Abstract
Approximately one in three persons will develop herpes zoster during their lifetime, and it can lead to serious complications such as postherpetic neuralgia. However, evidence on burden of herpes zoster and postherpetic neuralgia in Japan is limited. This prospective, observational, multicenter, physician practice‐based cohort study was conducted in Kushiro, Hokkaido, Japan (Clinicaltrials.gov identifier NCT01873365) to assess the incidence and hospitalization rates of herpes zoster, and the proportion, clinical burden and risk factors for postherpetic neuralgia in adults aged 60 years or more. Within the study area, 800 subjects developed herpes zoster and 412 were eligible for the study. Herpes zoster incidence was 10.2/1000 person‐years and higher among women and older subjects. Subjects with herpes zoster required on average 5.7 outpatient consultations. Herpes zoster‐associated hospitalization rate was 3.4% (27/800). The proportion of postherpetic neuralgia and other complications was 9.2% (38/412) and 26.5% (109/412), respectively. Statistically significant association with the development of postherpetic neuralgia was male sex (odds ratio [OR], 2.51; 95% confidence interval [CI], 1.17–5.38), age of 70–74 years (OR, 3.51; 95% CI, 1.09–11.3), immunosuppressive therapy (OR, 6.44; 95% CI, 1.26–32.9), severe herpes zoster pain at first consultation (OR, 3.08; 95% CI, 1.10–8.62) and rash on upper arms (vs no rash on upper arms; OR, 3.46; 95% CI, 1.10–10.9). Considerable herpes zoster and postherpetic neuralgia burden exists among elderly in Japan, and there may be predictive factors at the first visit which could be indicative of the risk of developing postherpetic neuralgia.
Keywords: burden of disease, epidemiology, herpes zoster, incidence, postherpetic neuralgia
Introduction
Herpes zoster (HZ) occurs when Varicella zoster virus reactivates from the sensory ganglia during waning of cell‐mediated immunity below a critical level.1 Over 95% of individuals aged 50 years or older have been infected with Varicella zoster virus and are, therefore, at risk of developing HZ.2, 3 The lifetime risk of developing HZ is between 25% and 30%, rising to 50% in those who reach 80 years of age.4, 5, 6 In a significant proportion of HZ patients, the most common complication is postherpetic neuralgia (PHN) that can occur months to years after onset of rash. HZ, and especially PHN, may significantly impact patients' quality of daily life and impose a considerable economic burden on the payers and the society.7, 8, 9, 10
Japan has the highest proportion of elderly people in the world; 32% of its population was 60 years or older in 2012, and this proportion is expected to increase to 41% by 2050,11 causing an increase in overall absolute number of HZ in this population, and therefore leading to substantial burden on the health‐care system and society. A recent publication described the incidence of HZ (10.9/1000 person‐years) and PHN (2.1/1000 person‐years) in the Japanese population aged 50 years or older.12 Clinical characteristics, risk factors, health‐resource utilization and quality of life of the subjects with HZ and PHN have, to date, not been studied in detail in Japan.
In this prospective observational cohort study in Japan, we assessed the incidence and clinical characteristics of HZ and the proportion of PHN in subjects aged 60 years or older. In addition, this study assessed resource utilization due to HZ as well as the quality of life of affected subjects, but these topics will be reported elsewhere.
Methods
Study design
This was a prospective, observational, multicenter, physician practice‐based cohort study in adults aged 60 years or older conducted by eight dermatologists and six internists in 14 centers in Kushiro, Hokkaido, Japan, between 29 June 2013 and 4 February 2015. The study is registered at ClinicalTrials.gov (NCT01873365).
The subjects were included into the prospective part of the study if they: (i) were aged 60 years or older; (ii) were diagnosed with HZ by the physician at the study participating sites at the initial or at the second consultation occurring within 7 days of the initial consultation; (iii) were capable of completing the required quality of life questionnaires at predefined time points; and (iv) provided signed informed consent.
Herpes zoster severity was assessed using a pain intensity scale (item 3 of the Zoster Brief Pain Inventory [ZBPI] questionnaire)7 by dividing the scale into three categories: (i) no or mild pain (0 ≤ pain < 3); (ii) moderate pain (3 ≤ pain < 7); or (iii) severe pain (7 ≤ pain ≤ 10).
Clinically relevant PHN was defined by the presence of HZ‐associated moderate to severe worst pain (rated as ≥3 in response to item 3 of the ZBPI questionnaire7) persisting or appearing more than 90 days after onset of the HZ rash. The duration of the study for each patient was at most 270 days (with ±7 days time window) after the initial visit, and it included follow up of subjects with HZ between initial visit and day 90 and subjects with PHN between days 90 and 270 after the initial visit.
This study was conducted in accordance with the Ethical Guidelines for Epidemiological Research, the Ethical Guidelines for Clinical Studies and applicable subject privacy requirements, and guideline principles of the Declaration of Helsinki, and was reviewed by an Institutional Review Board/Independent Ethics Committee. Written informed consent was obtained from all enrolled participants.
Study population
Eight hundred subjects aged 60 years or older developed HZ in the study area. Among them, 772 (96.5%) and 28 (3.5%) visited a dermatologist or internal medicine specialist, respectively. Residents of Kushiro city comprised 666 (83.3%) subjects and 134 (16.7%) subjects lived outside Kushiro city. A total of 448 (56%) subjects with HZ and who visited one of the study dermatology practices were enrolled in the prospective part of the study, 412 (91.9%) of whom were eligible for the study analysis and 36 (8.1%) excluded from the study due to either non‐compliance with the study inclusion criteria or violation of the informed consent process (Fig. 1).
