Abstract
Aims
To characterize gastrointestinal adverse events (AEs) with different glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs).
Methods
Two retrospective intention‐to‐treat analyses of 6‐month patient‐level data were conducted. Data from three studies comparing exenatide once weekly (n = 617) with exenatide twice daily (n = 606) were pooled, and one (DURATION‐6) comparing exenatide once weekly (n = 461) with liraglutide (n = 450) was analysed separately. Patient‐reported gastrointestinal AEs were classified as upper or lower, AE incidences and timing were determined, subgroups were analysed, and associations of gastrointestinal AEs with efficacy were examined.
Results
Nausea was the most common gastrointestinal AE for all treatments. Fewer exenatide once‐weekly‐treated vs exenatide twice‐daily‐ or liraglutide‐treated patients reported gastrointestinal AEs (34% vs 45% and 25% vs 41%, respectively; both P < .0001). Fewer exenatide once‐weekly‐treated patients reported upper plus lower events than liraglutide‐treated patients (P < .001); the difference between exenatide once weekly and twice daily was not significant. Within each group, more women than men reported gastrointestinal AEs. Events occurrred early and were predominantly mild. Glycated haemoglobin reductions were similar for patients with or without gastrointestinal AEs. Weight loss was greater for patients with gastrointestinal AEs with exenatide once weekly and exenatide twice daily (P < .05); no difference was observed in DURATION‐6.
Conclusions
Gastrointestinal AEs were less frequent with exenatide once weekly vs exenatide twice daily or liraglutide, and combined upper and lower events occurred less often. Gastrointestinal AEs were typically mild and occurred early. Gastrointestinal AEs did not affect glycaemic control but may be associated with greater weight loss.
Keywords: adverse events, exenatide, gastrointestinal disorders, GLP‐1 receptor agonists, liraglutide, type 2 diabetes
1. INTRODUCTION
In patients with type 2 diabetes (T2D), glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) lead to significant reductions in glycated haemoglobin (HbA1c) levels and body weight, and are now approved and used across the spectrum of T2D therapies, as an adjunct to diet and exercise, oral agents or insulin.1 GLP‐1RAs are associated with gastrointestinal adverse events (AEs), predominantly nausea, vomiting and diarrhoea. They act by stimulating insulin release and suppressing glucagon secretion in a glucose‐dependent fashion, while increasing satiety, slowing gastric emptying and inhibiting small intestinal motility.2, 3
Differences between individual GLP‐1RAs have been observed regarding the type and frequency of gastrointestinal events,1 which may be related to the specific agent and/or to pharmacokinetic differences. It is now understood that with short‐acting GLP‐1RAs, such as exenatide twice daily and lixisenatide, postprandial glucose reduction is associated with substantial slowing of gastric emptying, whereas the slowing of gastric emptying is much less pronounced with longer‐acting GLP‐1RAs, such as liraglutide and exenatide once weekly.4, 5 Similar incidence rates for nausea, vomiting and diarrhoea have been reported among patients receiving exenatide twice daily and liraglutide once daily, although nausea was less persistent with liraglutide compared with exenatide twice daily,6 whereas lower incidences have been reported with exenatide once weekly.4, 7, 8 More information is needed, however, on the specific gastrointestinal effects of different GLP‐1RAs, including broader categories of gastrointestinal symptoms and the possibility that multiple symptoms occur concurrently, and on comparisons between individual GLP‐1RAs. Furthermore, patient subgroups that may be at greater risk of gastrointestinal AEs have not been defined, and the implications of these events for glycaemic control and weight loss with different GLP‐1RAs have not been explored in large patient populations.
The present analysis characterized gastrointestinal AEs associated with GLP‐1RAs using systematically collected clinical trial data from the exenatide once‐weekly development programme, including the frequencies and co‐incidence of gastrointestinal AEs in patients and subgroups of patients with T2D treated with exenatide once weekly, exenatide twice daily or liraglutide. The potential impact of gastrointestinal AEs on treatment effects, including reductions in HbA1c and weight, was also investigated.
2. MATERIALS AND METHODS
This was a post hoc analysis of individual patient data from studies in the exenatide once‐weekly development programme, with 6‐month safety data in which exenatide once weekly was compared directly with another GLP‐1RA.
Two analyses of patient‐level data were performed: a pooled analysis of three studies comparing exenatide once weekly with exenatide twice daily (DURATION‐1, DURATION‐5 and NCT00917267),4, 7, 9 and a separate analysis of the DURATION‐6 study, which compared exenatide once weekly and liraglutide.8
The designs, inclusion and exclusion criteria, and primary findings of these studies have been reported elsewhere.4, 7, 8, 9 All studies were randomized, multicentre, open‐label trials designed to evaluate glycaemic control over treatment periods ranging from 24 to 30 weeks (Table S1, Supporting Information).4, 7, 8, 9 Typical titration schedules were followed for exenatide twice daily and liraglutide, with no titration required for exenatide once weekly (Table S1, Supporting Information). Final doses of study medication were exenatide once weekly 2 mg, exenatide twice daily 10 µg and liraglutide once daily 1.8 mg.
2.1. Recording of gastrointestinal AEs
The data comprised spontaneous, patient‐reported gastrointestinal AEs recorded at study visits. The nature and severity of AEs described by patients were recorded by the investigator and were not adjudicated or assessed for severity according to any systematic rating scale. Gastrointestinal AEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) terms.
