Abstract
Cefiderocol, a new injectable siderophore cephalosporin antibiotic, has promising in vitro and in vivo activity against Gram‐negative bacteria including multidrug‐resistant Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae. Cefiderocol is mainly renally eliminated. The pharmacokinetics and safety of cefiderocol in subjects with renal impairment were assessed following a single 1000‐mg intravenous 1‐hour infusion of cefiderocol. Subjects with mild, moderate, or severe renal impairment and end‐stage renal disease (ESRD) requiring hemodialysis were compared with demographically (age, body mass index, and sex) matched healthy subjects with normal renal function. The effect of hemodialysis on the clearance of cefiderocol was also assessed. Total drug clearance from plasma (CL) and terminal half‐life (t1/2) correlated with renal function. Ratios (90% confidence intervals) of area under the plasma concentration‐time curve from 0 to infinity (AUC) in mild, moderate, severe, and ESRD groups compared to those with normal renal function were 1.0 (0.8‐1.3), 1.5 (1.2‐1.9), 2.5 (2.0‐3.3), and 4.1 (3.3‐5.2), respectively. Maximum plasma concentration (Cmax) was similar between renal‐impairment groups and the normal‐renal‐function group. Approximately 60% of cefiderocol was removed by hemodialysis for 3 to 4 hours. The plasma‐protein‐unbound fraction was similar between various renal function groups. The incidence of adverse events did not appear to have any correlation with the degree of renal impairment. Single 1000‐mg intravenous doses of cefiderocol were generally well tolerated in subjects with impaired renal function except for 1 subject who discontinued due to urticaria. In conclusion, renal impairment impacted AUC, CL, and t1/2 without affecting Cmax. Cefiderocol was significantly removed by intermittent hemodialysis.
Keywords: cefiderocol, cephalosporin, pharmacokinetics, renal impairment, hemodialysis
Although siderophore antibiotics have been investigated for decades, none of them is available for clinical use in the treatment of bacterial infections.1 Cefiderocol (also known as S‐649266, Figure 1), a novel siderophore cephalosporin antibiotic having a catechol‐substituent, has a potent in vitro activity and in vivo efficacy against various multidrug‐resistant Gram‐negative bacteria including carbapenem‐resistant Enterobacteriaceae, P aeruginosa, and Acinetobacter baumannii.2, 3, 4, 5
Figure 1.
Chemical structure of cefiderocol.
The safety, tolerability, and pharmacokinetics (PK) of cefiderocol have been evaluated in a single‐ and multiple‐dose phase 1 study.6 Cefiderocol PK is linear over the range of 100 to 2000 mg. The terminal elimination half‐life of cefiderocol was 1.98 to 2.74 hours in these healthy volunteers. Cefiderocol is mainly renally excreted with 60% to 70% of the administered dose found in urine as unchanged parent drug. No accumulation was observed at the 2 g every 8 hours dose regimen, and this dose was well tolerated when administered over 10 days.
Because human PK data indicated that most of cefiderocol was excreted unchanged in the urine, subjects with impaired renal function receiving cefiderocol would likely require a reduction in dose or an increase in dosing interval to avoid drug accumulation. The purpose of this study was to evaluate the PK of the siderophore cephalosporin cefiderocol in clinically stable subjects with mild, moderate, and severe renal impairment and in subjects undergoing hemodialysis (HD). The effect of HD on the elimination of cefiderocol from blood was also investigated along with the safety and tolerability of single doses of cefiderocol in these renally impaired subjects.
Methods
Ethical Considerations
This study was conducted at DaVita Clinical Research in Minneapolis, Minnesota and Lakewood, Colorado following approval by the Independent Ethics Committee (Western Institutional Review Board, Puyallup, Washington). This study was carried out in accordance with the provision of the Declaration of Helsinki and all revisions thereof, in accordance with ICH GCP requirements. Signed informed consent forms were obtained from all subjects.
Study Subjects
Renally impaired subjects with various degrees of renal function were enrolled along with a matched healthy control group in this open‐label phase 1 study, which was designed in accordance with the FDA guideline on PK in patients with impaired renal function.7 A total of 38 males or females, 20 to 80 years of age, with body mass index between 18.5 and 38.0 kg/m2 were enrolled. Renal function classification was a simplified version of the FDA's draft Guidance,7 and renal impairment subjects were assigned according to their estimated glomerular filtration rate (eGFR), calculated using the modification of diet in renal disease (MDRD) formula8 at screening (day –28 to day –2 relative to day 1): mild impairment (eGFR 60 to <90 mL/[min·1.73 m2], n = 8), moderate impairment (eGFR 30 to < 60 mL/[min·1.73 m2], n = 8), severe impairment (eGFR < 30 mL/[min·1.73 m2], n = 6), and end‐stage renal disease (ESRD) on HD (n = 8). Renal function was to be stable, defined as ˂30% difference in eGFR assessed at screening and on admission on day –1. ESRD subjects on HD should receive stable HD at least 3 times a week for at least 6 months and have a clinically stable condition with respect to the underlying renal impairment. Normal renal function was identified using the Cockcroft‐Gault equation calculation9 with creatinine clearance (CLcr) ≥ 90 mL/min without adjusting for body surface area. Each of the 8 subjects with normal renal function (control subjects) was matched to the demographic characteristics of 1 of the 8 subjects with moderate renal impairment with respect to age (± 10 years), body mass index (± 20%), and sex. Main exclusion criteria included subjects with fluctuating or rapidly deteriorating renal function, with a history of hypersensitivity to cephalosporins or penicillins, or who required continuous treatment with methotrexate, procainamide, probenecid, or valproic acid.