Figure 1.
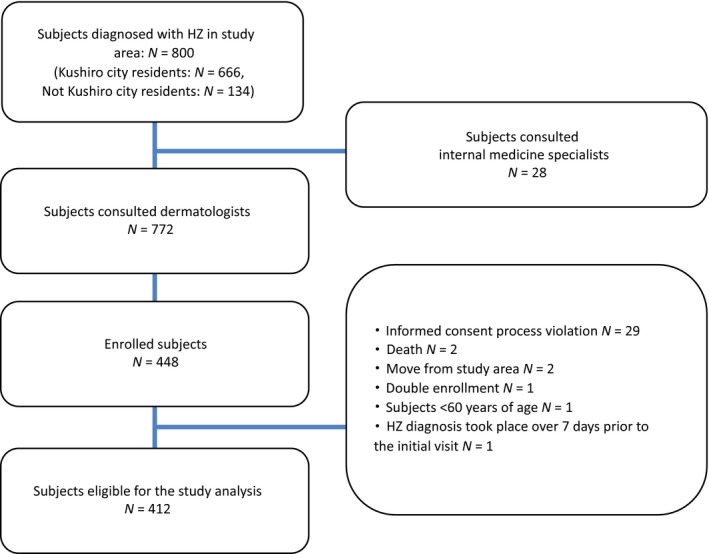
Subject flowchart with reasons for exclusion and number of patients followed at each time point. N, number of subjects.
Data collection
Aggregated data (number of subjects with HZ in each sex and age group, number of hospitalizations and outpatient visits) were collected on 800 subjects diagnosed with HZ in the study area. These aggregated data have been used to estimate HZ incidence and the proportion of hospitalization among subjects with HZ. Individual data (demographic, clinical and health‐resource utilization) on 412 subjects with HZ enrolled into the prospective part of the study were also collected at each subject's visit by the study investigator. In addition, data on quality of life of the subjects with HZ and PHN were collected from questionnaires completed by the subjects at the following time points: days 0, 15, 30, 60, 90, 120, 150, 180, 210, 240 and 270. The following questionnaires were included for the completion: (i) ZBPI questionnaire for the estimation of pain severity and persistence; (ii) Short‐Form Health Survey for the assessment of health status; (iii) EuroQol 5‐Dimension questionnaire for estimation of utility; and (iv) Short‐Form McGill Pain Questionnaire for collection of type of pain and intensity. Details of the questionnaires as well as the results of the questionnaire survey will be discussed elsewhere. Briefly, ZBPI questionnaire was used to quantify HZ pain and discomfort and measure selected activities of daily living and health. This questionnaire also has been used for the PHN case definition. The ZBPI was adapted from the Brief Pain Inventory to make it a HZ‐specific measure of pain severity that captures pain and discomfort (including allodynia and pruritus) caused by HZ.7 It uses an 11‐point Likert scale (0–10) to rate HZ pain and discomfort for four dimensions: worst, least and average during the past 24 h and now. The follow up of the patients rating pain as more than 1 in answer to item 3 of the ZBPI questionnaire on days 90 or 180 were completed accordingly at one of those dates. The follow up of the patients rating pain as 1 or more in answer to item 3 of the ZBPI questionnaire at days 90 or 180 was extended to days 120, 150 and 180, and 210, 240 and 270, respectively. Patients were asked to fill in the questionnaires and return them within 10 days.
Sample size
Sample size was estimated based on HZ incidence of a study conducted in Miyazaki prefecture by Toyama et al.13 For a total population of 180 000 at Kushiro city of which 64 880 people were aged 60 years or older, the number of eligible HZ cases expected during a 1‐year period was approximately 469. Assuming that 70% of these patients agreed to participate in this study, 328 subjects were expected to be enrolled. Among these subjects, approximately 30 were expected to develop PHN 90 days after the onset of HZ.
Statistical analyses
Analyses were conducted in several study populations. The incidence of HZ and the proportion of HZ hospitalizations were estimated based on 800 subjects with HZ for whom data were available in the aggregated format. The rest of the study end‐points were estimated on 412 subjects for whom individual demographic and clinical data was available. In case subjects had missing information at certain time point of observation, they were only excluded from the analysis for this specific time point.
The incidence was calculated as the number of HZ episodes per 1000 person‐years by dividing the total number of subjects with HZ diagnosed in the study area by the total number of adults aged 60 years or older residing in the study area during the recruitment period (obtained from the Kushiro city government records). The incidence rate of HZ cases was calculated by age group, sex and overall, and it was then tabulated with exact Poisson 95% confidence interval (CI).
The proportion of patients with any pain persisting or appearing after the onset of HZ was assessed with a 95% CI by age group and sex at days 90, 180 and 270. The proportion of PHN cases among patients who completed the questionnaire at days 90, 180 and 270 was also calculated. The proportion of HZ‐associated hospitalizations among the total number of HZ cases was tabulated with 95% CI overall, by age group and by sex. The medical history of patients with HZ and PHN and the characteristics of HZ and PHN episodes (severity, symptoms, onset date and presence of complications) were described.