Because investigators were not instructed as to how to define gastrointestinal AE terms, terms other than nausea, vomiting and diarrhoea were included. A list of all reported gastrointestinal AE terms, which did not specify treatment assignment, was reviewed by a gastroenterologist (C.K.R.). Oral events and those with known causes (eg, structural or infectious disorders) were excluded (Table S2, Supporting Information). Gastrointestinal AEs of interest (Table S2, Supporting Information) were then categorized anatomically as upper or lower, with review and consensus by all authors. A patient was categorized as having an “upper” or “lower” AE when only upper or lower gastrointestinal AEs, respectively, were reported and as having “upper + lower” gastrointestinal AEs when at least one upper and one lower gastrointestinal AE was reported either concurrently or at different times.
The intensity of gastrointestinal AEs was categorized as mild, moderate or severe, according to standard definitions, and standard criteria were used to identify serious AEs (Table S3, Supporting Information); patients could be included in up to three severity categories if they had ≥2 events of different intensity. Gastrointestinal AEs withdrawal rates were determined for each treatment, and specific AEs leading to discontinuation were recorded.
2.2. Analysis of patient subgroups
Patient subgroups were defined according to characteristics that could potentially influence the incidence of gastrointestinal AEs (sex, race/ethnicity [Asian/Hispanic/white], background metformin [use/non‐use]) or response to therapy (occurrence or absence of gastrointestinal AEs of interest).
2.3. Event timing
The timing of specific gastrointestinal AEs was studied with reference to treatment initiation (days on therapy) when an individual event was studied. The proportion of patients with an AE was calculated using the number of patients remaining in the study at each time interval as the denominator. When the timing of multiple gastrointestinal AEs was studied, one was defined as the reference event and the timing of other events was calculated as days before or after the first occurrence of this event.
2.4. Statistical analyses
All analyses were conducted for the intention‐to‐treat populations of the included studies, defined as all randomized patients who had taken ≥1 dose of study drug. Only gastrointestinal AEs with onset or worsening after the first randomized study medication dose were analysed. For the purpose of integration, all AEs were re‐coded using MedDRA version 16.0.
Incidences of various categories of gastrointestinal AEs were determined in terms of patient numbers, and percentages were calculated from the total size of the appropriate treatment population. Proportions of patients with specific gastrointestinal AEs were compared between treatments using Fisher's exact test, and two‐sided P values at the α levels of .05, .001 and .0001 are reported.
Baseline characteristics were calculated descriptively as mean ± standard deviation (s.d.). Changes in HbA1c and body weight were calculated as mean change ± standard error (s.e.) in the defined patient subpopulation. Changes from baseline in HbA1c and body weight at study endpoint were evaluated, with missing data for HbA1c and weight inferred using the last observation carried forward method. Within‐group comparisons of HbA1c and body weight were conducted using a paired t‐test, while between‐group comparisons were made using Student's t‐test; both reported two‐sided P values at the α level of .05.
Subgroups with <30 patients were considered to be of insufficient size for statistical analysis.
3. RESULTS
3.1. Study population
The pooled analysis population included 1223 patients (exenatide once weekly, n = 617; exenatide twice daily, n = 606) and the DURATION‐6 analysis included 911 patients (exenatide once weekly, n = 461; liraglutide, n = 450).
Patient characteristics in the two populations were generally similar; however, the pooled analysis included a greater proportion of Asian patients (56% vs 12%) and a smaller proportion of white patients (31% vs 65%) than DURATION‐6, and patients in the pooled analysis had numerically lower mean body weight and body mass index (Table 1).
Table 1.
Baseline patient characteristics for pooled analysis and DURATION‐6
| Pooled analysis 1 (N = 1223) | DURATION‐6 (N = 911) | |||
|---|---|---|---|---|
| Exenatide once weekly (n = 617) | Exenatide twice daily (n = 606) | Exenatide once weekly (n = 461) | Liraglutide (n = 450) | |
| Age, years | 55 ± 11 | 56 ± 10 | 56 ± 9 | 56 ± 10 |
| Men, n (%) | 342 (55) | 326 (54) | 254 (55) | 245 (54) |
| Race/ethnicity, n (%) | ||||
| White | 204 (33) | 173 (29) | 304 (66) | 287 (64) |
| Asian | 345 (56) | 344 (57) | 55 (12) | 56 (12) |
| Hispanic | 53 (9) | 61 (10) | 98 (21) | 99 (22) |
| Black | 15 (2) | 28 (5) | 3 (1) | 3 (1) |
| Other | 0 | 0 | 1 (<1) | 4 (1) |
| Body weight, kg | 83 ± 22 | 83 ± 21 | 91 ± 19 | 91 ± 19 |
| BMI, kg/m2 | 30 ± 6 | 30 ± 6 | 32 ± 6 | 32 ± 5 |
| HbA1c, % | 8.5 ± 1.1 | 8.5 ± 1.1 | 8.4 ± 1.0 | 8.4 ± 1.0 |
| FPG, mmol/L | 9.2 ± 2.5 | 9.2 ± 2.6 | 9.6 ± 2.5 | 9.8 ± 2.6 |
| Duration of T2D, years | 7 ± 5 | 8 ± 6 | 8 ± 6 | 9 ± 6 |
| Background metformin, n (%)2 | 506 (82) | 484 (80) | 436 (95) | 425 (94) |
Values are reported as mean ± s.d., unless otherwise stated.