Study Design
This was a nonrandomized open‐label cohort study in subjects with varying but stable renal function.
The subjects received a single intravenous administration of 1000 mg of cefiderocol over 1 hour. For subjects with hemodialysis‐dependent ESRD, cefiderocol was administered twice: the first dose approximately 1 hour after completion of the subject's HD session (period 1, without HD), and the second dose (after a 72‐hour washout period) approximately 2 hours prior to the subject's normally scheduled HD session (period 2, with HD). Subjects underwent 3‐ to 4‐hour HD. The dialyzer used was OptiFlux F180NR (Fresenius Medical Care, Bad Homburg vor der Höhe, Germany). The blood flow rate and the dialysate flow rate were 400 to 500 mL/min and 600 to 700 mL/min, respectively.
Blood and Urine Sample Collection
For quantification of cefiderocol concentrations in plasma, serial blood samples were drawn immediately before (–0.25 hours) and during infusion at 0.25, 0.5, and 1 hour (at the end of infusion) and at 1.5, 2, 3, 4, 5, 6, 8, 12, 16, 24, 36, 48, and 72 hours from start of infusion.
Urine samples were collected in the following time intervals: predose, 0 to 8, 8 to 16, 16 to 24, 24 to 36, 36 to 48, and 48 to 72 hours for subjects that could produce urine.
Plasma protein binding was determined from a separate blood sample obtained at 1 (the end of infusion) and 8 hours from initiation of infusion for all cohorts except for the second period of administration for ESRD subjects.
Arterial and venous PK samples and aliquots from the dialysate were collected at 3, 4, 5, and 6 hours or at the end of HD. Dialysate samples were collected at 2 to 3 (at the start of HD), 3 to 4, 4 to 5, and 5 to 6 (at the end of HD) hours.
Bioanalytical Methods
The composite samples were prepared by treating each matrix (plasma, urine, and dialysate) with a buffer (0.2 mol/L ammonium acetate, pH 5) in 1:1 volume ratio and used for measurement of cefiderocol concentrations. Cefiderocol concentrations in plasma were determined by a validated high‐performance liquid chromatography‐tandem mass spectrometry (HPLC‐MS/MS; QPS, LLC, Newark, Delaware) assay, with a lower limit of quantification of 0.1, 1, and 0.05 μg/mL for plasma, urine, and dialysate, respectively. The assay was linear from 0.1 to 100, 1 to 1000, and 0.05 to 50 μg/mL for plasma, urine, and dialysate, respectively. The precision of the assay was 1.2% to 6.2%, 1.7% to 9.3%, and 2.4% to 7.6% for plasma, urine, and dialysate, respectively. The accuracy of the assay was –5.3% to 2.1%, –8.3% to 5.8%, and –8.7% to 6.8% for plasma, urine, and dialysate, respectively.
To evaluate the fraction of cefiderocol unbound to plasma proteins, the plasma was preincubated at 37°C for 15 minutes. The plasma (1 mL) was dispensed into ultrafiltration devices (Centrifree YM‐30) at 1 mL per device and centrifuged (1800g, 37ºC, 15 minutes), and the filtrate was collected (approximately 200 μL). An equal volume of 0.2 mol/L ammonium acetate buffer (pH 5) was added into an aliquot of filtrate. The mixture was used for measurement of cefiderocol concentrations.
Safety Assessment
All subjects who received any dose of study drug were included in the safety evaluation. The safety and tolerability of cefiderocol were assessed by monitoring adverse events (AEs), physical examinations (PEs), vital signs, 12‐lead electrocardiographs (ECGs), continuous dual‐lead ECG up to 6 (normal) or 12 hours (renally impaired) after initiation of infusion, and clinical laboratory evaluations (including hematology, blood chemistry tests, and urinalysis).