Univariate and multivariate logistic regression analyses were conducted to describe the association between PHN occurrence and independent variables (age, sex, current immunosuppressive therapy, initial HZ pain severity, number of locations of rash and location of rash) in order to identify potential risk factors. Analysis was conducted among the subjects who had completed the questionnaire at day 90. Adjusted and unadjusted odds ratios (OR) were calculated for the potential risk factors.
All statistical analyses were performed using the SAS Drug and Development web portal and Microsoft Excel (Microsoft, Redmond, WA, USA).
Results
The mean age of the 412 subjects was 71.7 years (standard deviation, ±7.5). Women accounted for 60.2% (248/412) of the subjects. A large percentage of patients, 71.8% (296/412) were already under treatment for another disease at the time of enrollment. Other subject characteristics are described in Table 1.
Table 1.
Baseline characteristics of subjects with HZ
| Total (n = 412) | PHN cases (n = 38) | |
|---|---|---|
| n (%) | n (%) | |
| Age stratification (age at rash onset) | ||
| Mean age ± SD, years | 71.7 ± 7.5 | 68.4 ± 6.8 |
| 60–64 | 92 (22.3) | 5 (13.2) |
| 65–69 | 85 (20.6) | 7 (18.4) |
| 70–74 | 89 (21.6) | 14 (36.8) |
| 75–79 | 74 (18.0) | 6 (15.8) |
| ≥80 | 72 (17.5) | 6 (15.8) |
| Sex | ||
| Male | 164 (39.8) | 17 (44.7) |
| Female | 248 (60.2) | 21 (55.3) |
| Residence | ||
| Kushiro city | 352 (85.4) | 29 (76.3) |
| Outside Kushiro city | 60 (14.6) | 9 (23.7) |
| Severity at initial visit | ||
| No or mild pain | 137 (33.3) | 7 (18.4) |
| Moderate pain | 160 (38.8) | 13 (34.2) |
| Severe pain | 109 (26.5) | 16 (42.1) |
| Missing | 6 (1.5) | 2 (0.5) |
| Underlying diseasesa | ||
| Total with disease under treatment | 296 (71.8) | 29 (76.3) |
| Diabetes mellitus | 61 (20.6) | 4 (10.5) |
| Liver disease | 4 (1.4) | 0 (0) |
| Renal failure/dialysis | 9 (3.0) | 1 (2.6) |
| Current emotional problems: stress or depression | 2 (0.7) | 1 (2.6) |
| Blood malignancy | 2 (0.7) | 0 (0) |
| Solid malignancy | 5 (1.7) | 1 (2.6) |
| Autoimmune disease active and under therapy | 7 (2.4) | 1 (2.6) |
| Other | 246 (83.1) | 25 (65.8) |
| Immunosuppressive medication | ||
| Yes | 13 (3.2) | 3 (7.9) |
| Oral or parenteral corticosteroids | 8 (2.0) | 1 (2.6) |
| Cytostatic/chemotherapy treatment | 3 (0.7) | 1 (2.6) |
| Other | 2 (0.5) | 1 (2.6) |
n refer to number of subjects
Some subjects had more than one underlying disease. HZ, herpes zoster; PHN, postherpetic neuralgia; SD, standard deviation.
An overall HZ incidence of 10.2/1000 person‐years (95% CI, 9.4–11.0) was estimated among the Kushiro population aged 60 years and older (Table 2). The incidence rate of HZ was higher for the 80 years or older age group (12.1/1000 person‐years; 95% CI, 10.3–14.1) than for the other age groups. The incidence rate was also higher among women (11.6/1000 person‐years; 95% CI, 10.5–12.7) than men (8.4/1000 person‐years; 95% CI, 7.3–9.5).
Table 2.
Sex‐ and age‐specific incidence rates of HZ per 1000 person‐years
| Age (years) | Men | Women | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Casesa | Person‐years | Incidence rate (per 1000 person‐years) (95% CI) | Casesa | Person‐years | Incidence rate (per 1000 person‐years) (95% CI) | Casesa | Person‐years | Incidence rate (per 1000 person‐years) (95% CI) | |
| 60–69 | 106 | 13,779.4 | 7.7 (6.3–9.3) | 167 | 15,898.2 | 10.5 (9.0–12.2) | 273 | 29,677.5 | 9.2 (8.2–10.4) |
| 70–79 | 79 | 9704.2 | 8.2 (6.5–10.2) | 152 | 12,533.5 | 12.1 (10.3–14.2) | 231 | 22,237.7 | 10.4 (9.1–11.8) |
| ≥80 | 51 | 4730.9 | 10.8 (8.0–14.2) | 111 | 8697.6 | 12.8 (10.5–15.4) | 162 | 13,428.5 | 12.1 (10.3–14.1) |
| Total | 236 | 28,214.5 | 8.4 (7.3–9.5) | 430 | 37,129.3 | 11.6 (10.5–12.7) | 666 | 65,343.8 | 10.2 (9.4–11.0) |
Number includes the number of HZ cases from the aggregated logbook. 95% CI, exact Poisson confidence interval; HZ, herpes zoster.
The mean delay between the date of the subject's HZ rash onset and the date of their first visit to the doctor was 4.4 days (range, 0–26) with 8% (33/412) of the subjects visiting the doctor 10 days or more after rash onset. There were no differences in mean delays between different age groups slightly varying from 4.3 days in the age groups 60–64 and 70–79 years to 4.7 days in the age groups 65–69 and 80 years or older.
