.731). Then it was explored if participants in studies with more women were younger, but the opposite was true as age and percentage of women was positive correlated (r = 0.43, p = 0.027). Finally, five different regression models were tested, in which percentage of women predicted the five individual outcomes. Higher percentage of female participants was associated with larger effects on physical well‐being (B < 0.01, p = 0.029) and mastery (B = 0.01, p = 0.001), but was not associated with psychological well‐being (B < −0.01, p = 0.616), interpersonal well‐being (B < 0.01, p = 0.493), or generic quality of life (B = 0.01, p = 0.184).
A number of intervention characteristics were also evaluated as moderators. Studies stating to be mainly oriented towards a cognitive‐behavioral framework (K = 12) obtained a small, non‐significant effect size (g = 0.09, p = 0.125). An effect size for studies not claiming to adhere to a cognitive‐behavioral framework (K = 24) obtained an effect size of similar magnitude (g = 0.08, p = 0.045), and the between‐study difference did not reach statistical significance (p = 0.132). Concerning delivery mode, face‐to‐face therapy (g = 0.11, p = 0.037) obtained a numerically larger effect size than therapy delivered over the phone or the Internet (g = 0.03, p = 0.637), but the difference was not statistically significant (p = 0.516). Studies using combined delivery modes also obtained a small, non‐significant effect size (g = 0.09, p = 0.063). The effects of studies providing therapy for the IC only (g = 0.12, p = 0.064) or for the couples/dyads (g = 0.08, p = 0.050) were both of a small magnitude and did not differ between studies (p = 0.272). The same was true for treatment delivered individually (g = 0.07, p = 0.059) or in groups (g = 0.16, p = 0.045), where no between‐study difference was detected (p = 0.309). Number of treatment sessions (B = −0.01, p = 0.484), treatment duration (B = −0.01, p = 0.154), and number of CBT components (B = 0.02, p 0.454) were not associated with the magnitude of the effects.
Patient characteristics were also explored as potential moderators. Patient disease stage did not moderate the effect. ICs of survivors obtained a numerically larger effect (g = 0.31, p = 0.021) than ICs of patients with early stage cancer (g = 0.08, p = 0.396), late stage (g = 0.05, p = 0.509), and mixed stages (g = 0.09, p = 0.046). Only the effect for ICs of patients with mixed stages and survivors reached statistical significance. Time since diagnosis was not statistically significantly associated with the magnitude of the effect (B = 0.02, p = 0.431).
Retrospective power analyses showed that the statistical power to detect significant associations between the individual proposed moderators and the effect size varied between 0.1 and 0.9.
Publication bias
For all statistically significant results, the risk of publication bias was evaluated. For nine of the 23 analyses, effect sizes were asymmetrically distributed, as determined by a significant Egger's test, indicating possible publication bias. Adjusted effect sizes can be found in Table 1. Furthermore, all analyses failed to meet the criterion for the fail‐safe N, indicating a lack of robustness of the results.
Discussion
Overall, the effect of CBTs for ICs was negligible. Although the effect across all outcome types was statistically significant, and the between study variance was largely homogenous, the robustness of the effect was poor, as indicated by a small failsafe number. The largest and statistically significant effects were found for psychological well‐being, physical well‐being, and interpersonal well‐being. However, the robustness was also poor for these effects. There could be several reasons for these findings.
First, the magnitude of the effect could be associated with study quality characteristics. Comparing studies that used a randomized controlled versus an open design revealed that RCTs obtained a non‐significant and smaller effect than OTs. Because an RCT design is a more rigorous test of an intervention's effect, it may be less surprising that the effect was smaller in the RCTs. No moderating effect was found for the remaining study quality variables, including control type and overall quality of the study (modified Jadad score). This is in line with other findings, where quality scores have generally been found to be poor predictors of study results 32, 45.
Second, the evaluated studies, although considered CBTs, varied in main theoretical framework, and it is possible that interventions that did not state to be mainly oriented towards a cognitive‐behavioral framework might not be a true CBT. However, the most commonly employed treatment components were coping‐skills training, problem‐solving techniques, cognitive restructuring, the use of structured home‐work, and relaxation techniques, which are all frequently used techniques across CBTs [e.g. 46]. Furthermore, self‐claimed therapeutic framework did not moderate the effect. Other intervention characteristics could also be hypothesized to moderate the effect. For instance, one could argue that the flexibility characterizing phone and web interventions would be very suitable for the caregiver population who, because of caregiver responsibilities, may not be able to leave the home. However, the effect of CBTs delivered over the phone or web was non‐significant and numerically smaller than that obtained for face‐to‐face CBTs, albeit not significantly different. The finding that treatment recipient did not moderate the effect is somewhat surprising because it has often been proposed that coping with cancer occurs within an interpersonal system, that is, the affected couple engages in dyadic coping [e.g. 47, 48]. However, the present data suggest that – across outcomes – the effect of CBTs does not differ between interventions for the IC only or the couple.