Pharmacokinetic Analysis
Mean and standard deviation (SD) for plasma concentrations were calculated by group and sampling time. Data below the lower limit of quantification (BLQ) were treated as 0 for the calculation of mean and SD for plasma concentrations. The following PK parameters were calculated from the plasma concentration data for cefiderocol using the noncompartmental methods in Phoenix WinNonlin® version 6.3 (Certara USA, Inc, Princeton, New Jersey): maximum plasma concentration (Cmax), time to Cmax (tmax), area under the plasma concentration‐time curve from 0 to the time of the last quantifiable concentration (AUC(0‐t)), area under the plasma concentration‐time curve from time 0 to infinity (AUC), terminal half‐life (t1/2), total drug clearance from plasma (CL), and volume of distribution under steady‐state conditions (Vss). Fraction of total drug that is unbound in plasma (fu) was also assessed. AUC(0‐t) and AUC were calculated with the linear‐up/log‐down trapezoidal method for the extrapolation. Cumulative amount of drug excreted unchanged in urine (Ae), fraction of dose excreted unchanged into urine (fe), and renal clearance of drug (CLR) were estimated for each subject with urinary excretion data. For subjects on hemodialysis, cumulative amount of drug recovered unchanged in dialysate (AR), hemodialysis clearance (CLhd), and fraction of the total body pool of drug removed by hemodialysis (Fr) were determined.10
Statistical Analysis
An analyses of variance (ANOVA) was performed for ln‐transformed values for PK parameters including terms for renal status as a fixed effect by using Proc Mixed with SAS version 9.1 (SAS Institute Inc, Cary, North Carolina).
Point estimates and 90% confidence intervals (CIs) were calculated for the ratios of parameters in subjects with mild, moderate, and severe renal impairment and ESRD (after‐dialysis dosing) compared with healthy control subjects.
Results
Demographics
Demographic data are summarized in Table 1. Twenty‐five subjects were male (65.8%), the mean age was 54.6 years (range 27 to 74 years), and the total number of white and black or African‐American subjects were the same (18 subjects each, 47.4%). Overall, demographic data were similar among the 5 cohorts.
Table 1.
Baseline Demographics
| Renal Function | ||||||
|---|---|---|---|---|---|---|
| Matched Healthy | Mild Impairment | Moderate Impairment | Severe Impairment | End‐Stage Renal Disease | ||
| Characteristic (unit) | (n = 8) | (n = 8) | (n = 8) | (n = 6) | (n = 8) | Total (n = 38) |
| Sex, n (%) | ||||||
| Male | 5 (62.5) | 6 (75.0) | 5 (62.5) | 4 (66.7) | 5 (62.5) | 25 ( 65.8 ) |
| Female | 3 (37.5) | 2 (25.0) | 3 (37.5) | 2 (33.3) | 3 (37.5) | 13 ( 34.2) |
| Race, n (%) | ||||||
| White | 3 (37.5) | 5 (62.5) | 5 (62.5) | 3 (50.0) | 2 (25.0) | 18 (47.4) |
| Black or African American | 4 (50.0) | 3 (37.5) | 3 (37.5) | 2 (33.3) | 6 (75.0) | 18 (47.4) |
| Native American or Alaska Native | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (2.6) |
| Other | 0 | 0 | 0 | 1 ( 16.7 ) | 0 | 1 (2.6) |
| Mean age, year (range) | 56.5 | 58.1 | 60.1 | 51.8 | 45.8 | 54.6 |
| (44‐67) | (44‐74) | (40‐71) | (35‐63) | (27‐58) | (27‐74) | |
| Mean body mass index, kg/m2 (range) | 27.3 | 30.9 | 29.4 | 29.4 | 30.9 | 29.6 |
| (22.9‐31.0) | (22.4‐37.9) | (22.1‐36.3) | (24.4‐37.4) | (25.0‐35.6) | (22.1‐37.9) | |
| Mean eGFR, mL/[min·1.73 m2] (range) | 98.5 | 72.1 | 48.1 | 20.8 | 7.5 | 50.9 |
| (81.0‐124.0) | (66.0‐1.0) | (40.0‐59.0) | (13.0‐29.0) | (4.0‐16.0) | (4.0‐124.0) | |
| Mean CLcr, mL/[min·1.73 m2] (range)a | 100.5 | 78.2 | 55.3 | 27.7 | 11.7 | 56.1 |
| (76.4‐122.3) | (67.3‐91.5) | (38.6‐79.3) | (15.6‐39.1) | (6.0‐23.1) | (6.0‐122.3) | |
The body surface area calculated with the formula of DuBois and DuBois20 was used for the adjustment.
All subjects received a 1000‐mg dose of cefiderocol, infused over 1 hour. One subject from the moderate impairment cohort was prematurely withdrawn from the study due to an AE of urticaria; the subject received only 74.2 mL of the reconstituted study drug instead of total 100 mL. The PK data from this subject were excluded from summaries and statistical analyses. All of the other 37 subjects completed the study.
Pharmacokinetics
Mean plasma concentration profiles following a single intravenous infusion of 1000 mg over 1 hour are presented in Figure 2. The plasma concentration profiles were altered significantly with increasing degrees of renal impairment. A summary of the PK parameters following single doses of cefiderocol is presented in Table 2. The t1/2 values in normal, mild, moderate, severe, and hemodialysis (dosing posthemodialysis, without HD) groups were 2.8, 3.0, 4.1, 6.9, and 9.6 hours, respectively. The comparisons of PK parameters between renal impairment groups and the normal group are presented in Table 3. Ratios (90% CIs) of AUC in the mild, moderate, severe, and ESRD requiring hemodialysis (without HD) groups to that in the normal group were 1.0 (0.8‐1.3), 1.5 (1.2‐1.9), 2.5 (2.0‐3.3), and 4.1 (3.3‐5.2), respectively, indicating that cefiderocol exposure increased in subjects with moderate and severe renal impairment and ESRD compared with normal renal function. Geometric mean values of Cmax and Vss were similar among renal function groups. The fu at 1 and 8 hours was similar among renal function groups.