There was a mean of 5.7 visits (median, 4; range, 1–94) for treatment of HZ/PHN per subject, and it increased with both advanced age and severity of pain (Table 3).
Table 3.
Number of visits by age group, HZ severity and location of rash
| n | Mean ± SD | Median (range) | |
|---|---|---|---|
| All | 412 | 5.7 ± 7.7 | 4 (1–94) |
| Age, years | |||
| 60–64 | 92 | 4.8 ± 5.3 | 4 (1–45) |
| 65–69 | 85 | 4.8 ± 3.6 | 4 (1–18) |
| 70–74 | 89 | 5.6 ± 3.8 | 5 (1–17) |
| 75–79 | 74 | 6.8 ± 11.4 | 4 (1–94) |
| ≥80 | 72 | 7.1 ± 11.7 | 4 (1–82) |
| Severity of HZ at initial visit | |||
| No or mild pain | 137 | 4.4 ± 5.0 | 3 (1–55) |
| Moderate pain | 160 | 6.0 ± 8.4 | 4 (1–94) |
| Severe pain | 109 | 7.0 ± 9.2 | 5 (1–82) |
| Location of rash | |||
| Head | 75 | 6.6 ± 11.0 | 4 (1–94) |
| Neck | 33 | 5.8 ± 5.0 | 4 (1–27) |
| Upper back | 119 | 6.0 ± 6.6 | 4 (1–55) |
| Lower back | 84 | 4.8 ± 2.9 | 4 (1–18) |
| Upper arms | 43 | 7.7 ± 13.0 | 4 (1–82) |
| Lower arms | 22 | 6.6 ± 6.9 | 5 (1–34) |
| Hands | 10 | 14.7 ± 25.5 | 4 (2–82) |
| Chest | 121 | 6.3 ± 6.6 | 4 (1–55) |
| Abdomen | 88 | 5.2 ± 5.2 | 4 (1–45) |
| Upper legs | 59 | 6.2 ± 6.1 | 5 (1–45) |
| Knees | 9 | 4.4 ± 3.0 | 3 (1–11) |
| Lower legs | 22 | 4.6 ± 2.3 | 4 (1–11) |
| Eye | 10 | 12.8 ± 28.7 | 3 (1–94) |
| Ear | 4 | 6.0 ± 5.4 | 3.5 (3–14) |
There was a total of 412 subjects. HZ, herpes zoster; SD, standard deviation.
n refers to number of subjects
A total of 3.4% (27/800) of the subjects required hospitalization for HZ: 11 subjects among those aged 60–69 years, 10 aged 70–79 years and six aged 80 years or older groups. The proportion of hospitalization was higher among men (4.8%) than women (2.7%).
Prodromal symptoms and complications were experienced by 77.2% (318/412) of the subjects before rash onset: there was higher prodromal pain (71.1% [27/38] vs 68.2% [255/374]), fever (10.5% [4/38] vs 4.6% [17/374]), malaise (21.1% [8/38] vs 12.6% [47/374]) and other symptoms (7.9% [3/38] vs 9.1% [34/374]) among subjects with PHN than subjects without PHN, respectively. At the initial visit, subjects reported five cutaneous, six ocular, 20 neurological and two other types of complications. During the study at any subject's visit, cutaneous complications were reported by 21.1% (8/38) and 3.5% (13/374), ocular complications by 5.3% (2/38) and 1.1% (4/374), neurological complications by 68.4% (26/38) and 20.9% (78/374), and other complications by 10.5% (4/38) and 2.9% (11/374) of subjects with and without PHN, respectively.
A total of 65% of the subjects reported moderate to severe pain associated with the onset of HZ at the initial visit, and this proportion of subjects steadily decreased to 9.2% at day 90 (Fig. 2), corresponding to the proportion of PHN. Nearly all patients reported some pain at the initial visit, but by day 90, the total percentage reporting any pain declined to 20%. Subjects aged 70–74 years experienced more moderate to severe pain at any time point than the other age groups.
Figure 2.
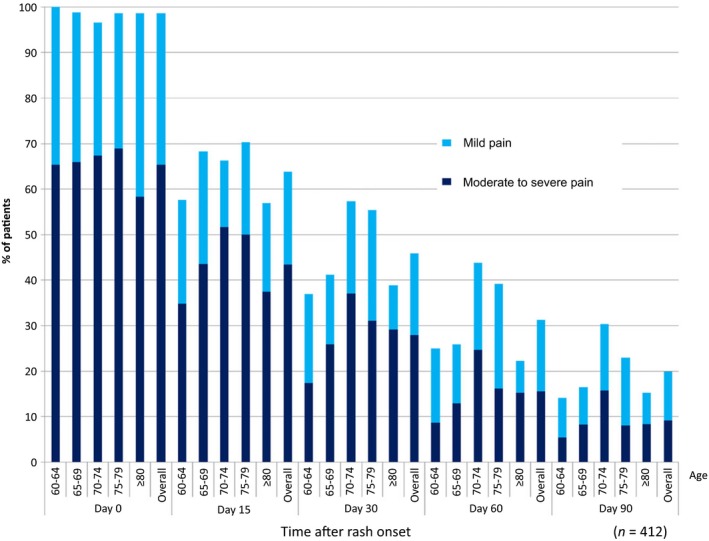
Proportion of HZ subjects who had HZ‐related pain from onset of rash after 90 days (HZ‐associated worst pain intensity in the last 24 h). HZ, herpes zoster; n, number of subjects.