Abbreviations: BMI, body mass index; FPG, fasting plasma glucose.
Studies included DURATION‐1, DURATION‐5 and NCT00917267.
Use of metformin alone or in combination with sulphonylurea, thiazolidinedione, insulin, or other glucose‐lowering therapy.
3.2. Incidences of gastrointestinal AEs
Overall, gastrointestinal AEs of interest occurred in a smaller proportion of patients treated with exenatide once weekly vs exenatide twice daily (34% vs 45%; P < .0001), and with exenatide once weekly vs liraglutide (25% vs 41%; P < .0001; Figure 1A and B).
Figure 1.
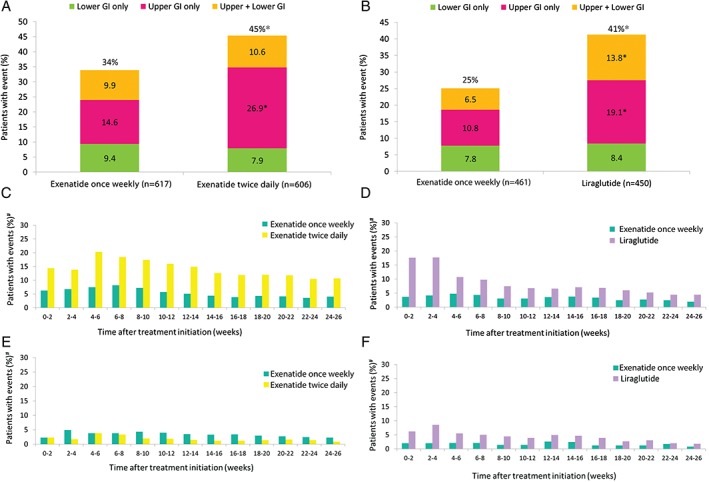
Incidences of gastrointestinal AEs in patients receiving GLP‐1RAs in A, pooled analysis and B, DURATION‐6; timing of nausea events in C, pooled analysis and D, DURATION‐6; timing of diarrhoea events in E, pooled analysis and F, DURATION‐6. GI, gastrointestinal. *P < .001. #Proportion of patients remaining on study at each time interval.
Fewer upper gastrointestinal AEs were noted for exenatide once weekly compared with exenatide twice daily and liraglutide, respectively (Figure 1A and B); this applied to patients reporting only upper gastrointestinal AEs with exenatide once weekly vs exenatide twice daily (P < .0001), exenatide once weekly vs liraglutide (P < .001), and to those reporting both upper and lower gastrointestinal AEs with exenatide once weekly vs liraglutide (P < .001). There were no between‐group differences in the proportion of patients with only lower gastrointestinal AEs in either analysis.
Analysis of lower gastrointestinal AEs by type (diarrhoea, constipation or both) showed no difference between exenatide once weekly and exenatide twice daily, although fewer patients treated with exenatide once weekly vs liraglutide reported lower gastrointestinal AEs (P < .01; Table 2). The only significant between‐group difference for individual lower gastrointestinal AEs was that fewer patients reported diarrhoea with exenatide once weekly than with liraglutide (P < .001).
Table 2.
Incidences of specific lower gastrointestinal AEs in patients treated with GLP‐1RAs, and comparisons of gastrointestinal AEs between patient subgroups
| Pooled analysis | DURATION‐6 | |||
|---|---|---|---|---|
| Exenatide once weekly (n = 617) | Exenatide twice daily (n = 606) | Exenatide once weekly (n = 461) | Liraglutide (n = 450) | |
| Subset of lower gastrointestinal AEs, n (%) | ||||
| Total | 98 (15.9) | 86 (14.2) | 50 (10.8) | 84 (18.7)‡ |
| Diarrhoea + constipation | 7 (1.1) | 4 (0.7) | 3 (0.7) | 3 (0.7) |
| Diarrhoea only | 62 (10.0) | 47 (7.8) | 28 (6.1) | 61 (13.6)* |
| Constipation only | 29 (4.7) | 35 (5.8) | 19 (4.1) | 20 (4.4) |
| Sex: women/men, n (%) 1 | ||||
| N (women/men) | 275/342 | 280/326 | 207/254 | 205/245 |
| Total | ||||
| Women | 106 (38.5)‡ | 157 (56.1)* | 66 (31.9)† | 99 (48.3)† |
| Men | 103 (30.1) | 118 (36.2) | 50 (19.7) | 87 (35.5) |
| Upper + lower | ||||
| Women | 34 (12.4) | 37 (13.2) | 16 (7.7) | 34 (16.6) |
| Men | 27 (7.9) | 27 (8.3) | 14 (5.5) | 28 (11.4) |
| Upper only | ||||
| Women | 46 (16.7) | 101 (36.1)* | 31 (15.0)‡ | 49 (23.9)‡ |
| Men | 44 (12.9) | 62 (19.0) | 19 (7.5) | 37 (15.1) |
| Lower only | ||||
| Women | 26 (9.5) | 19 (6.8) | 19 (9.2) | 16 (7.8) |
| Men | 32 (9.4) | 29 (8.9) | 17 (6.7) | 22 (9.0) |
| Race/ethnicity: Asian/Hispanic/white, n (%) 1 , 2 | ||||
| N (Asian/Hispanic/white) | 345/53/204 | 344/61/173 | 55/98/304 | 56/99/287 |
| Total | ||||
| Asian | 102 (29.6)* | 154 (44.8) | 21 (38.2)‡ | 33 (58.9)† |
| Hispanic | 9 (17.0)* | 22 (36.1) | 24 (24.5) | 36 (36.4) |
| White | 93 (45.6) | 88 (50.9) | 69 (22.7) | 113 (39.4) |
| Upper + lower | ||||
| Asian | 29 (8.4)‡ | 35 (10.2) | 8 (14.5)‡ | 12 (21.4) |
| Hispanic | 1 (1.9)† | 2 (3.3)‡ | 7 (7.1) | 14 (14.1) |
| White | 29 (14.2) | 24 (13.9) | 15 (4.9) | 34 (11.8) |
| Upper only | ||||
| Asian | 41 (11.9)† | 87 (25.3) | 7 (12.7) | 13 (23.2) |