Figure 2.
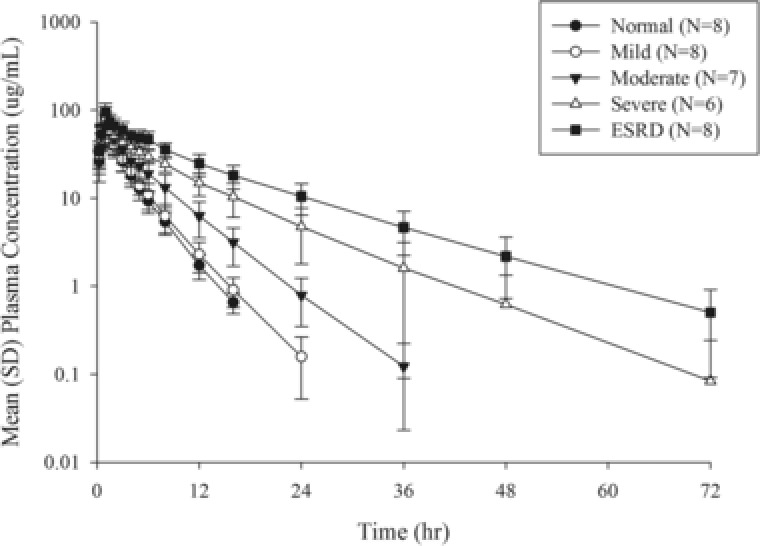
Mean plasma cefiderocol concentration profiles following single intravenous infusion of 1000 mg of cefiderocol over 1 hour. Semilogarithmic scale. Mean ± SD. Abbreviation: ESRD, end‐stage renal disease (dosing posthemodialysis). The BLQ data were treated as 0 for the calculation of mean and SD for plasma concentrations. The data at 24 and 36 hours in the normal‐renal‐function group were removed from the figure because the mean values were lower than 0.1 μg/mL, the lower limit of quantification for plasma.
Table 2.
Summary of Pharmacokinetic Parameters of Cefiderocol Following Single Intravenous Infusion of 1000 mg Over 1 Hour
| Renal Function Group | ||||||
|---|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | ESRD (w/o HD) | ESRD (with HD) | |
| PK Parameter | (N = 8) | (N = 8) | (N = 7) | (N = 6) | (N = 8) | (N = 8) |
| Cmax (μg/mL) | 81.0 (27.4) | 73.4 (21.3) | 78.0 (31.1) | 80.1 (19.8) | 93.0 (27.8) | 75.4 (31.1) |
| tmax (h) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.1) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.5) |
| AUC(0‐t) (μg·h/mL) | 212.0 (26.7) | 217.8 (22.2) | 311.0 (38.6) | 540.3 (23.6) | 872.5 (23.9) | 314.9 (20.3) |
| AUC (μg·h/mL) | 213.4 (26.5) | 218.7 (22.2) | 312.3 (38.4) | 543.2 (23.6) | 880.7 (24.2) | 318.1 (20.3) |
| t1/2 (h) | 2.8 (16.5) | 3.0 (8.4) | 4.1 (12.6) | 6.9 (30.6) | 9.6 (33.4) | 9.5 (32.8) |
| CL (L/h) | 4.7 (26.5) | 4.6 (22.2) | 3.2 (38.4) | 1.8 (23.6) | 1.1 (24.2) | 3.1 (20.3) |
| Vss (L) | 13.5 (30.2) | 14.8 (17.7) | 15.4 (28.7) | 16.4 (23.4) | 14.2 (22.5) | 26.6 (33.5) |
| fe (%) | 68.6 (17.3) | 68.3 (14.0) | 55.5 (19.6) | –a | –a | –a |
| CLR (L/h) | 3.2 (28.0) | 3.1 (30.3) | 1.8 (41.9) | –a | –a | –a |
| fu (1 h) | 0.42 (12.7) | 0.37 (43.5) | 0.35 (38.9) | 0.36 (31.4) | 0.42 (26.6) | 0.47 (19.8) |
| fu (8 h) | 0.44 (9.8) | 0.42 (19.1) | 0.45 (18.5) | 0.44 (10.1) | 0.37 (27.0) | 0.42 (21.5) |
Geometric mean (CV% geometric mean) is shown except for tmax, where median and range (minimum, maximum) are shown. Abbreviations: ESRD, end‐stage renal disease; w/o HD, without hemodialysis (dosing posthemodialysis); with HD, dosing prior to hemodialysis; Cmax, maximum plasma concentration; tmax, time to Cmax; AUC(0‐t), area under the plasma concentration‐time curve from 0 to the time of the last quantifiable concentration; AUC, area under the plasma concentration‐time curve from time 0 to infinity; t1/2, terminal half‐life; CL, total drug clearance from plasma; Vss, volume of distribution under steady‐state conditions; fe, fraction of dose excreted unchanged into urine; CLR, renal clearance of drug; fu, fraction of total drug that is unbound in plasma.