Among the 412 subjects, there were 38 (9.2%; 95% CI, 6.6–12.4) who presented with PHN 90 or more days after rash onset. This proportion was slightly higher when assessed among subjects who completed a questionnaire more than 90 days after rash onset (70%, 287/412) and accounted for 13.2% (38/287). Women tend to developed less PHN (6.9%; 95% CI, 4.0–10.7) than men (12.8%; 95% CI, 8.1–18.9), and a higher number of PHN (14 cases, 15.7%; 95% CI, 8.9–25.0) were diagnosed in the 70–74 years age group than in the other age groups. Among 38 PHN subjects, 39.5% reported moderate to severe pain more than 180 days and 10.5% reported moderate to severe pain 270 days after rash onset. Approximately 20% (95% CI, 16.1–24.1) of the subjects presented with any HZ‐related pain 90 days after rash onset (Table 4).
Table 4.
Proportion of subjects with PHN or any pain among 412 subjects with HZ by sex and age group
| Sex | Age at rash onset, years | n | PHN (moderate/severe pain) | Any pain | ||
|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | |||
| Male | 60–64 | 40 | 2 | 5.0 (0.6–16.9) | 7 | 17.5 (7.3–32.8) |
| 65–69 | 34 | 5 | 14.7 (5–31.1) | 8 | 23.5 (10.8–41.2) | |
| 70–74 | 35 | 9 | 25.7 (12.5–43.3) | 11 | 31.4 (16.9–49.3) | |
| 75–79 | 30 | 2 | 6.7 (0.8–22.0) | 9 | 30.0 (14.7–49.4) | |
| ≥80 | 25 | 3 | 12.0 (2.6–31.2) | 4 | 16.0 (4.5–36.1) | |
| All males | 164 | 21 | 12.8 (8.1–18.9) | 39 | 23.8 (17.5–31.0) | |
| Female | 60–64 | 52 | 3 | 5.8 (1.2–16) | 6 | 11.5 (4.4–2.4) |
| 65–69 | 51 | 2 | 3.9 (0.5–13.5) | 6 | 11.8 (4.4–23.9) | |
| 70–74 | 54 | 5 | 9.3 (3.1–20.3) | 16 | 29.6 (18.0–43.6) | |
| 75–79 | 44 | 4 | 9.1 (2.5–21.7) | 8 | 18.2 (8.2–32.7) | |
| ≥80 | 47 | 3 | 6.4 (1.3–17.5) | 7 | 14.9 (6.2–28.3) | |
| All females | 248 | 17 | 6.9 (4.0–10.8) | 43 | 17.3 (12.8–22.6) | |
n refers to number of subjects
95% CI, exact confidence interval; HZ, herpes zoster; PHN, postherpetic neuralgia.
Male sex (OR, 2.51; 95% CI, 1.17–5.38), age group of 70–74 years (OR, 3.51; 95% CI, 1.10–11.4), concomitant immunosuppressive therapy (OR, 6.44; 95% CI, 1.26–32.9), severe HZ pain at first consultation (vs no or mild pain; OR, 3.08; 95% CI, 1.10–8.62) and rash on upper arms (vs no rash on upper arms; OR, 3.46; 95% CI, 1.10–10.9) were statistically significantly associated with the development of PHN (Table 5).
Table 5.
Risk factors for PHN among the HZ subjects who completed day 90 questionnaire (N = 282)
| Variable | Category | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| Odds ratios | (LL, UL) | P‐value | Odds ratios | (LL, UL) | P‐value | ||
| Sex | Male | 2.00 | (1.01, 3.99) | 0.05 | 2.51 | (1.17, 5.38) | 0.02 |
| Female | (ref) | (ref) | |||||
| Age group (age) | ≥80 | 1.65 | (0.47, 5.79) | 0.43 | 2.54 | (0.68, 9.57) | 0.17 |
| 75–79 | 1.40 | (0.40, 4.90) | 0.59 | 1.41 | (0.38, 5.17) | 0.61 | |
| 70–74 | 3.14 | (1.06, 9.36) | 0.04 | 3.51 | (1.09, 11.3) | 0.04 | |
| 65–69 | 1.33 | (0.40, 4.43) | 0.65 | 1.11 | (0.30, 4.12) | 0.88 | |
| 60–64 | (ref) | (ref) | |||||
| Current immunosuppressive therapy | Yes | 3.46 | (0.83, 14.46) | 0.09 | 6.44 | (1.26, 32.9) | 0.03 |
| No | (ref) | (ref) | |||||
| HZ severity at first consultationa | Severe pain | 2.78 | (1.08, 7.15) | 0.03 | 3.08 | (1.10, 8.62) | 0.03 |
| Moderate pain | 1.66 | (0.63, 4.36) | 0.30 | 1.88 | (0.67, 5.32) | 0.23 | |
| No or mild pain | (ref) | (ref) | |||||
| Number of location of rash | ≥3 | 2.04 | (0.76, 5.42) | 0.16 | 0.86 | (0.23, 3.18) | 0.82 |
| 1 or 2 | (ref) | (ref) | |||||
| Location of rash | Upper Arms | 3.05 | (1.29, 7.22) | 0.01 | 3.46 | (1.10, 10.9) | 0.03 |
| No rash on upper arms | (ref) | – | (ref) | ||||
(ref): reference. N = 282
HZ, Herpes Zoster; LL, lower limit; UL, upper limit; PHN, post‐herpetic neuralgia.