| Hispanic | 4 (7.5)‡ | 16 (26.2) | 9 (9.2) | 15 (15.2) |
| White | 42 (20.6) | 54 (31.2) | 34 (11.2) | 58 (20.2) |
| Lower only | ||||
| Asian | 32 (9.3) | 32 (9.3) | 6 (10.9) | 8 (14.3) |
| Hispanic | 4 (7.5) | 4 (6.6) | 8 (8.2) | 7 (7.1) |
| White | 22 (10.8) | 10 (5.8) | 20 (6.6) | 21 (7.3) |
| Background metformin: use 3 /non‐use, n (%) 1 | ||||
| N (metformin/no metformin) | 506/111 | 484/122 | ||
| Total | ||||
| Metformin | 166 (32.8) | 225 (46.5) | ||
| No metformin | 43 (38.7) | 50 (41.0) | ||
| Upper + lower | ||||
| Metformin | 48 (9.5) | 55 (11.4) | ||
| No metformin | 13 (11.7) | 9 (7.4) | ||
| Upper only | ||||
| Metformin | 68 (13.4) | 135 (27.9) | ||
| No metformin | 22 (19.8) | 28 (23.0) | ||
| Lower only | ||||
| Metformin | 50 (9.9) | 35 (7.2) | ||
| No metformin | 8 (7.2) | 13 (10.7) | ||
P < .001.
P < .01.
P < .05.
Statistical comparisons for subgroups are within each treatment group.
White subgroup used as reference for comparisons.
Metformin alone or in combination with other oral glucose‐lowering drugs or insulin.
“Pancreatitis” or “acute pancreatitis” was recorded for one patient each in both the pooled analysis and DURATION‐6; these patients were in the exenatide once‐weekly group. Individual gastrointestinal AEs reported in each treatment group are presented in Table S4, Supporting Information.
3.3. Patient subgroups
3.3.1. Sex
A greater proportion of women than men reported gastrointestinal AEs in both the pooled analysis (P < .05 for exenatide once weekly; P < .0001 for exenatide twice daily) and DURATION‐6 (P < .01 for exenatide once weekly; P < .01 for liraglutide; Table 2).
3.3.2. Race/ethnicity
There were differences in the proportion of patients reporting gastrointestinal AEs when categorized by race/ethnicity in both analyses, but the direction of the difference was inconclusive (Table 2). In the pooled analysis, the proportion of patients with gastrointestinal AEs in the exenatide once‐weekly group was lower for Asian and Hispanic patients than for white patients (both P < .001), without any difference for exenatide twice‐daily‐treated patients. In DURATION‐6, the proportion of patients with gastrointestinal AEs was higher for Asian than for white patients with both exenatide once weekly (P < .05) and liraglutide (P < .01), with no differences between Hispanic and white patients.
3.3.3. Background metformin use
Continued use of metformin was not associated with increased gastrointestinal AEs in the pooled population (Table 2).
3.4. Timing of gastrointestinal AEs
Nausea was the most common gastrointestinal AE throughout the treatment period but differed between the GLP‐1RAs. For exenatide once weekly, few patients reported nausea during each 2‐week interval, and the proportion was consistently lower than with exenatide twice daily or liraglutide (Figure 1C and D). With exenatide twice daily, nausea incidence peaked at weeks 4 to 6 and declined thereafter; for liraglutide, the proportion of patients reporting nausea was highest during the first 4 weeks, before decreasing.
The proportions of patients reporting vomiting, diarrhoea and constipation during any 2‐week interval were <7%, <9% and <5%, respectively, for all treatments.
Vomiting remained infrequent throughout treatment with exenatide once weekly in the pooled analysis, while there was a peak at weeks 4 to 6 for exenatide twice daily. Similarly in DURATION‐6, vomiting remained uncommon for exenatide once weekly throughout, but peaked during weeks 0 to 4 for liraglutide.
Diarrhoea was more frequent with exenatide once weekly (peaking at weeks 2–4) than with exenatide twice daily (peaking at weeks 4–6) and declined thereafter for each (Figure 1E). In DURATION‐6, diarrhoea was more frequent with liraglutide (peaking at weeks 0–4) than with exenatide once weekly (Figure 1F). The proportion of patients reporting constipation remained low throughout treatment in all groups.