The parameter derived from urine concentrations of cefiderocol is not presented for the subject population (severe and ESRD) including subjects who did not have sufficient ability to produce urine.
Table 3.
Comparisons of Cefiderocol Pharmacokinetic Parameters of Renal Impairment Groups to Those of Normal Renal Function Group
| Ratio of Geometric Least‐Squares Mean (90% Confidence Interval) | ||||
| PK Parameter | Mild vs Normal | Moderate vs Normal | Severe vs Normal | ESRD (w/o HD) vs Normal |
| Cmax | 0.905 (0.729‐1.124) | 0.962 (0.769‐1.204) | 0.989 (0.783‐1.249) | 1.148 (0.925‐1.425) |
| AUC(0‐t) | 1.027 (0.818‐1.290) | 1.467 (1.159‐1.857) | 2.549 (1.993‐3.260) | 4.116 (3.728‐5.169) |
| AUC | 1.025 (0.817‐1.287) | 1.464 (1.157‐1.852) | 2.546 (1.992‐3.254) | 4.128 (3.289‐5.181) |
| t1/2 | 1.058 (0.879‐1.272) | 1.465 (1.210‐1.773) | 2.454 (2.010‐2.996) | 3.409 (2.834‐4.100) |
| CL | 0.975 (0.777‐1.224) | 0.683 (0.540‐0.864) | 0.393 (0.307‐0.502) | 0.242 (0.193‐0.304) |
| Vss | 1.096 (0.891‐1.348) | 1.138 (0.918‐1.410) | 1.211 (0.968‐1.514) | 1.048 (0.852‐1.289) |
| CLR | 0.970 (0.323‐2.912) | 0.552 (0.177‐1.722) | –a | –a |
Abbreviations: ESRD, end‐stage renal disease; w/o HD, dosing posthemodialysis; Cmax, maximum plasma concentration; AUC(0‐t), area under the plasma concentration‐time curve from 0 to the time of the last quantifiable concentration; AUC, area under the plasma concentration‐time curve from time 0 to infinity; t1/2, terminal half‐life; CL, total drug clearance from plasma; Vss, volume of distribution under steady‐state conditions; CLR, renal clearance of drug.
The parameter derived from urine concentrations of cefiderocol is not presented for the subject population (severe and ESRD) including subjects who did not have sufficient ability to produce urine.
Mean plasma concentration profiles without and with hemodialysis following single intravenous infusion of 1000 mg over 60 minutes are presented in Figure 3. Cefiderocol was removed by HD immediately after the start of HD. Geometric mean and coefficient of variation for geometric mean (CV% geometric mean) of CLhd were 7.5 L/h (9.8%). Geometric mean (CV% geometric mean) of AR relative to cefiderocol dose was 56.1% (12.2%), and Fr was 62.3% (8.4%), suggesting that intermittent HD removed approximately 60% of the cefiderocol dose.
Figure 3.
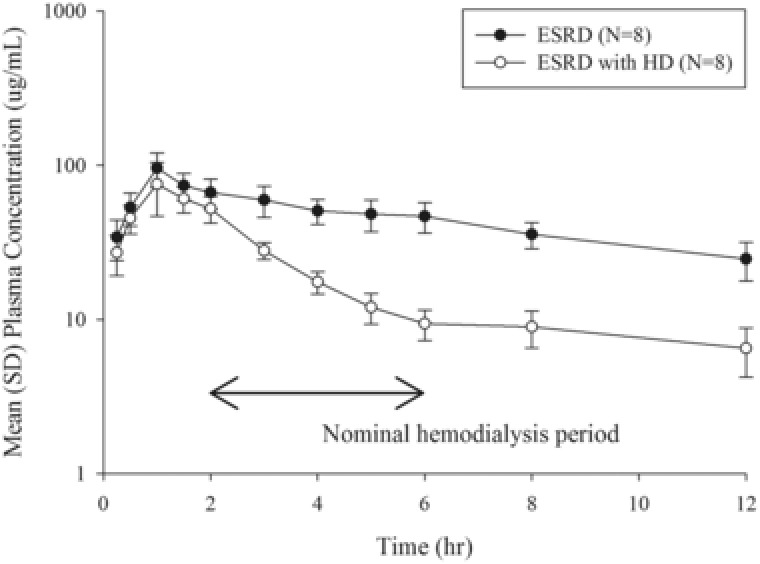
Mean plasma cefiderocol concentration profiles before and after hemodialysis following single intravenous infusion of 1000 mg of cefiderocol over 1 hour. Semilogarithmic scale. Mean ± SD. Abbreviations: ESRD, end‐stage renal disease (dosing posthemodialysis); ESRD with HD, end‐stage renal disease (dosing prior to hemodialysis). The BLQ data were treated as 0 for the calculation of mean and SD for plasma concentrations.