Severity is defined by the pain as per ZBPI questionnaire at the time of initial visit.
Discussion
This is the first study to assess the burden of HZ and PHN including incidence and clinical characteristics of HZ, proportion and risk factors of PHN, severity of pain related to HZ and PHN, and health‐resource utilization in Japanese adults aged 60 years or older.
Our study confirmed that approximately 1 in 100 subjects aged 60 years or older had HZ with higher incidence in women and older age groups. Approximately 10% of the subjects with HZ developed PHN 90 days or more after rash onset requiring long term anti‐pain treatment. On average, subjects consulted a doctor approximately six times per HZ episode and more than 3% of them had been hospitalized. There may be also some predictive clinical factors for PHN development such as rash location, immunosuppressive therapy and severity of the HZ pain at the initial visit to the doctor, which the doctor should look at when consulting subjects presenting with HZ.
The HZ incidence rate reported here is consistent with recently published data from a prospective Japanese study (the SHEZ study).12 Similar values for HZ incidence were found in both our and the SHEZ studies, respectively: 9.2/1000 versus 9.6/1000 person‐years for the 60–69 years age group, 10.4/1000 versus 12.9/1000 person‐years for the 70–79 years age group and 12.1/1000 versus 12.6/1000 person‐years for the 80 years or older age group. HZ incidence in our study is also consistent with incidence found in the Miyazaki study, a 10‐year survey conducted in Japan between 1997 and 2006.13 However, the study in Miyazaki tends to underestimate the incidence of HZ in 80 years or older age group that could be due to the specificity of their study setting related to limitations in accruing a sufficient number of elderly subjects. In contrary, our and the SHEZ studies observed an increase in HZ incidence with age that could be associated with a decrease in the cell‐mediated immune response against Varicella zoster virus,12, 14 but additional studies are needed to confirm this hypothesis. Both our and the SHEZ studies estimated a higher incidence of HZ among the female population, with consistently higher values across all age groups, and we note that a tendency of increased HZ incidence rates in women in Europe was also highlighted in a systematic review by Pinchinat et al.15
There are limited data on HZ‐related hospitalizations in Japan, but a recent systematic review summarized results of studies conducted in North America and parts of the European Union.16 Although these results may not be entirely comparable with ours due to many factors, such as health‐care systems and study settings, considerably higher hospitalization rates were also found in the elderly population, especially in the 80 years or older age group. The estimated rate in the current study (3.4%) is in agreement with rates ranging 1–3% that were reported in Germany,17 the USA18 and Australia.19 We do note that a recent study conducted in Taiwan among 150 subjects aged 50 years or older reported a 20.7% rate of hospitalization, but the initial severity of HZ‐associated pain was higher in this study where 98% of the subjects experienced HZ‐associated pain at enrollment,20 whereas in our study 33% of the subjects experienced no or mild pain at enrollment.
Studies assessing PHN and associated risk factors are difficult to compare, because they are not uniform in the definition of PHN and design, and so the reported proportion of PHN cases in HZ patients can vary considerably.16 In the present study, PHN was defined as moderate or severe pain persisting or appearing at 90 days after HZ onset. A proportion of 9.2% among all eligible HZ patients and 13.2% of patients who completed questionnaires reported PHN‐related pain at day 90. The latter value may overestimate PHN proportion, because patients experiencing pain may be more inclined to complete the questionnaires. However, these proportions are in line with previously reported data from a population‐based study conducted in the USA in adults. In that study, 10% of the study participants reported pain at 90 days after HZ diagnosis, and the duration and intensity of the pain was found to increase with age.18 In a retrospective study encompassing 25 002 HZ patients aged 50 years or older in the UK, Gauthier et al.21 reported that 13.7% of patients developed PHN (defined as pain persisting up to 3 months), and they observed similar PHN proportions among men and women. Gialloreti et al.22 estimated the proportion of HZ cases developing into PHN at 7.7% for Italian patients aged 50 years or older and concluded that PHN was more common in women than in men. In the SHEZ study,12 a definition similar to that of Gauthier et al. for PHN was employed to estimate an overall proportion of 20.83% among HZ patients aged 60 years or older and similar PHN proportions were found for men and women, although these varied considerably with the age groups. For instance, in patients aged 60–69 years, 19.4% of men and only 10.8% of women developed PHN, while in the 70–79 years age group, the proportions of PHN cases were 12.5% and 24.7% in men and women, respectively.12 The present study observed that the proportion of men developing PHN was significantly higher than that of women. Because the study design or definition of PHN vary across studies, these contradictory results may warrant further investigation or could be due to specificity of the study setting.