Third, as mentioned in the introduction, there is great variation in levels of distress within this population, and some ICs' distress levels may have been rather low to begin with, leaving little room for improvement. Related to this, attrition rates could have affected the outcome in the sense that the most distressed ICs may have dropped out of treatment. However, when available, the N used in the calculation was the N in the final analysis for each outcome, thereby adjusting the analyses for this potential issue.
Finally, in the traditional cognitive treatment model, the client's emotional struggles are primarily viewed as a result of erroneous and maladaptive cognitions 49. However, when working with ICs of patients with cancer, there is often validity to the negative thoughts, and traditional cognitive methods may therefore be experienced by the client as invalidating 50. This could be one explanation for the larger effect size detected for survivors, where the situation is characterized by a factually better outlook.
The present findings could encourage future investigations of psychological interventions within a cognitive‐behavioral framework to look beyond traditional methods. We believe that recent advances in the basic affective sciences may elucidate caregiver treatment. These advances emphasize functional and motivational aspects of emotional responses, and how these motivations can be in conflict and lead to distress 51, 52. A variety of motivational conflicts may arise as a result of taking responsibility for the care of a patient with cancer. A prominent conflict for ICs is balancing self‐care and care for the patient, in which the IC may, for example, feel the need to leave the house or the hospital and at the same time experience fear and guilt in thinking about leaving the patient. The IC may also wish to have positive experiences with the patient while concurrently feeling distress about the anticipated loss of their loved one 53. Such conflicts can manifest themselves as intense and painful emotions that may be difficult to handle. For instance, when faced with intense or painful emotions, individuals with anxiety and depression – emotional distress characteristic of ICs – often use emotion regulation strategies aimed at diminishing or avoiding these emotions, resulting in increased negative emotions and exacerbated stress‐responses 54, 55, 56. One could therefore argue that it would be relevant to offer ICs psychotherapy specifically aimed at handling motivational conflicts and emotion regulation. Indeed, these emotional processes are a core feature of the ‘third wave’ behavior therapies as well as other contemporary CBTs [e.g. 57, 58, 59], in which realistic reappraisals are granted no or a minor role. Congruently, the authors of the present paper are currently running a trial investigating Emotion Regulation Therapy for ICs 58, 60 (ClinicalTrials.gov identifier: NCT02322905), an approach that draws directly from this basic affect science framework and offers specific interventions aimed at improving motivational awareness and emotion regulatory ability.
In addition to the search for other, more effective interventions for ICs, attention should also be paid to caregiver characteristics that may be associated with better treatment response. In the present study, the percentage of female participants was positively associated with the effect of CBT, and – when considered together with percentage of women – age was negatively associated with the magnitude of the effect. In further exploring the effect of gender, it was found that the number of female participants was associated with larger effects concerning physical well‐being and mastery. Identifying characteristics associated with better treatment response may help target different treatments to different subgroups of ICs.
Limitations of the present review include a broad definition of CBT, leading to the inclusion of a diverse set of therapies. However, the definition of CBTs reflects the large variety in what historically have been and currently are considered cognitive behavioral therapies [e.g. 46], holding central elements hypothesized to drive the treatment effect in both traditional and contemporary CBTs 61, 62. Furthermore, the inclusion criteria regarding the IC population varied between studies, limiting the internal validity. In some cases the IC was the primary caregiver responsible for many elements of physical and emotional care, whereas other studies defined ICs as an intimate partner without regard to actual caregiving demands. Similarly, some studies enrolled the ‘closest relative’, a category that included individuals in varying relationships with the patient (e.g. spouse or child). However, despite including studies representing variation in intervention type and ICs, results indicated relative homogeneity among studies. Additional data were requested from 15 authors, and data were only received from five. A number of proposed moderator variables were explored, but only few were significantly associated with the effect, which may in part be because of low statistical power. This, combined with the exploratory nature of the analyses, suggests that the results should therefore be interpreted with caution. Furthermore, the temporal burden experienced by ICs may have limited their adherence to homework assignments, thereby mitigating treatment response. Finally, four of the five outcome groups were categorized as ‘well‐being’. However, for the psychological well‐being outcome group, the vast majority of measures concerned distress, for instance anxiety and depression. The lack of distress does not necessarily constitute psychological well‐being. There is now emerging evidence for the potential rewards of providing care, including gaining meaning in life and increased appreciation of others [e.g. 63, 64]. The effect of CBTs on such growth or gains remains uninvestigated.