Safety and Tolerability
No deaths or serious AEs were reported during the study. One subject from the moderate impairment cohort had an AE of urticaria during administration, which led to discontinuation of the study medication and premature withdrawal from the study. This AE was considered related to the study drug. The subject was treated with diphenhydramine, and the event of urticaria resolved approximately 30 minutes after its onset.
Cefiderocol was generally well tolerated in the study subjects. By cohort, half or fewer subjects experienced at least 1 AE. This included 4 subjects (50.0%) in the moderate impairment cohort, 3 (37.5%) in the ESRD cohort (predialysis, period 1), 2 (25.0%) in the mild and severe impairment cohorts, and 1 (12.5%) each in the healthy and ESRD (postdialysis, period 2) cohorts.
The most frequently reported AE was contact dermatitis (7.9%), reported by 1 subject each in the mild, severe, and ESRD (period 1) cohorts. All of these were assessed as unrelated to the study drug. Other skin and subcutaneous tissue disorders were reported in 2 subjects in the moderate impairment cohort (maculopapular rash and urticaria, Table 4). Except for contact dermatitis, only nausea was reported as an AE by more than 1 subject per cohort.
Table 4.
Adverse Events by Renal Function
| Number (%) of Subjects With Renal Function Classified As | |||||||
|---|---|---|---|---|---|---|---|
| End‐Stage Renal Disease | |||||||
| Moderate | Severe | ||||||
| Matched Healthy | Mild Impairment | Impairment | Impairment | Without HD | With HD | Total | |
| Type of AE | (n = 8) | (n = 8) | (n = 8) | (n = 6) | (n = 8) | (n = 8) | (n = 38) |
| Any AE | 1 (12.5) | 2 (25.0) | 4 (50.0) | 2 (33.3) | 3 (37.5) | 1 (12.5) | 12 (31.6) |
| Any AE causally related to study druga | 1 (12.5) | 0 | 3 (37.5) | 0 | 1 (12.5) | 0 | 5 (13.2) |
| Any AE leading to withdrawal of study drug | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (2.6) |
| AEs by system organ classb | |||||||
| Gastrointestinal disorders | 0 | 1 (12.5) | 1 (12.5) | 0 | 1 (12.5) | 0 | 3 (7.9) |
| Constipation | 0 | 1 (12.5) | 0 | 0 | 0 | 0 | 1 (2.6) |
| Nausea | 0 | 0 | 1 (12.5) | 0 | 1 (12.5) | 0 | 2 (5.3) |
| Infections and infestations | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (2.6) |
| Upper respiratory tract infection | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (2.6) |
| Injury, poisoning, and procedural complications | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (12.5) | 1 (2.6) |
| Arteriovenous fistula site complication | 0 | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (2.6) |
| Postoperative wound complication | 0 | 0 | 0 | 0 | 1 (12.5) | 1 (12.5) | 1 (2.6) |
| Metabolism and nutrition disorders | 0 | 0 | 1 (12.5) | 0 | 1 (12.5) | 0 | 2 (5.3) |
| Gout | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (2.6) |
| Hypoglycemia | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.6) |
| Musculoskeletal and connective tissue disorders | 1 (12.5) | 0 | 1 (12.5) | 0 | 0 | 0 | 2 (5.3) |
| Flank pain | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (2.6) |
| Myalgia | 1 (12.5) | 0 | 0 | 0 | 0 | 0 | 1 (2.6) |
| Nervous system disorders | 0 | 0 | 1 (12.5) | 0 | 1 (12.5) | 0 | 2 (5.3) |
| Dizziness | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (2.6) |
| Headache | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.6) |
| Paresthesia | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.6) |
| Renal and urinary disorders | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.6) |
| Polyuria | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.6) |
| Skin and subcutaneous tissue disorders | 0 | 1 (12.5) | 2 (25.0) | 1 (16.7) | 1 (12.5) | 0 | 5 (13.2) |
| Dermatitis, contact | 0 | 1 (12.5) | 0 | 1 (16.7) | 1 (12.5) | 0 | 3 (7.9) |
| Rash, maculopapular | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (2.6) |
| Urticaria | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (2.6) |
| Vascular disorders | 0 | 0 | 1 (12.5) | 0 | 1 (12.5) | 0 | 2 (5.3) |
| Flushing | 0 | 0 | 0 | 0 | 1 (12.5) | 0 | 1 (2.6) |
| Phlebitis | 0 | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (2.6) |
Abbreviation: HD, hemodialysis.
aInvestigator‐defined AEs.
bMore than 1 AE can occur in a single subject.
Five subjects (13.2%) experienced 5 drug‐related AEs, which included nausea, maculopapular rash, or urticaria for 3 subjects in the moderate impairment cohort, myalgia in a matched healthy subject, and polyuria in an ESRD subject (period 1, without HD).
No clinically significant changes to physical examinations, vital signs, 12‐lead ECG, continuous dual‐lead ECG, QTcF/QTcB, or clinical laboratory evaluations (including hematology, blood chemistry, and urinalysis) were observed.