Among the risk factors of PHN, older age, severity of acute pain and severity of rash are the most frequently cited in the published work.3, 23 In the present study, increasing age was not a predictor for PHN within the targeted age groups, while male sex was significantly associated with higher risk of developing PHN among adults aged 60 years or older. The reason that males exhibited a higher proportion of PHN is unclear. In this study, rash on upper arms was identified as a risk factor for developing PHN. The brachial plexus is a dense nerve system in the upper arm; therefore, the subjects with rash on upper arms may have persisting moderate to severe pain. We observed that rashes located on the upper arms were often connected with rashes located on the upper back and sometimes with rashes located on the lower arms. A greater number of different rash locations may be indicative of the severity of HZ, and as such be associated with a higher proportion of PHN; however, the number of rash locations itself did not show a significant risk for developing PHN in this study. We also observed that the severity of HZ pain at the initial visit to the doctor is positively associated with the risk of PHN; it seems that the higher magnitude of pain presented at the initial visit may have tendency to stay the same at day 90 or later after rash onset, whereas other subjects with less severe pain do recover from HZ‐related pain by that time. More detailed assessment with other variables will be needed in the future studies.
A recent pooled analysis assessing PHN predictors from prospective cohort studies conducted in North and Latin America and Asia among HZ patients older than 50 years found no sex difference with respect to the PHN incidence rates.23 Concomitant immunosuppressive therapy and pain severity at HZ onset were also associated with higher risk of developing PHN in our study, and this is consistent with prior findings.24, 25
We observed an average of 5.7 visits to the doctor per HZ episode. This represents a significant burden not only for the patients themselves but also for their family and health‐care system. This is particularly a burden for elderly patients. The current study also showed that 10% of patients visited the doctor 10 days or more after rash onset. A delay in the start of antiviral therapy may cause an increase in the severity of HZ, however, at the same time more severe subjects may tend to come to the doctor earlier for HZ treatment. Given the aging society in Japan and a potential increase in absolute number of HZ cases in the Japanese elderly population as its consequence, prevention activities or raising awareness for the need to seek prompt consultation after rash onset will be needed.
The current study has strengths and limitations that could potentially affect the robustness of the results. The major strength of this study is its prospective study design with data collection from all medical specialists (internal medicine specialists and dermatologists) who see subjects with HZ in real‐life settings.13 In addition, PHN case definition was based on the validated ZBPI questionnaire which accounted for various degrees of pain intensity.7 The study also has several limitations. First, although almost all dermatologists in Kushiro city and five sites outside of Kushiro city participated in this study, incidence of HZ might have been underestimated, because some subjects with HZ might have visited to other clinics outside of this study. Second, only patients who provided signed informed consent were included in the study for most of the outcomes, and that population represented only 62% of all HZ diagnosed in the study area. That may cause certain biases as it could be that this population could be slightly different from one which was not a part of our prospective study. Third, if the subjects who had severe pain tend to continue follow up in this study, the proportion of PHN may be also biased.
In conclusion, our study confirmed that approximately 1 in 100 subjects aged 60 years or older had HZ with higher incidence in women and older age groups. Approximately 10% of subjects with HZ developed PHN 90 days or more after rash onset requiring long‐term anti‐pain treatment. On average, subjects consulted doctors approximately six times per HZ episode and more than 3% of them were hospitalized. There may be also some predictive clinical factors for PHN development such as rash location, immunosuppressive therapy and severity of the HZ pain at the initial visit to the doctor, which the doctor should look at when consulting subjects presenting with HZ.
In Japan's aging society, the burden of HZ will increase and, therefore, appropriate treatment and prevention of HZ will become increasingly important in the future.
Conflict of Interest
K. Adachi and K. Asano received a study grant from the GSK group of companies, personal fees for medical advice during the conduct of the study and personal fees for lecturing from the GSK group of companies outside the submitted work. R. A. and M. K. reported a study grant and personal fees for medical advice from the GSK group of companies during the conduct of the study. H. N. reported a study grant, personal fees for medical advice and travel support to a congress presentation from the GSK group of companies during the conduct of the study. K. K. reported a study grant from the GSK group of companies during the conduct of the study. A. W. reported a study grant from GSK during the conduct of the study and personal fees for lecturing from the GSK group of companies outside the submitted work. K. S., T. K. and T. M. received funding from Japan Vaccine Co., Ltd. (a 50%/50% Joint Venture of GSK/Daiichi Sankyo Company, Ltd.) for the conduct of the study. K. H., K. S. and T. K. are employees of the GSK group of companies and hold stock options or restricted shares in the GSK group of companies as part of his/her employee remuneration. T. M. and K. G. are employees of the GSK group of companies.
Acknowledgments
The authors thank Satoru Takaya, Keizo Nakata, Shigeharu Sugimoto, Naoki Yamamoto, Hideyuki Harada, Takuto Miyagishima (Investigator Internal Medicine Specialist), Yasuhiro Ito (Investigator Dermatologist), Kenji Iida (Investigator Dermatologist), Yoriko Morioka (Japan Vaccines Corporation employee), Karolien Peeters (Study Delivery Lead, GSK Vaccines), Anand Kumar (Statistician, GSK), Kanhaiyalal Chauhan (Data manager, GSK), Akiko Mizukami (GSK K.K.), Emad Yanni (GSK Vaccines), Desmond Curran (GSK Vaccines) and Sean Matthews (Freelance Consultant). The authors would like to thank the Business and Decision Life Sciences platform (on behalf of GSK Vaccines) for editorial assistance and manuscript coordination. Gregory Collet coordinated manuscript development and editorial support and John Bean (Bean Medical Writing) provided medical writing services. GlaxoSmithKline Biologicals SA was the funding source and was involved in all study activities and overall data management (collection, analysis and interpretation). GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and the corresponding author was responsible for submission of the publication. Author contributions are as follows: K. Adachi, K. H., K. T., K. S. participated to the conception and the design of the study; K. Adachi., R. A., K. Asano, K. G., K. H., T. K., M. K., K. K., T. M., H. N., K. S. and A. W. participated in the collection or generation of the study data; K. Adachi, R. A., K. Asano, K. G., K. H., T. K., M. K., K. K., T. M., H. N., K. S. and A. W. performed the study; K. G., K. H., T. K. and K. S. contributed to the material; and K. G., K. H., T. K. and S. K. were involved in the analysis or interpretation of the data.