The first gastrointestinal AE of any kind occurred most frequently during the first 5 days for all treatments, with another peak between days 25 and 30 for exenatide twice daily. Few first events occurred after 3 months.
During the first 5 days of treatment, there were 40 first gastrointestinal AEs with exenatide once weekly, compared with 93 with exenatide twice daily. First events occurred in 20 exenatide once‐weekly‐treated patients, compared with 65 liraglutide‐treated patients (Figure 2A and B). First events were more frequently upper than lower gastrointestinal AEs. Among patients who reported both upper gastrointestinal AEs and diarrhoea, these events generally occurred within 10 days of each other (Figure 2C and D).
Figure 2.
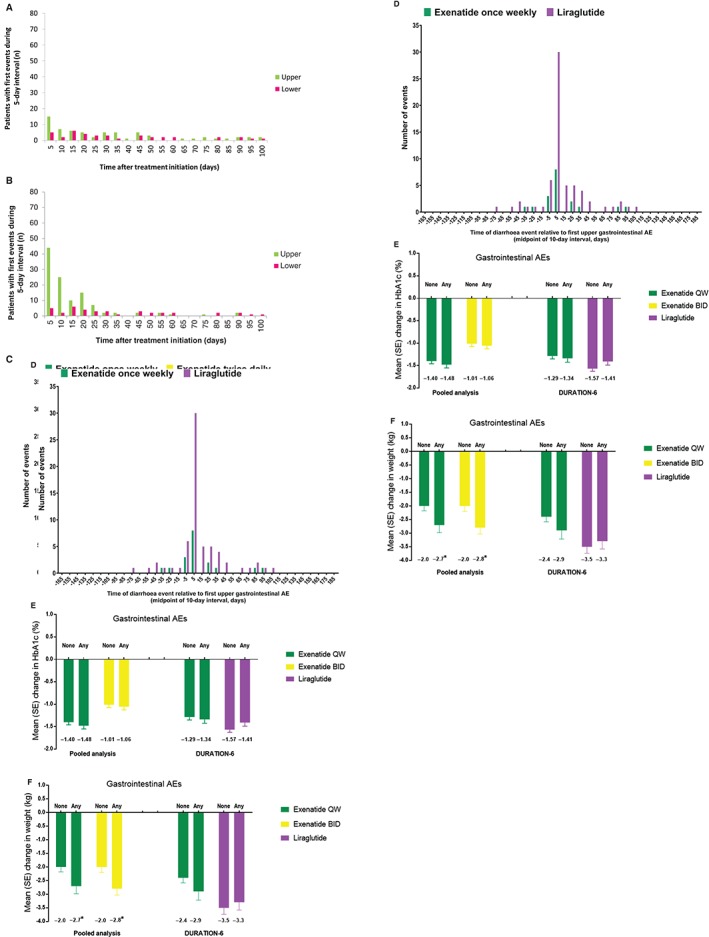
Timing of first gastrointestinal AEs in the DURATION‐6 study in patients receiving: A, exenatide once weekly and B, liraglutide (events shown for 5‐day intervals from day 0 to day 100); temporal association between diarrhoea events and the first upper gastrointestinal AE in C, pooled analysis and D, DURATION‐6; impact of gastrointestinal AEs on change from baseline in E, HbA1c and F, body weight in the pooled analysis and DURATION‐6. *P < .05 vs no gastrointestinal events.
3.5. Severity, serious events and events leading to withdrawal
Gastrointestinal AEs were predominantly mild (74.8% for exenatide once weekly and 75.5% for exenatide twice daily; 83.0% for exenatide once weekly and 74.3% for liraglutide).
In the pooled analysis, mild, moderate and severe gastrointestinal AEs were reported by 30.3%, 9.7% and 1.3% of exenatide once‐weekly‐treated patients and by 38.6%, 12.4% and 1.7% of exenatide twice‐daily‐treated patients, respectively. In DURATION‐6, the corresponding proportions were 23.0%, 4.8% and 1.3% for exenatide once weekly and 34.2%, 12.9% and 2.9% for liraglutide.
Few serious gastrointestinal AEs were reported for any of the GLP‐1RAs (four with exenatide once weekly and two with exenatide twice daily in the pooled analysis; three with exenatide once weekly and three with liraglutide in DURATION‐6 [Table S5, Supporting Information]).
Discontinuation because of gastrointestinal AEs was infrequent and occurred less with exenatide once weekly vs exenatide twice daily (1% vs 6% of patients; P < .0001) and exenatide once weekly vs liraglutide (1% vs 4% of patients; P < .05). Nausea and vomiting were the gastrointestinal AEs that most often led to withdrawal of exenatide twice daily and liraglutide; no trend was apparent for exenatide once weekly (Table S6, Supporting Information). In the exenatide twice‐daily and liraglutide groups, nausea events that led to withdrawal mainly occurred within the first month of treatment.