Discussion
The catechol moiety of cefiderocol contributes to its potent antimicrobial activity against Gram‐negative bacteria including those resistant to other antibiotics by functioning as a siderophore, an iron‐complexing protein, to form a chelating complex with ferric iron and utilize the bacterial iron transport system to penetrate the outer membrane of the Gram‐negative organisms.11 This concept of carrying compounds into other cells such as bacteria has been likened to the “Trojan horse.”12
Cefiderocol exposure increased in subjects with moderate and severe renal impairment and ESRD compared to those with normal renal function. Therefore, dose adjustments based on renal function would be needed so that the dose regimens will provide drug exposures comparable to that in subjects with normal renal function.
A dose of 2 g every 8 hours (daily dosage of 6 g), which would be a standard dose regimen, was tolerable in the previous phase 1 study.6 The significant effect of renal impairment on the PK of cefiderocol was expected because cefiderocol is mainly renally excreted. Therefore, in the renal impairment study, the single dose of 1 g was selected as a tested dose regimen that would have sufficient margin for exposure and prolonged elimination.
The MDRD equation and the Cockcroft‐Gault equation were used to categorize the renal function groups. The MDRD equation was reported to provide more accurate estimates of glomerular filtration rate (GFR) for impaired renal function than the Cockcroft‐Gault equation.8 However, the MDRD equation tended to underestimate GFR in the higher GFR range. This trend potentially causes a biased evaluation for an effect of renal impairment on cefiderocol PK when renal impairment groups are compared with the normal function group. In addition, the data with GFR > 90 mL/[min·1.73 m2] were limited in the development of the MDRD equation. On the other hand, CLcr calculated using the Cockcroft‐Gault equation was used to describe the measured CLcr in the normal‐renal‐function range.9
For cefiderocol, which exerts bactericidal activity dependent on the duration of exposure, the pharmacokinetic/pharmacodynamic (PK/PD) index that best correlates with the pharmacodynamic effect is the fraction of time for which the free drug concentration in plasma exceeds the minimum inhibitory concentration of the infecting microorganism over the dosing interval (Tf >MIC) as well as other cephalosporins.13, 14 Based on animal infection models, for cefiderocol the target of 55% to 80% Tf >MIC provides ≥1‐log10 reduction in bacteria, whereas 40% to 70% Tf >MIC inhibited bacterial growth.15, 16, 17
Cefiderocol was significantly removed by a 3‐ to 4‐hour HD session, as the ratio of cumulative amount of drug recovered unchanged in dialysate relative to dose was 56.1% and the fraction of the total body pool of drug removed by hemodialysis was 62.3%. The significant effect of intermittent HD on cefiderocol PK is reasonable because the fu of cefiderocol was 0.35 to 0.47, the molecular weight is 752.2, and Vss of 13.5 to 16.4 L was similar to the volume of extracellular fluid calculated as 20% of total body weight of the subjects (17.4 L for mean body weight of 87.2 kg).18 The significant removal of cefiderocol with HD suggests the need for a supplemental dose after the intermittent HD, which is applied to maintain the above pharmacodynamic effect.
The unbound fraction of drug is an important factor to consider the efficacy for antibiotics.19 The fu, which relates to Tf >MIC, at 1 and 8 hours after the start of infusion, was comparable among renal function groups. The serum concentrations of albumin, to which cefiderocol was predominantly bound, were comparable among the renal function groups (mean 3.9 to 4.2 g/L at baseline). The fu was also comparable at pre‐HD and post‐HD in the subjects requiring HD. These results suggested renal impairment and HD did not affect the protein binding of cefiderocol.
No notable safety findings in subjects with renal impairment were observed except that 1 subject of moderate renal impairment discontinued the study due to urticaria during the study drug administration. Overall, the safety profile observed in this study was consistent with that reported in the cefiderocol clinical trial program in subjects without renal impairment.6
One subject in the moderate renal impairment cohort who discontinued due to urticaria had an ongoing medical history of lactose intolerance and seasonal allergies. Although the subject's laboratory values for eosinophils were normal, the value for eosinophils/leukocytes was high (11.9% and 11.4%) at screening and on day 1 (reference range 0.0% to 0.6%). The blood samples were obtained from the subject before and after the administration of cefiderocol including approximately 37 minutes after the onset of urticaria, and these were examined for immunoglobulin E and immunoglobulin G antibody isotypes against cefiderocol. No antibodies against cefiderocol were detected in any of the samples. This result supports that the urticaria was not caused by antibodies against cefiderocol or unknown antibodies that might potentially show cross‐reactivity to the former antibodies.
Although the number of subjects for each group is small, there was no apparent difference in AEs even though the exposure of cefiderocol increased with increasing degree of renal impairment. The AEs observed in 2 or more groups were nausea (1 each in moderate and ESRD without HD) and contact dermatitis (1 each in mild, severe, and ESRD without HD), and these were all mild in severity. Geometric mean Cmax in the ESRD without HD group was modestly higher than those in the other groups. The differences were less than 25%, and the numbers of subjects who experienced the AEs were comparable to those in other groups. One subject in the ESRD without HD group experienced nausea, headache, paresthesia, polyuria, and flushing, and had an ongoing medical condition of type 2 diabetes mellitus with diabetic retinopathy. The incidence of AEs did not appear to have any correlation with the degree of renal impairment.