Conclusions
Overall, the results indicated that CBTs for ICs had a negligible effect. A number of moderators were explored of which only few reached statistical significance suggesting that CBTs may be more effective for younger, female ICs. However, when compared to a control group in randomized designs, the effect of CBTs did not reach statistical significance. A number of reasons could be hypothesized to underlie this negligible effect, and it is suggested that future studies move beyond traditional CBT methods and orient towards recent advances in the basic affective sciences in order to better understand and treat the emotional struggles experienced by ICs.
Conflict of interest
The authors declare that there are no conflicts of interest.
Funding resources
This work was supported by the Danish Cancer Society.
Supporting information
Supporting Info Item
O'Toole, M. S. , Zachariae, R. , Renna, M. E. , Mennin, D. S. , and Applebaum, A. (2017) Cognitive behavioral therapies for informal caregivers of patients with cancer and cancer survivors: a systematic review and meta‐analysis. Psycho‐Oncology, 26: 428–437. doi: 10.1002/pon.4144.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics [Internet]. 2014. [cited 2015 Nov 17]. p. 9–29. Available from: http://www.wcrf.org/cancer_statistics/cancer_facts/index.php
- 2. Given BA, Kozachik S, Collins CE, DeVoss DN, Given CW. Caregiver role strain: a nursing diagnosis In Nursing Diagnoses and Interventions for the Elderly, Maas M, Buckwalter KC, Hardy M, Tripp‐Reimer T, Titler M. (eds.), Mosby: St. Louis, 2001;679–695. [Google Scholar]
- 3. Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta‐analysis. Psychol Aging 2003;18:250–267. [DOI] [PubMed] [Google Scholar]
- 4. Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta‐analysis. Psychol Bull 2003;129:946–972. [DOI] [PubMed] [Google Scholar]
- 5. Girgis A, Lambert SD, McElduff P, et al Some things change, some things stay the same: a longitudinal analysis of cancer caregivers' unmet supportive care needs. Psycho-Oncology 2013;22:1557–1564. [DOI] [PubMed] [Google Scholar]
- 6. Kim Y, Shaffer KM, Carver CS, Cannady RS. Prevalence and predictors of depressive symptoms among cancer caregivers 5 years after the relative's cancer diagnosis. J Consult Clin Psychol 2014;82:1–8. [DOI] [PubMed] [Google Scholar]
- 7. Lethborg CE, Kissane D, Burns WI. ‘It's not the easy part’: the experience of significant others of women with early stage breast cancer, at treatment completion. Soc Work Health Care 2003;37:63–85. [DOI] [PubMed] [Google Scholar]
- 8. Lambert SD, Jones BL, Girgis A, Lecathelinais C. Distressed partners and caregivers do not recover easily: adjustment trajectories among partners and caregivers of cancer survivors. Ann Behav Med 2012;44:225–235. [DOI] [PubMed] [Google Scholar]
- 9. Lambert SD, Girgis A, Lecathelinais C, Stacey F. Walking a mile in their shoes: anxiety and depression among partners and caregivers of cancer survivors at 6 and 12 months post‐diagnosis. Support Care Cancer 2013;21:75–85. [DOI] [PubMed] [Google Scholar]
- 10. Jensen S, Given BA. Fatigue affecting family caregivers of cancer patients. Cancer Nurs 1991;14:181–187. [PubMed] [Google Scholar]
- 11. Hearson B, McClement S. Sleep disturbance in family caregivers of patients with advanced cancer. Int J Palliat Nurs 2007;13:495–501. [DOI] [PubMed] [Google Scholar]
- 12. Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med 2002;64:418–435. [DOI] [PubMed] [Google Scholar]
- 13. von Kanel R, Mausbach BT, Patterson TL, et al Increased Framingham Coronary Heart Disease Risk Score in dementia caregivers relative to non‐caregiving controls. Gerontology 2008;54:131–137. [DOI] [PubMed] [Google Scholar]
- 14. Vitaliano PP, Scanlan JM, Ochs HD, Syrjala K, Siegler IC, Snyder EA. Psychosocial stress moderates the relationship of cancer history with natural killer cell activity. Ann Behav Med 1998;20:199–208. [DOI] [PubMed] [Google Scholar]
- 15. Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: dysregulation of pro‐ and anti‐inflammatory signaling pathways. J Clin Oncol 2009;27:2909–2915. [DOI] [PubMed] [Google Scholar]
- 16. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA 1999;282:2215–2219. [DOI] [PubMed] [Google Scholar]