3.6. Association between gastrointestinal AEs and treatment effects
Reductions from baseline in HbA1c and body weight occurred with all treatments at week 26 (all P < .05 vs baseline). The reduction in HbA1c did not differ between patients with upper and/or lower gastrointestinal AEs and those without in any analysis (Figure 2E). Weight loss was greater in patients who reported gastrointestinal AEs than in those who did not in the exenatide once‐weekly (−2.7 kg vs −2.0 kg; P < .05) and exenatide twice‐daily groups (−2.8 kg vs −2.0 kg; P < .05) in the pooled analysis (Figure 2F). There were no differences in weight loss for exenatide once weekly or liraglutide in DURATION‐6 (Figure 2F).
4. DISCUSSION
Gastrointestinal symptoms occur frequently in patients with T2D and represent a substantial cause of morbidity,10 so it is important to understand the potential effects of medications on these symptoms. GLP‐1RAs have been shown to reduce HbA1c and body weight, with low intrinsic risk of hypoglycaemia. One potential AE associated with these agents is an increase in gastrointestinal events that is transient in most cases.1 As such, understanding the specific gastrointestinal AE profile and how it varies across the GLP‐1RA class is of interest for patients and physicians, and may be useful in guiding therapy.
Both pooled patient‐level data and a single trial were studied in the present analysis, so that data for three separate GLP‐1RAs were available. The most common gastrointestinal AEs reported were nausea, vomiting and diarrhoea. Other diagnoses, including dyspepsia and gastroesophageal reflux disease, were also associated with the GLP‐1RAs studied, but differed between individual GLP‐1RAs and were reported less frequently (Table S4, Supporting Information). The results also suggest that some patients experience both upper and lower gastrointestinal AEs, most commonly nausea followed within 10 days by diarrhoea—an observation not, to our knowledge, previously reported.
Incidences of gastrointestinal AEs differed between the GLP‐1RAs investigated. Upper gastrointestinal AEs were less common for exenatide once weekly, compared with exenatide twice daily and liraglutide. In contrast, the frequency of lower gastrointestinal AEs was similar across treatment groups, although diarrhoea occurred more frequently with liraglutide than with exenatide once weekly. Constipation was uncommon, and most patients reporting constipation also reported diarrhoea at a different time during treatment. While constipation has been reported by patients treated with GLP‐1RAs, its incidence in clinical trials has varied considerably between agents.11, 12, 13, 14, 15, 16 Patients who reported both upper and lower gastrointestinal AEs were more commonly treated with liraglutide than with exenatide once weekly; however, there was no significant difference between exenatide once weekly and exenatide twice daily. It is likely that a similar analysis with other GLP‐1RAs would also show differences in the incidence of gastrointestinal AEs. In head‐to‐head studies, albiglutide was associated with lower rates of nausea and vomiting compared with liraglutide,17 while gastrointestinal AE rates were similar for dulaglutide 1.5 mg vs exenatide twice daily or liraglutide and lower for dulaglutide 0.75 mg vs exenatide twice daily.18, 19
Several studies have suggested a possible relationship between gastrointestinal symptoms and HbA1c in patients with T2D,10, 20, 21, 22 with symptoms occurring more frequently in patients with poor glycaemic control and higher HbA1c levels, possibly because of the impact of long‐term poor glycaemic control on diabetic complications, such as autonomic neuropathy; however, this association was not observed in the present analyses. Additionally, the presence of gastrointestinal AEs as a whole did not influence changes in HbA1c in response to therapy; however, in the pooled analysis, patients who reported gastrointestinal AEs had significantly greater weight loss than those who did not in both the exenatide once‐weekly and exenatide twice‐daily groups, although absolute differences in mean weight loss were <1 kg. This is consistent with the outcome of a recent analysis of 12 studies that found that weight loss among exenatide once‐weekly‐treated patients was greater for those with nausea/vomiting at 24 weeks than those without (−3.1 kg vs −2.2 kg; P < .05).23
Although it is commonly perceived that GLP‐1RAs cause gastrointestinal AEs by slowing gastric emptying, there is, in general, a weak relationship of symptoms with delayed gastric emptying.24 Moreover, symptoms occur in the fasted state25; therefore, it is likely that centrally mediated effects are important. While the effects of GLP‐1RAs on regions of the gut other than the stomach have not been extensively studied, the potential for exenatide twice daily to inhibit small intestinal motor function was recently demonstrated,3 and such effects may contribute to lower gastrointestinal symptoms such as diarrhoea or constipation.
Possible reasons for differences in gastrointestinal AEs between GLP‐1RAs include differences in drug concentration profiles; the half‐life and time to minimal effective and steady‐state concentrations are much longer for exenatide once weekly than for exenatide twice daily or liraglutide.11, 26, 27 GLP‐1RAs also differ in their ability to cross the blood–brain barrier, with small‐molecule GLP‐1RAs such as exenatide, liraglutide and lixisenatide being able to enter the brain, whereas larger GLP‐1RAs such as albiglutide cannot27, 28, 29; it is possible that these differences impact centrally mediated AEs. It was recently established that tachyphylaxis occurs for the effects of exogenous glucagon‐like peptide‐1 on the slowing of gastric emptying with prolonged exposure.30 Thus, while short‐acting GLP‐1RAs such as exenatide twice daily are likely to have a sustained effect to slow gastric emptying substantially, this effect is likely to be markedly diminished with longer‐acting formulations such as exenatide once weekly or liraglutide.4, 26 It is unknown whether a similar phenomenon applies to the effects of GLP‐1RAs on other gut regions (eg, the small or large intestine), or for the central effects of these agents.