References
- 1. Arvin A, Gershon A. Varicella‐Zoster Virus – Virology and Clinical Management, 1st edn Cambridge: Cambridge University Press, 2000. [Google Scholar]
- 2. Kurokawa I, Murakawa K, Kumano K. The change in zoster‐associated pain treated with oral valaciclovir in immunocompetent patients with acute herpes zoster. Int J Clin Pract 2007; 61: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 3. Tontodonati M, Ursini T, Polilli E et al Post‐herpetic neuralgia. Int J Gen Med 2012; 5: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Studahl M, Petzold M, Cassel T. Disease burden of herpes zoster in Sweden – predominance in the elderly and in women – a register based study. BMC Infect Dis 2013; 13(1): 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology 2013; 81: 928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S‐Y, Suaya JA, Li Q et al Incidence of herpes zoster in patients with altered immune function. Infection 2014; 42: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coplan PM, Schmader K, Nikas A et al Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain 2004; 5: 344–356. [DOI] [PubMed] [Google Scholar]
- 8. Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health‐related quality of life. Clin Infect Dis 2004; 39: 342–348. [DOI] [PubMed] [Google Scholar]
- 9. Drolet M, Brisson M, Schmader KE et al The impact of herpes zoster and postherpetic neuralgia on health‐related quality of life: A prospective study . CMAJ 2010; 182: 1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volpi A, Gatti A, Pica F, Bellino S, Marsella LT, Sabato AF. Clinical and psychosocial correlates of post‐herpetic neuralgia. J Med Virol 2008; 80: 1646–1652. [DOI] [PubMed] [Google Scholar]
- 11. World Population Ageing and Development , 2012. [Accessed 9 Sept 2016.] Available from URL: http://www.un.org/esa/population/publications/2012PopAgeingDev_Chart/2012PopAgeingandDev_WallChart.pdf
- 12. Takao Y, Miyazaki Y, Okeda M et al Incidences of herpes zoster and postherpetic neuralgia in Japanese adults aged 50 years and older from a community‐based prospective cohort study: the SHEZ study. J Epidemiol 2015; 25: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toyama N, Shiraki K. Society of the Miyazaki Prefecture Dermatologists. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10‐year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol 2009; 81: 2053–2058. [DOI] [PubMed] [Google Scholar]
- 14. Levin MJ, Smith JG, Kaufhold RM et al Decline in varicella‐zoster virus (VZV)‐specific cell‐mediated immunity with increasing age and boosting with a high‐dose VZV vaccine. J Infect Dis 2003; 188: 1336–1344. [DOI] [PubMed] [Google Scholar]
- 15. Pinchinat S, Cebrián‐Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis 2013; 13: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4: e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ultsch B, Köster I, Reinhold T et al Epidemiology and cost of herpes zoster and postherpetic neuralgia in Germany. Eur J Health Econ 2013; 14: 1015–1026. [DOI] [PubMed] [Google Scholar]
- 18. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population‐based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82: 1341–1349. [DOI] [PubMed] [Google Scholar]
- 19. Stein AN, Britt H, Harrison C, Conway EL, Cunningham A, Macintyre CR. Herpes zoster burden of illness and health care resource utilisation in the Australian population aged 50 years and older. Vaccine 2009; 27: 520–529. [DOI] [PubMed] [Google Scholar]
- 20. Tsai T‐F, Yao C‐A, Yu H‐S et al Herpes zoster‐associated severity and duration of pain, health‐related quality of life, and healthcare utilization in Taiwan: a prospective observational study. Int J Dermatol 2015; 54: 529–536. [DOI] [PubMed] [Google Scholar]
- 21. Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post‐herpetic neuralgia in the United Kingdom. Epidemiol Infect 2009; 137(1): 38–47. [DOI] [PubMed] [Google Scholar]
- 22. Gialloreti LE, Merito M, Pezzotti P et al Epidemiology and economic burden of herpes zoster and post‐herpetic neuralgia in Italy: a retrospective, population‐based study. BMC Infect Dis 2010; 10: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawai K, Rampakakis E, Tsai T‐F et al Predictors of postherpetic neuralgia in patients with herpes zoster: a pooled analysis of prospective cohort studies from North and Latin America and Asia. Int J Infect Dis 2015; 34: 126–131. [DOI] [PubMed] [Google Scholar]
- 24. Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect 2013; 67: 463–469. [DOI] [PubMed] [Google Scholar]
- 25. Drolet M, Brisson M, Schmader K et al Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain 2010; 11: 1211–1221. [DOI] [PubMed] [Google Scholar]