- 17. Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. N Engl J Med 2006;354:719–730. [DOI] [PubMed] [Google Scholar]
- 18. National Alliance for Caregiving. Caregiving in the U.S. [Internet] . National Alliance for Caregiving and AARP, Inc. 2005. [cited 2015 Nov 1]. p. 1–25. Available from: http://assets.aarp.org/rgcenter/il/us_caregiving_1.pdf
- 19. Applebaum AJ, Breitbart W. Care for the cancer caregiver: a systematic review. Palliat Support Care 2013;11:231–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofmann SG, Smits JAJ. Cognitive‐behavioral therapy for adult anxiety disorders: a meta‐analysis of randomized placebo‐controlled trials. J Clin Psychiatry 2008;69:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta‐analyses. Cognit Ther Res 2012;36:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hudson P. A critical review of supportive interventions for family caregivers of patients with palliative‐stage cancer. J Psychosoc Oncol 2005;22:77–92. [Google Scholar]
- 23. Honea NJ, Brintnall R, Given B, et al Putting evidence into practice: nursing assessment and interventions to reduce family caregiver strain and burden. Clin J Oncol Nurs 2008;12:507–516. [DOI] [PubMed] [Google Scholar]
- 24. Harding R, List S, Epiphaniou E, Jones H. How can informal caregivers in cancer and palliative care be supported? An updated systematic literature review of interventions and their effectiveness. Palliat Med 2012;26:7–22. [DOI] [PubMed] [Google Scholar]
- 25. Waldron EA, Janke EA, Bechtel CF, Ramirez M, Cohen A. A systematic review of psychosocial interventions to improve cancer caregiver quality of life. Psycho–Oncology 2013;22:1200–1207. [DOI] [PubMed] [Google Scholar]
- 26. O'Toole MS, Mennin DS, Renna ME, Zachariae R, Applebaum A. Cognitive behavioral therapy for informal cancer caregivers: a review of the literature and future directions In Cognitive Behavioral Therapy: New Research, Myers B. (ed.), Nova Science Publications: Hauppage, NY, 2014. [Google Scholar]
- 27. Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with family caregivers of cancer patients: meta‐analysis of randomized trials. CA Cancer J Clin 2010;60:317–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nenova M, Morris L, Paul L, Li Y, Applebaum A, DuHamel K. Psychosocial interventions with cognitive‐behavioral components for the treatment of cancer‐related traumatic stress symptoms: a review of randomized controlled trials. J Cogn Psychother 2013;27:258–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liberati A, Altman DG, Tetzlaff J, et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J 2009;339:2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jadad AR, Moore RA, Carroll D, et al Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 31. Sterne JAC, Egger M, Moher D. Addressing reporting biases In Cochrane Handbook for Systematic Reviews of Intervention, Higgins JPT, Green S. (eds.), Wiley‐Blackwell Publishing: Chichester, UK, 2008;297–333. [Google Scholar]
- 32. Greenland S, O'Rourke K. On the bias produced by quality scores in meta‐analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2:463–471. [DOI] [PubMed] [Google Scholar]
- 33. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis, West Sussex, UK: John Wiley & Sons Ltd, 2009. [Google Scholar]
- 34. Hedges L, Olkin I. Statistical Methods for Meta‐Analysis, Academic Press: New York, 1995. [Google Scholar]
- 35. Poole C, Greenland S. Random‐effects meta‐analyses are not always conservative. Am J Epidemiol 1999;150:469–475. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 37. Light RJ, Singer JD, Willett JB. The visual presentation and interpretation of meta‐analyses In The Handbook of Research Synthesis, Cooper M, Hedges LV. (eds.), Russel Sage Foundation: New York, 1994. [Google Scholar]
- 38. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. Br Med J 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 40. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull 1979;86:638–641. [Google Scholar]
- 41. Rosenthal R. Meta‐analysis: a review. Psychosom Med 1991;53:247–271. [DOI] [PubMed] [Google Scholar]
- 42. Comprehensive meta‐analysis. Eaglewood, NJ: Biostat; 2014.