In the present analysis we also examined whether any patient characteristics influenced the gastrointestinal effects of GLP‐1RAs. For all treatments, upper gastrointestinal AEs were reported more frequently by women than men; this is consistent with pooled analyses of 7 studies of exenatide once weekly31 and 16 studies involving exenatide twice daily.32 Even aside from drug studies, gastrointestinal symptoms are consistently reported more frequently in women than men, with or without diabetes,10 for reasons that are not well understood.
When analysed by race/ethnicity, there were inconsistent outcomes for comparisons of exenatide once weekly vs exenatide twice daily and exenatide once weekly vs liraglutide. This suggests that race/ethnicity has little, if any, effect on the occurrence of gastrointestinal AEs and is consistent with previous pooled analyses of 7 exenatide once‐weekly studies31 and 16 exenatide twice‐daily studies.32 Finally, metformin use had no significant effect on the proportion of patients experiencing gastrointestinal AEs. This contrasts with a previous study indicating a strong association of diarrhoea and faecal incontinence with metformin use in T2D.33 Given that metformin use was stable before study entry, it is possible that individuals who had AEs from metformin were not represented in the study populations.
Limitations inherent in post hoc analyses include their retrospective nature and the potential for selection bias resulting from differences between patients eligible for clinical trials and those seen in clinical practice. Furthermore, we focused on three specific GLP‐1RAs evaluated in the exenatide once‐weekly development programme, rather than the class as a whole, and the studies were not placebo‐controlled (ie, the lack of a placebo group made it difficult to determine how many gastrointestinal AEs were unrelated to study medication); moreover, patient histories of gastrointestinal disorders before study entry were not systematically recorded. Recording of gastrointestinal AEs was based on patient reports, which are likely to represent an underestimate of the true incidences.34 Furthermore, gastrointestinal AE data were not adjudicated, study investigators were not instructed in common gastrointestinal terminology, patients did not use a rating scale for severity of symptoms, and the timing and duration of events were not recorded precisely. Nevertheless, the proportions of patients reporting gastrointestinal AEs are in line with previous studies involving GLP‐1RAs.1
In conclusion, the present study provides detailed information on the gastrointestinal tolerability of three GLP‐1RAs based on patient‐level data from the exenatide once‐weekly clinical trial programme. Gastrointestinal AEs were usually mild and transient and particularly affected the upper gastrointestinal tract, and occurred more frequently with exenatide twice daily and liraglutide than with exenatide once weekly. These insights inform physician and patient expectations of potential experiences when initiating GLP‐1RA therapy.
Supporting information
Table S1. Study details for pooled analysis and DURATION‐6.
Table S2. Gastrointestinal adverse events observed in the trials in any treatment group.
Table S3. Definitions for assessment of adverse event intensity and serious adverse events.
Table S4. Gastrointestinal adverse events reported by patients, by drug.
Table S5. Serious gastrointestinal adverse events, and gastrointestinal adverse events reported by ≥2% of patients in each treatment group, according to frequency (highest to lowest).
Table S6. Gastrointestinal adverse events leading to treatment withdrawal.
ACKNOWLEDGMENTS
Raewyn Poole and Mollie Marko of inScience Communications, Springer Healthcare, provided medical writing support funded by AstraZeneca. The manuscript was reviewed by Mary Beth DeYoung of AstraZeneca LP.
Conflict of interest
M. H. has participated in advisory boards and/or symposia for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi and has received honoraria for this activity. V. R. A. has received research funding from AstraZeneca/Bristol‐Myers Squibb, Calibra, Eisai, Elcelyx, Janssen, Novo Nordisk, Sanofi and Theracos, and has performed consulting activities for Adocia, the American Diabetes Association, AstraZeneca, Janssen, Medscape, Novo Nordisk, Sanofi and Tufts. J. H. has received payment for consulting work from AstraZeneca. E. H. is an employee and stockholder of AstraZeneca. C. K. R. has received research funding from AstraZeneca, Merck Sharp & Dohme, Novartis, and Sanofi.
Author contributions
M. H., V. R. A., J. H., E. H. and C. K. R. conceived the analysis and participated in the writing of the manuscript. All authors approved the final version of the manuscript.
Horowitz M, Aroda VR, Han J, Hardy E and Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon‐like peptide‐1 receptor agonists: Incidence and consequences. Diabetes Obes Metab. 2017;19:672–681. https://doi.org/10.1111/dom.12872
Funding information The statistical analyses and development of the manuscript were supported by AstraZeneca.