Conclusions
Renal impairment impacted AUC, CL, and t1/2 without affecting Cmax. Cefiderocol was significantly removed by intermittent hemodialysis. The results of this study suggested the need for dose adjustment based on renal function in patients with moderate and severe renal impairment and ESRD subjects and the need for a supplemental dose in patients receiving intermittent HD.
Acknowledgments
The authors thank Shionogi employees Yutaka Saisho and Demetrias Carnegia for management of this study and Yukitoshi Narukawa for manuscript preparation. This study was funded by Shionogi.
Declaration of Conflicting Interests
T.K., J.C.A.F., and H.K.K. are employees of Shionogi. R.E. is a consultant to Shionogi and was compensated for supporting this research. J.K.B. and C.G. conducted this research at DaVita Clinical Research (Minneapolis, Minnesota and Lakewood, Colorado) in the course of their employment, and their employers were compensated by Shionogi for conducting this study.
References
- 1. Gorska A, Sloderbach A, Marszaff MP. Siderophore‐drug complexes: potential medicinal applications of the “Trojan horse” strategy. Trends Pharmacol Sci 2014;35(9):442–449. [DOI] [PubMed] [Google Scholar]
- 2. Ito A, Kohira N, Bouchillon SK, et al. In vitro antimicrobial activity of S‐649266, a catechol substituted siderophore cephalosporin, when tested against non‐fermenting Gram‐negative bacteria. J Antimicrob Chemother. 2016;71(3):670–677. [DOI] [PubMed] [Google Scholar]
- 3. Kohira N, West J, Ito A, et al. In vitro antimicrobial activity of a siderophore cephalosporin, S‐649266, against Enterobacteriaceae clinical isolates, including carbapenem‐resistant strains. Antimicrob Agents Chemother. 2015;60(2):729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito A, Toba S, Nishikawa T, et al. S‐649266, a novel siderophore cephalosporin: binding affinity to PBP and in vitro bactericidal activity. Presented at 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID 2015); April 25‐28, 2015; Copenhagen. Poster P0250.
- 5. Nakamura R, Toba S, Tsuji M, Yamano Y, Shimada J. A novel siderophore cephalosporin: IV. In vivo efficacy in various murine infection models. Presented at 54th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC 2014); September 5‐9, 2014; Washington, DC. Poster F‐1558.
- 6. Shimada J, Saisho Y, Katsube T, White S, Fukase H. S‐649266, a novel siderophore cephalosporin for Gram negative bacterial infections: pharmacokinetics, safety and tolerability in healthy subjects. Presented at 54th Interscience Conference on Antimicrobial Agents Chemotherapy (ICAAC 2014); September 5‐9, 2014; Washington, DC. Poster F‐1564. [DOI] [PMC free article] [PubMed]
- 7. Federal Drug Administration . Pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. Fed Register. 2010;75(155):13562–13563. [Google Scholar]
- 8. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. [DOI] [PubMed] [Google Scholar]
- 9. Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 10. Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DR, Pittman KA. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther. 1990;48(3):268–276. [DOI] [PubMed] [Google Scholar]
- 11. Ito A, Nishikawa T, Matsumoto S, et al. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa [published online ahead of print 2016]. Antimicrob Agents Chemother. In press. doi:10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tillotson GS. Trojan horse antibiotics—a novel way to circumvent Gram‐negative bacterial resistance? Infect Dis Res Treatment. 2016;9:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad‐spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22(1‐2):89–96. [DOI] [PubMed] [Google Scholar]
- 14. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura R, Toba S, Ito A, Tsuji M, Yamano Y, Shimada J. A novel siderophore cephalosporin: V. Pharmacodynamic assessment in murine thigh infection models. Presented at 54th Interscience Conference on Antimicrobial Agents Chemotherapy (ICAAC 2014); September 5‐9, 2014; Washington, DC. Poster F‐1559.
- 16. Horiyama T, Toba S, Nakamura R, Tsuji M, Yamano Y, Shimada J. A novel siderophore cephalosporin: VI. Magnitude of PK/PD parameter required for efficacy in murine lung infection model. Presented at 54th Interscience Conference on Antimicrobial Agents Chemotherapy (ICAAC 2014); September 5‐9, 2014; Washington, DC. Poster F‐1560.
- 17. Horiyama T, Toba S, Nakamura R, Tsuji M, Yamano Y, Shimada J. 2014. A novel siderophore cephalosporin: VII. Magnitude of PK/PD parameter required for efficacy in murine thigh infection model. Presented at 54th Interscience Conference on Antimicrobial Agents Chemotherapy (ICAAC 2014); September 5‐9, 2014; Washington, DC. Poster F‐1561.
- 18. Friis‐Hansen B. Water distribution in the foetus and newborn infant. Acta Paediatr Scand Suppl. 1983;305:7–11. [DOI] [PubMed] [Google Scholar]
- 19. Drusano GL. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis. 2003;36(suppl 1):S42–S50. [DOI] [PubMed] [Google Scholar]
- 20. DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]