- 43. Heinrich RL, Schag CC. Stress and activity management: group treatment for cancer patients and spouses. J Consult Clin Psychol 1985;53:439–446. [DOI] [PubMed] [Google Scholar]
- 44. Lipsey MW, Wilson DB. Practical Meta‐Analysis (Vol.49), Sage Publications, Inc: London, 2001. [Google Scholar]
- 45. Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282:1054–1060. [DOI] [PubMed] [Google Scholar]
- 46. Dobson KS, Dozois DJA. Historical and philosophical bases of the cognitive‐behavioral therapies In Handbook of Cognitive‐Behavioral Therapies (2nd ed.), Dobson KS. (ed.), Guilford Press: New York, 2001;3–39. [Google Scholar]
- 47. Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: a meta‐analysis and critical review of role and gender effects. Psychol Bull 2008;134:1–30. [DOI] [PubMed] [Google Scholar]
- 48. Traa MJ, De Vries J, Bodenmann G, Den Oudsten BL. Dyadic coping and relationship functioning in couples coping with cancer: a systematic review. Br J Health Psychol 2015;20:85–114. [DOI] [PubMed] [Google Scholar]
- 49. Beck AT. Cognitive Therapy and the Emotional Disorders, Penguin Books: New York, 1979. [Google Scholar]
- 50. Levin TT, Applebaum AJ. Acute cancer cognitive therapy. Cogn Behav Pract 2014;21:404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo‐Hippocampal System (2nd ed.), Oxford University Press: Oxford, 2000. [Google Scholar]
- 52. Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry 1998/12/23 ed 1998;44:1248–1263. [DOI] [PubMed] [Google Scholar]
- 53. Applebaum AJ, Kulikowski JR, Breitbart W. Meaning‐centered psychotherapy for cancer caregivers (MCP‐C): rationale and overview. Palliat Support Care 2015;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Campbell‐Sills L, Ellard KK, Barlow DH. Emotion regulation in anxiety disorders In Handbook of Emotion Regulation (2nd ed.), Gross JJ. (ed.), Guilford Press: New York, 2014;393–412. [Google Scholar]
- 55. Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well‐being. J Pers Soc Psychol American Psychological Association 2003;85:348–362. [DOI] [PubMed] [Google Scholar]
- 56. Joormann J, Siemer M. Emotion regulation in mood disorders In Handbook of Emotion Regulation, Guilford Press: New York, 2014;413–427. [Google Scholar]
- 57. Barlow DH, Farchione TJ, Fairholme CP, et al Unified Protocol for Transdiagnostic Treatment of Emotional Disorders: Therapist Guide, Oxford University Press, New York, NY: Treatments that work, 2011. [Google Scholar]
- 58. Mennin DS, Fresco DM. Emotion regulation therapy In Handbook of Emotion Regulation (2nd ed.), Gross JJ. (ed.), Guilford Press: New York, 2014;469–490. [Google Scholar]
- 59. Roemer L, Orsillo SM. Mindfulness‐ & Acceptance‐Based Behavioral Therapies in Practice, The Guilford Press: New York, 2009. [Google Scholar]
- 60. O'Toole MS, Mennin DS, Fresco DM. Emotion regulation therapy: an experiential approach to chronic anxiety and recurring depression In Working with Emotion in Cognitive‐Behavioral Therapy: Techniques for Clinical Practice, Thoma N, McKay D. (eds.), Guford Press: New York, 2015;310–330. [Google Scholar]
- 61. Kahl KG, Winter L, Schweiger U. The third wave of cognitive behavioural therapies: what is new and what is effective? Curr Opin Psychiatry2012/09/21 ed 2012;25:522–528. [DOI] [PubMed] [Google Scholar]
- 62. Mennin DS, Ellard KK, Fresco DM, Gross JJ. United we stand: emphasizing commonalities across cognitive‐behavioral therapies. Behav Ther 2013;44:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown SL, Nesse RM, Vinokur AD, Smith DM. Providing social support may be more beneficial than receiving it: results from a prospective study of mortality. Psychol Sci 2003;14:320–327. [DOI] [PubMed] [Google Scholar]
- 64. Applebaum AJ, Farran CJ, Marziliano AM, Pasternak AR, Breitbart W. Preliminary study of themes of meaning and psychosocial service use among informal cancer caregivers. Palliat Support Care 2014;12:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Info Item