REFERENCES
- 1. Sun F, Chai S, Yu K, et al. Gastrointestinal adverse events of glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Technol Ther. 2015;17:35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin‐4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77‐88. [DOI] [PubMed] [Google Scholar]
- 3. Thazhath SS, Marathe CS, Wu T, et al. The glucagon‐like peptide 1 receptor agonist exenatide inhibits small intestinal motility, flow, transit, and absorption of glucose in healthy subjects and patients with type 2 diabetes: a randomized controlled trial. Diabetes. 2016;65:269‐275. [DOI] [PubMed] [Google Scholar]
- 4. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet. 2008;372:1240‐1250. [DOI] [PubMed] [Google Scholar]
- 5. Meier JJ, Rosenstock J, Hincelin‐Méry A, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38:1263‐1273. [DOI] [PubMed] [Google Scholar]
- 6. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374:39‐47. [DOI] [PubMed] [Google Scholar]
- 7. Blevins T, Pullman J, Malloy J, et al. DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301‐1310. [DOI] [PubMed] [Google Scholar]
- 8. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet. 2013;381:117‐124. [DOI] [PubMed] [Google Scholar]
- 9. Ji L, Onishi Y, Ahn CW, et al. Efficacy and safety of exenatide once‐weekly vs exenatide twice‐daily in Asian patients with type 2 diabetes mellitus. J Diabetes Invest. 2013;4:53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population‐based survey of 15,000 adults. Arch Intern Med. 2001;161:1989‐1996. [DOI] [PubMed] [Google Scholar]
- 11.BYETTA (exenatide) injection [package insert]. Wilmington, DE: AstraZeneca; 2015.
- 12.VICTOZA (liraglutide) injection, for subcutaneous use [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; 2016.
- 13.BYDUREON (exenatide extended release) for injectable suspension [package insert]. Wilmington, DE: AstraZeneca; 2015.
- 14.TANZEUM (albiglutide) for injection, for subcutaneous use [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2015.
- 15.TRULICITY (dulaglutide) injection [package insert]. Indianapolis, IN: Eli Lilly and Company; 2015.
- 16.Lyxumia (lixisenatide) summary of product characteristics. Paris, France: Sanofi‐Aventis Groupe; 2013.
- 17. Pratley RE, Nauck MA, Barnett AH, et al. Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289‐297. [DOI] [PubMed] [Google Scholar]
- 18. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349‐1357. [DOI] [PubMed] [Google Scholar]
- 19. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37:2159‐2167. [DOI] [PubMed] [Google Scholar]
- 20. Abid S, Rizvi A, Jahan F, et al. Poor glycaemic control is the major factor associated with increased frequency of gastrointestinal symptoms in patients with diabetes mellitus. J Pak Med Assoc. 2007;57:345‐349. [PubMed] [Google Scholar]
- 21. Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604‐611. [DOI] [PubMed] [Google Scholar]
- 22. Kim JH, Park HS, Ko SY, et al. Diabetic factors associated with gastrointestinal symptoms in patients with type 2 diabetes. World J Gastroenterol. 2010;16:1782‐1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trautmann ME, Han J, Ruggles J. Early pharmacodynamic effects of exenatide once weekly in type 2 diabetes are independent of weight loss: a pooled analysis of patient‐level data. Clin Ther. 2016;38:1464‐1473. [DOI] [PubMed] [Google Scholar]
- 24. Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264‐1269. [DOI] [PubMed] [Google Scholar]
- 25. Nauck MA, Wollschlager D, Werner J, et al. Effects of subcutaneous glucagon‐like peptide 1 (GLP‐1 [7–36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546‐1553. [DOI] [PubMed] [Google Scholar]
- 26. Fineman M, Flanagan S, Taylor K, et al. Pharmacokinetics and pharmacodynamics of exenatide extended‐release after single and multiple dosing. Clin Pharmacokinet. 2011;50:65‐74. [DOI] [PubMed] [Google Scholar]
- 27. Gerich J. Pathogenesis and management of postprandial hyperglycemia: role of incretin‐based therapies. Int J Gen Med. 2013;6:877‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kastin AJ, Akerstrom V. Entry of exendin‐4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313‐318. [DOI] [PubMed] [Google Scholar]
- 30. Umapathysivam MM, Lee MY, Jones KL, et al. Comparative effects of prolonged and intermittent stimulation of the glucagon‐like peptide 1 receptor on gastric emptying and glycemia. Diabetes. 2014;63:785‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pencek R, Blickensderfer A, Li Y, Brunell SC, Chen S. Exenatide once weekly for the treatment of type 2 diabetes: effectiveness and tolerability in patient subpopulations. Int J Clin Pract. 2012;66:1021‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pencek R, Blickensderfer A, Li Y, Brunell SC, Anderson PW. Exenatide twice daily: analysis of effectiveness and safety data stratified by age, sex, race, duration of diabetes, and body mass index. Postgrad Med. 2012;124:21‐32. [DOI] [PubMed] [Google Scholar]
- 33. Bytzer P, Talley NJ, Jones MP, Horowitz M. Oral hypoglycaemic drugs and gastrointestinal symptoms in diabetes mellitus. Aliment Pharmacol Ther. 2001;15:137‐142. [DOI] [PubMed] [Google Scholar]
- 34. Molinder H, Agreus L, Kjellstrom L, et al. How individuals with the irritable bowel syndrome describe their own symptoms before formal diagnosis. Ups J Med Sci. 2015;120:276‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study details for pooled analysis and DURATION‐6.
Table S2. Gastrointestinal adverse events observed in the trials in any treatment group.
Table S3. Definitions for assessment of adverse event intensity and serious adverse events.
Table S4. Gastrointestinal adverse events reported by patients, by drug.
Table S5. Serious gastrointestinal adverse events, and gastrointestinal adverse events reported by ≥2% of patients in each treatment group, according to frequency (highest to lowest).
Table S6. Gastrointestinal adverse events leading to treatment withdrawal.


