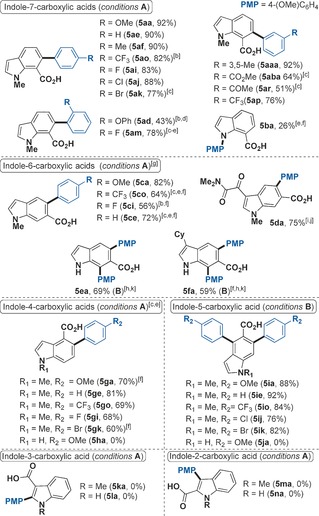
Table 4.
Scope of the Ru‐catalyzed arylation of indole carboxylic acids 4 a–n with iodoarenes 2.[a]

|
|---|
|
[a] Reaction conditions A: 4 (0.3 mmol), 2 (2.0 equiv), [Ru(tBuCN)6](BF4)2 (3 mol %), K2CO3 (2.0 equiv), KOC(CF3)3 (1.0 equiv) and tBuCN (8.0 equiv) stirred under Ar in a closed vessel at 140 °C for 16 h. Yields are of pure, isolated products. Reaction conditions B: 4 (0.3 mmol), 2 (4.0 equiv), [Ru(tBuCN)6](BF4)2 (6 mol %), K2CO3 (3.0 equiv), KOC(CF3)3 (1.5 equiv), tBuCN (12.0 equiv) and H2O (3.0 equiv) stirred under Ar in a closed vessel at 140 °C for 16 h. Yields are of pure, isolated products. [b] Reaction time 8 h. [c] Reaction time 3 h. [d] [Ru(tBuCN)6](BF4)2 (6 mol %). [e] 3.0 equiv of H2O were added. [f] Isolated as the corresponding methyl ester after derivatization with MeI. [g] [Ru(tBuCN)6](BF4)2 (5 mol %). [h] No H2O was added. [i] Reaction time 5 h. [j] Isolated as the corresponding benzyl ester after derivatization with BnCl. [k] Reaction time 12 h.
